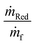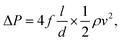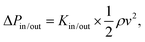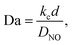Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Post-combustion emissions control in aero-gas turbine engines†
Prakash
Prashanth
 ,
Raymond L.
Speth
,
Sebastian D.
Eastham
,
Raymond L.
Speth
,
Sebastian D.
Eastham
 ,
Jayant S.
Sabnis
and
Steven R. H.
Barrett
,
Jayant S.
Sabnis
and
Steven R. H.
Barrett
 *
*
Laboratory for Aviation and the Environment, Department of Aeronautics and Astronautics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. E-mail: sbarrett@mit.edu
First published on 7th December 2020
Abstract
Emissions of nitrogen oxides (NOx) from aircraft cause air quality degradation and climate change. Efforts to improve the efficiency of aircraft propulsion systems are leading to small, power-dense engine cores with higher overall pressure ratios and combustion temperatures, which can result in higher NOx emissions. The trend towards smaller engine cores with smaller mass flow rates in the core stream, presents new opportunities for emissions control. Specifically, we propose and assess using a selective catalytic reduction (SCR) system that was previously infeasible when mass flow rates in the core were an order of magnitude larger than heavy-duty diesel engines for road based applications. SCR systems would reduce NOx emissions at the cost of increased aircraft weight and specific fuel consumption due to the pressure drop in the core stream induced by the catalyst. We quantify the effects of these trade-offs in terms of emissions reduction and fuel burn increase using representative engine cycle models provided by a major aero-gas turbine manufacturer. Due to its size, any SCR system will likely need to be housed in the aircraft body, potentially making it most suitable for future hybrid- or turbo-electric aircraft designs. Furthermore, SCR systems require ultra-low sulfur (ULS) fuel to prevent catalytic fouling. We find that employing an ammonia-based SCR results in an approximately 95% reduction in NOx emissions in exchange for a ∼0.5% increase in block fuel burn. The performance of the post-combustion emissions control (PCEC) system is shown to improve for smaller-core engines, such as those proposed in the NASA N + 3 time-line (2030–2035). Using a global chemistry-transport model we estimate that PCEC used with ULS fuel, could avert ∼92% of aviation air pollution related early deaths each year. Using a simplified climate model and accounting for changes in emissions (including life cycle emissions) and radiative forcing we estimate that PCEC with ULS fuel increases climate damages by ∼7.5%. We estimate that the net benefit of using PCEC accounting for air quality and climate impacts is 304 USD (2015) per metric tonne of jet fuel burned, or a reduction of ∼52% in monetized air quality and climate damages.
Broader contextEmissions of nitrogen-oxides (NOx) from the aviation industry have an impact on global climate change and air quality. It is well documented that NOx is a precursor to fine particulate matter and ozone, which have an adverse impact on human health. The continued growth of the aviation industry will further increase the absolute and relative contribution of aviation emissions to global pollution. Moreover, the current techniques used to reduce NOx emissions from aero-gas turbine engines are approaching their limit. Leveraging the trends in aircraft engine design and novel aircraft configurations such as turbo-electric designs, our work is the first proposal and assessment of post-combustion emissions control methods for aircraft gas turbine engines for a future commercial aircraft. Our findings indicate that using post-combustion emissions control can virtually eliminate aviation related air-quality damages at the cost of small increase in aviation climate impacts. While detailed investigations of various aspects and implications of post-combustion emissions control need to be undertaken, this work opens up a new area of study in the design of the next generation of aircraft and maybe a step towards the sustainable development of the aviation industry. |
1 Introduction
Emissions of oxides of nitrogen (NOx) adversely impact air quality and human health.1,2 NOx emitted at cruise altitude produces ozone that upon reaching the surface alters the background chemistry to increase the concentration of fine particulate matter (PM2.5). PM2.5 and ozone cause asthma, cardiovascular, and respiratory diseases,1,2 and increase risk of early death. Previous estimates (using 2006 data) suggest that NOx emissions from global aviation results in ∼16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 premature mortalities annually.3 With the current growth rate of the aviation industry at an average of 5% per year,4 the absolute and relative contribution of aviation NOx emissions to air pollution is likely to increase over the coming decades. Furthermore, local air quality degradation near airports inhibits airport expansion. NOx also has an impact on the climate, causing a short-term northern hemispheric warming effect on the order of aviation CO2, with a long-term global cooling effect due to methane destruction.5
000 premature mortalities annually.3 With the current growth rate of the aviation industry at an average of 5% per year,4 the absolute and relative contribution of aviation NOx emissions to air pollution is likely to increase over the coming decades. Furthermore, local air quality degradation near airports inhibits airport expansion. NOx also has an impact on the climate, causing a short-term northern hemispheric warming effect on the order of aviation CO2, with a long-term global cooling effect due to methane destruction.5
In the commercial aviation sector, gas turbines have been the primary choice of power plant since the early 1950s6 due to their high power density (relative to reciprocating engines) and suitability for high subsonic speeds. The thermodynamic efficiency of the gas turbine increases with higher overall pressure ratio (OPR). A higher OPR leads to increased thermal NOx production as the compressor exit temperature increases with OPR.7 Various combustor design strategies such as RQL (rich-quench-lean) combustion chambers have provided ∼50% reduction in NOx emissions compared to annular combustors8 but their effectiveness decreases as OPR of the engines increase.9 We propose that post-combustion treatment of the NOx emissions could offer a solution by eliminating >90% NOx emissions. It may also expand the design space for new engine architectures by partly decoupling combustor design from NOx control.
1.1 Post-combustion emissions control in other industries
Heavy-duty diesel engines and the power generation industry routinely use post-combustion emissions control to reduce their emissions. NOx emissions from aero-derivative engines (used for power generation) are approximately an order of magnitude lower than the original engines used in an aircraft.8 This is in part due to the choice of fuel. A liquid fuel will result in local regions of stoichiometric conditions as the fuel droplets evaporate,10 resulting in local high temperature pockets, that increase NOx formation whereas natural gas used in ground-based power plants tends to lead to lower NOx emissions.8 However, the bulk of the emission reduction in ground-based power plants (over 90%) comes from post-combustion emissions control that is primarily in the form of selective catalytic reduction (SCR).Prior to 1991, diesel engines in automobiles in the United States (US) did not require after-treatment and the average engine out NOx emissions were 4.6 g kW−1 h−1. By 2013 emissions regulations required all on-road engines in the US to use after-treatment measures to control emissions. The average NOx emissions from diesel engines was reduced to 0.27 g kW−1 h−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 over 20 years using SCR. This corresponds to approximately a 94% reduction in NOx emissions. SCR systems in modern engines remove 95% to 98%12 of NOx across the catalyst.
11 over 20 years using SCR. This corresponds to approximately a 94% reduction in NOx emissions. SCR systems in modern engines remove 95% to 98%12 of NOx across the catalyst.
1.2 Selective catalytic reduction (SCR)
SCR converts oxides of nitrogen (NO and NO2) to N2 and H2O in the presence of a catalyst using an ammonia based reducing agent. The following section describes the reaction pathways and characteristics of the catalysts used.| 4NO + 4NH3 + O2 → 4N2 + 6H2O |
| NO + NO2 + 2NH3 → 2N2 + 3H2O |
Greater than 90% of NOx emissions from typical diesel engines (and gas turbines) consists of NO.13 Since gas turbine emissions are also predominantly NO (approximately 95%),15 except at low thrust conditions16 as used in approach and taxi operations, the first of the two reactions is the primary reaction for deNOx (conversion of NOx to N2 and H2O) with ammonia.13
1.3 Challenges to implementing SCR on aircraft engines
Implementing SCR on aircraft engines will result in increased pressure drop in the core air stream, and aircraft fuel consumption is sensitive to such a pressure drop. The mass flow rates through the core of a gas turbine engine used to power an Airbus A320 size aircraft during cruise is 25–30 kg s−1 (based on cycle calculations and ref. 23). This is the mass flow that needs to be treated by the catalyst. For comparison, a heavy-duty diesel engine has a mass flow rate on the order of 1 kg s−1 (calculated at peak power for 4 stroke engines such as the Paccar MX1324). The higher mass flow rate in an aircraft engine increases the deleterious effect of a pressure drop in the core stream. The ideal operating temperature range for ion-exchanged zeolite SCR catalysts is approximately 550–650 K.25 This temperature range generally occurs after the low pressure turbine (LPT) for the engine class under consideration. Installing a catalyst downstream of the LPT will cause a pressure drop downstream of the turbine, thus reducing the work that it can extract. In order to maintain the required work output, the fuel flow to the engine needs to be increased from the baseline case (with no catalyst), thus increasing the thrust specific fuel consumption (SFC). Furthermore, aircraft fuel consumption is more sensitive to vehicle mass than is the case with road vehicles. In the past, these weight and SFC concerns had discouraged any investigation into the use of SCR in aircraft.8Today the core size of the engine is becoming smaller in new engine architectures such as the Pratt and Whitney geared turbofan and proposed small core engines.26 The smaller, power dense core implies that a smaller mass of exhaust gas needs to be treated for a fixed engine thrust. This reduces the impact of a pressure drop in the core stream on the engine SFC. Furthermore, these cores contribute little to the overall engine thrust. For example, approximately 8.0% of the gross thrust in the modeled geared turbofan comes from the core exhaust and we estimate that for a small core engine as described by Lord et al.26 the core flow will contribute 3.6% of the gross thrust.
Approaches designed to improve engine efficiency such as increased pressure ratios also increase NOx emissions. Present low-NOx combustor designs, which attempt to change the flame structure within the combustor to reduce residence time in high temperature regions9 will be less effective as the OPR increases.9 Post-combustion emissions control could provide an alternative approach.
Efforts to improve the overall efficiency of the aircraft have led to novel architectures and configurations. For example propulsion, airframe integration, distributed propulsion, turbo-/hybrid-electric propulsion, and boundary layer ingestion. The work done in these areas have been primarily aimed at improving the system level efficiency of the aircraft. These changes in configuration also present a new opportunity to implement an SCR based system to reduce the NOx emissions from the engine. For example, an SCR based system could be used in a turbo-/hybrid-electric aircraft with fuselage embedded gas turbines, or mechanical transmission in other configurations.
This work quantifies the additional fuel burn (which is proportional to CO2 emissions) incurred as a function of NOx reduction relative to a baseline design. We evaluate the environmental costs and benefits of lower NOx and increased CO2 emissions by quantifying air quality and climate impacts. We include the life cycle emissions of CO2 for the fuel (accounting for the desulfurization process) and ammonia (for SCR based post-combustion emissions control) in the analysis. The uncertainties in the analysis are propagated using a Monte Carlo approach, where feasible.
2 Methods
This section outlines the approach taken to evaluate the implementation of ammonia-based SCR of NOx on aircraft gas turbine engines, which is detailed in subsequent subsections. After sizing the catalyst, we quantify the pressure drop through the catalyst and use an engine model to calculate the increase in SFC. We then calculate the increase in fuel burn from the baseline case due to the additional weight of the reducing agent and the catalyst and the increased SFC due to the pressure loss in the catalyst. Using global atmospheric modeling tools and the calculated reduction in NOx we estimate the effect this has on ground level PM2.5 and ozone concentrations. Air quality impacts are estimated using epidemiological studies that relate the health impacts to the change in exposure to PM2.5 and ozone. Country-specific values of statistical life (VSL) are used to monetize the impacts. The changes in radiative forcing (RF) due to post-combustion emissions control are estimated using a radiative transfer model coupled to a global atmospheric chemistry-transport model. The changes in RF and CO2 (due to increased fuel burn) are used to quantify the monetized climate impacts using a simplified climate model (Section 2.5.1). These costs and benefits are then aggregated to estimate the overall monetized impact of adopting post-combustion emissions control.2.1 Mass transfer in monolithic catalyst and SCR model
The SCR process consists of bulk mass transfer, diffusion through the pores of the catalyst wash coat, followed by chemical reaction at the catalytic site. Each of these processes is temperature dependent – as the temperature increases, the chemical reaction rate increases exponentially27 while the diffusion coefficients of the gas increases approximately with T3/2,27 where T is the temperature of the gas. Therefore, at sufficiently high temperatures (T > 500 K), the bulk diffusion or mass transfer becomes the limiting process.14 This operating regime is referred to as the mass transfer-limited regime.In this section we describe a lumped parameter model of the monolithic reactor. Tronconi and Forzatti28 showed the adequacy of lumped parameter models for simulating SCR reactors, finding an average error between experiments and the lumped one-dimensional model of 1.3%. In this model, average values of velocity and non-dimensional species concentration over the channel cross-section are used. The non-dimensional NO concentration is represented by Γ = [NO]/[NO]0, where [NO] is the local concentration of NO and [NO]0 is the concentration of NO at the inlet to the catalyst channel.
Based on the work done by Tronconi and Forzatti28 we can express the efficiency of the catalyst in removing NOx in the exhaust (deNOx) as
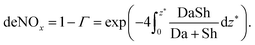 | (1) |
2.2 Pressure drop in monolithic catalysts
Installing an SCR catalyst downstream of the turbines introduces a pressure drop associated with the flow through a catalyst monolith. We estimate the pressure drop the fluid experiences with29where f is the Fanning friction factor, l is the length of the channel, d is the hydraulic diameter of the channel and
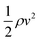 is the dynamic pressure of the flow. If the flow regime is laminar (as is almost always the case29) then the friction factor
is the dynamic pressure of the flow. If the flow regime is laminar (as is almost always the case29) then the friction factor 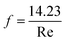 (for square channels), where
(for square channels), where 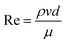 , ρ is the density, μ is the dynamic viscosity, and v is the local flow velocity of the exhaust gas. The pressure loss associated with the inlet and outlet of the channel are estimated as
, ρ is the density, μ is the dynamic viscosity, and v is the local flow velocity of the exhaust gas. The pressure loss associated with the inlet and outlet of the channel are estimated aswhere Kin/out is the inlet or outlet loss coefficients,29 which are given by
| Kin = −0.415 × OFA + 1.08 |
| Kout = (1 − OFA)2, |
2.3 Estimating the increase in SFC due to a pressure loss in the catalyst
A gas turbine cycle deck is used to estimate the increase in SFC due to the pressure loss through the catalyst monolith. In this work we use a GasTurb 13 engine model provided by Pratt and Whitney to evaluate the impact on SFC due to a pressure drop downstream of the LPT.The implications for three engines were assessed, a representative turbofan (110 kN (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lbf) thrust class), a geared turbofan for the same thrust class and a small core engine (58 kN (13
000 lbf) thrust class), a geared turbofan for the same thrust class and a small core engine (58 kN (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lbf) thrust class). The lower thrust of the small core engine reflects the lift to drag ratio (L/D ≈ 20)30 benefits from future airframes.
000 lbf) thrust class). The lower thrust of the small core engine reflects the lift to drag ratio (L/D ≈ 20)30 benefits from future airframes.
The effect of the pressure drop through the catalyst is modeled by varying the turbine exit duct pressure loss in a series of calculations. GasTurb was run iteratively such that the engine produces the same design point thrust for each turbine exit duct pressure drop by adjusting the combustor exit temperature. This corresponds to increasing the fuel flow rate and hence the SFC. The increase in the maximum landing mass and SFC, is used to calculate the percentage increase in fuel burn from eqn (2) as described in Section 2.4.
We size the catalyst by first considering effective bulk dimensions as shown in Fig. 2. The catalyst for this purpose is characterized by three parameters – the catalyst substrate, total frontal area (A) of the catalyst and the reacting length (l) of each channel in the catalyst. The catalyst substrate sets the hydraulic diameter of each channel, the bulk density and the open frontal area (OFA) of the catalyst. The total frontal area, A sets the local velocity of the flow in each channel by continuity and the reacting length of the channel sets the residence time of the exhaust gases within the catalyst.
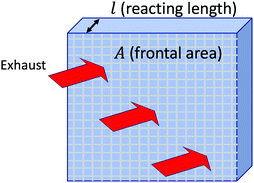 | ||
| Fig. 2 Bulk effective dimensions of the lumped catalyst model. Frontal area is defined as the area perpendicular to the flow through the catalyst. | ||
The above three parameters also indirectly affect the SFC of the engine. Once values are chosen for the substrate, flow through area and the reacting length we compute the pressure drop and the NOx conversion fraction. The pressure drop and additional weight is then used to calculate the increase in fuel burn from the baseline case (where no after-treatment is used and no additional weight is carried).
2.4 Estimating the fuel burn penalty
To evaluate the fuel burn penalty associated with a certain level of NOx removal we estimate the increase in SFC (due to the pressure drop) and mass of the aircraft (due to the mass of the SCR catalyst, reductant carried and associated components such as reductant tanks and pumps). We do not consider other changes in mass, assuming they are relatively small and that the change in mass occurs relative to some future baseline design, e.g. a turbo-electric aircraft. Given an aircraft's range (R), flight speed (V), L/D, maximum landing mass (MLW), and the propulsion system's SFC, the Breguet range equation can be used to estimate cruise fuel burn.31 To calculate the fuel burn for an aircraft with ammonia based SCR, the Breguet range equation needs to be modified, as detailed in the ESI,† to account for the consumption of the reductant during flight. For an aircraft with a given SFC, carrying and consuming fuel and reductant at the rate of ṁf and ṁRed, respectively, the mass of fuel required is,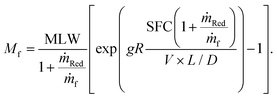 | (2) |
2.5 Modeling impacts of aviation emissions changes on global climate and air quality
The atmospheric chemistry and transport of various chemical species is calculated by using the GEOS-Chem global atmospheric chemistry-transport model (version 12.0.2).32 The standard mechanism is employed, including tropospheric and stratospheric chemistry and physics.33 The spatial resolution used is a 4° × 5° global grid, with 72 non-uniform vertical layers (from sea-level up to a pressure of 1 Pa). The MERRA-234 meteorological data from the Global Modeling and Assimilation Office (GMAO) at NASA's Goddard Space Flight Center is used. GEOS-Chem solves global chemistry and transport equations to estimate the global atmospheric composition at 20 minute and 10 minute time steps respectively.The baseline impact of aviation on radiative forcing and surface air quality is determined by performing two GEOS-Chem simulations, one with and one without aviation emissions for 2015, such that the differences in atmospheric composition between the two cases (after a spin up period of one year) are attributable to baseline aviation emissions. Similarly, the impact of post-combustion emissions control (PCEC) and ultra-low sulfur fuel (ULS) is the difference in atmospheric composition between simulations where aviation emissions are at their baseline values and simulations where the aviation emissions have been adjusted for a comparison scenario of fleet-wide use of PCEC with ULS fuel. The emissions are obtained by scaling down aviation NOx emissions and introducing ammonia emissions (NH3) to capture the effect of ammonia slip (any ammonia that remains un-reacted downstream of the catalyst) when PCEC is used. The fleet-wide application is not intended to be representative of an introduction scenario, but as with comparable analyses22 to enable calculation of a representative average of the environmental impacts of PCEC. The effect of ULS fuel is modeled by reducing the fuel sulfur content from 600 ppm (typical jet fuel) to 15 ppm. The CO2 emissions from the life-cycle of the fuel and ammonia (in the PCEC scenario) are also considered in the analysis.
The anthropogenic, biogenic, and natural emissions inventories in GEOS-Chem used for all scenarios are shown in Table 2. However, we note that the marginal benefits of NOx reduction from aviation may be higher if a future cleaner atmosphere were used as the background.35,36 Details of the aviation emissions inventory for each scenario considered, including life-cycle emissions are provided in Table 3. In the simulation year (2015) aviation emissions accounted for 2.1% of the global NOx emissions from all sources compared to ∼12% from lightning. If we consider the Northern Hemisphere above 1 km in altitude, aviation accounts for ∼20% of all NOx emissions, with the remainder being produced by lightning. NOx emissions in this region are associated with increased ozone production and climate impacts relative to surface NOx emissions.37 The NOx burden is provided in Section 3.7.
| Region | Inventory | Species |
|---|---|---|
| Global | EDGARv4340 | NOx, SOx, SO4, CO, NH3 |
| Global | BOND41 | BC, OC |
| Global | RETRO42 | NMVOCs except C2H6 and C3H8 |
| Global | SHIP43 | NOx, SO2, CO |
| Global | ParaNOx44 | O3, HNO3 |
| Global | C2H6_201045 | C2H6 |
| Global | POET46 | C2H5OH |
| Asia | MIX47 | NOx, SO2, CO, BC, OC, NMVOCs, NH3 |
| US | NEI201148 | NOx, SO2, CO, BC, OC, NMVOCs, NH3 |
| Canada | APEI49 | NOx, SOx, CO, BC, OC, NMVOCs, NH3 |
| Mexico | BRAVO50 | NOx, SO2, CO |
| Europe | EMEP51 | NOx, SO2, CO |
| Global | VOLCANO | SO2 |
| Global | Lightning52 | NO |
| Global | SoilNOx53 | NO |
| Global | BROMOCARB54 | CHBr3, CH2Br2 |
| Global | IODOCARB55 | CH3I, CH2I2, CH2ICl, CH2IBr |
| Global | MEGAN56 | Biogenic hydrocarbons |
The well-to-tank emissions for conventional jet fuel and ULS fuel are taken from Stratton et al.38 While the combustion CO2 emissions of ULS fuel are lower (by ∼0.4%) than conventional jet fuel, due to a change in the hydrogen to carbon ratio during the desulfurization process, the life-cycle CO2 emissions (well-to-wake) from ULS fuel are ∼2% higher than conventional jet fuel, which we account for.22,38 The global average estimate of life cycle emissions for ammonia are taken from Bicer et al.39
In this work a single year of aviation emissions (for the year 2015) and its integrated impact into the future is considered. This is carried out for each scenario outlined in Table 3. While post-combustion emissions control may not be applicable in all aircraft, this analysis will allow future research to scale the benefits and costs based on the percentage of aviation fuel burn where post-combustion emissions control is practical. A discount rate of 3% is used to discount the damages occurring in the future and the NPV is used to compare the climate damages from the two scenarios.
The premature mortalities due to aviation attributable PM2.5 and ozone are estimated using log-linear concentration response functions (CRF). The ozone impacts are estimated using the relative risk from Jerrett et al.58 This study found a 4% [95% CI: 1.3% to 6.7%] increase in risk of respiratory disease related mortality per 10 ppbv increase in the daily 1 hour maximum ozone concentration (MDA1) during local ozone season. The health impacts due to PM2.5 exposure are estimated using the relative risk from Hoek et al.59 This meta-analysis of epidemiological studies reports a 11% [95% CI: 5.0% to 16%] increase in cardiovascular mortality rates per 10 mg increase in annual average PM2.5 exposure.
An EPA-recommended60 cessation lag of 20 years is used. It assumes that 30% of the premature mortalities occur in the first year, 50% of the mortalities in the next 4 years and the final 20% over the remaining 15 years. Consistent with the method used in AMPT-IC, a discount rate of 3% is used when monetizing impacts. The damages due to premature mortalities are calculated based on the US EPA estimates of the value of statistical life (VSL).61 The resulting mean US VSL (scaled from 1990 income levels using an income elasticity of 0.7) is $10.2 million (in 2015 US dollars). The VSL for other countries is calculated from the US value using the gross domestic product per capita (PPP basis) and adjusted using an income elasticity of 0.7.62
3 Results and discussion
We obtain an estimate of the effectiveness of post-combustion emissions control for NOx reduction in aircraft gas turbine engines. The results shown here are for a geared turbofan configuration (based on data provided by a major aero-gas turbine manufacturer) with the SCR catalyst installed downstream of the LPT. The core exhaust is assumed to be accelerated downstream of the catalyst in a propelling nozzle to produce thrust. However, we envision that the actual application of post-combustion emissions control with a clean-sheet engine and aircraft design may be configured so that all the thrust is delivered by separate propulsors. This may be in a turbo-electric configuration or by mechanical transmission.3.1 Mass transfer limited regime
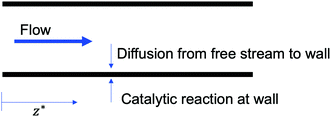
To verify that the catalyst is operating in the mass transfer limited regime we calculate the Damköhler numberwhere kc is the rate constant for the chemical reaction63 and DNO is the diffusivity of NO at a particular temperature and pressure which is calculated based on Tang et al.64 At temperatures of ∼450 °C the catalytic reactions are confined to a 5–10 μm layer of the wash-coat.13 We account for the effective diffusivity of the reactants using a porosity of ∼0.56 and tortuosity factor of 2 per Beeckman.65 The effective diffusion66 is Deff = θ × D/τ, where θ is the porosity and τ is the tortuosity. Therefore, Deff = 0.56 × D/2 ≈ 0.3 × D.
At the temperatures and pressures found downstream of the LPT, we find Da ≈ 1.6 × 1010, which indicates that the chemical reactions are several orders of magnitude faster than the mass transfer from the free stream to the wall.
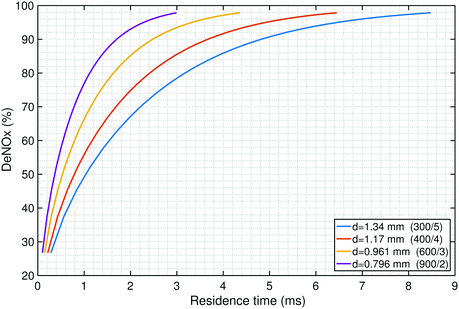
DeNOx is thus only dependent on the non-dimensional parameter z* = (zDNO)/(ud2). Thus the required residence time (τ = z/u) for a certain level of deNOx is dependent only on the square of the hydraulic diameter of the channel, d2 (for a given diffusivity DNO). A smaller channel diameter implies a shorter residence time is required as compared to a larger channel diameter (see Fig. 3).
3.2 Estimating fuel specific reductant consumption
The fuel-specific reductant consumption (FSRC) for various reductants is calculated based on Xm,NH3 (moles of NH3 per kg reductant) and an average cruise NOx emissions index (EI(NOx)) of 14 g kg−1.67 From the results in Table 1 we see that pure anhydrous liquid ammonia has the lowest reductant consumption as it has the highest ammonia content per unit mass. We note that post-combustion emissions control is also applicable to the landing and takeoff cycle, but here we first consider the cruise EI(NOx) as this dominates NOx emissions and corresponding reductant consumption.The capacity of the reductant storage tank and hence the weight of the storage system is estimated using eqn (2). The total mass of fuel spent for a 1500 km range mission is approximately 4.1 tonnes, which would require 21 kg of anhydrous NH3 to treat the NOx emissions (based on the FSRC calculated in Table 1). Using the density of anhydrous liquid NH3 the volume of the storage tank required is 35 L (9.25 gal). Storage tanks for anhydrous NH3 are typically filled to ∼85% of the total volume (∼15% vapour space must be maintained to account for expansion).68 Therefore for the design range the storage tank has a volume of ∼42 L (cylindrical tank of inner radius of 15 cm and a length of 0.6 m) and is designed for a gauge pressure of 250 psi (∼1725 kPa) (based on safety recommendations for ammonia storage69). This results in an empty tank weight of approximately 8 kg per aircraft. Anhydrous ammonia pumps for the required flow rates weigh approximately 60 kg70 per engine. Assuming that any additional mass requirements for piping and injectors are small, we use 128 kg (a pump for each engine in a two-engine aircraft and a single NH3 storage tank) as the total additional mass due to the reductant storage and delivery systems.
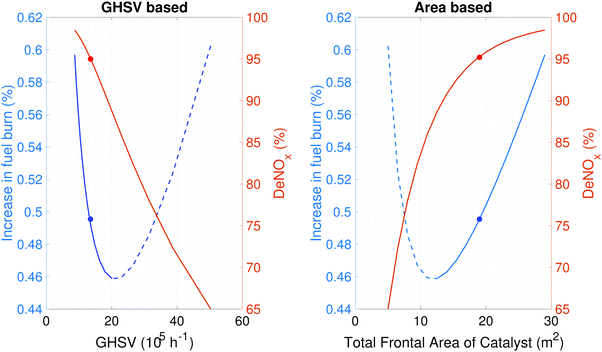
3.3 Effect of catalyst size on deNOx and fuel burn penalty
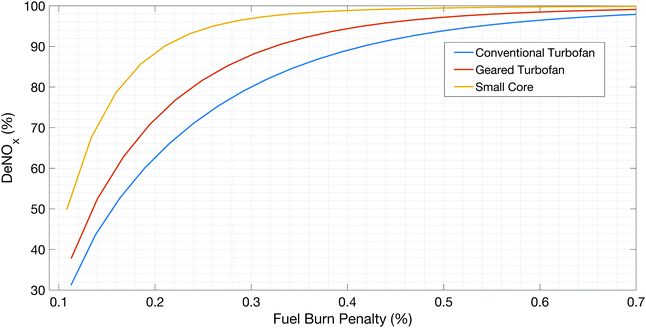
The effect of catalyst size on deNOx and the associated fuel burn penalty is shown in Fig. 4. The reacting length was fixed at 1.25 cm in this analysis as this results in a packed size that could fit in two of the typical seven containers of the cargo hold in an A320 aircraft.The gas hourly space velocity (GHSV) is defined as the ratio of the volume flow rate per hour of the exhaust gas to the bulk volume of the catalyst and is inversely proportional to the residence time in the catalyst. A large catalyst corresponds to a smaller GHSV (longer residence time) and hence shows a greater conversion of NOx. Fig. 4 shows that post-combustion emissions control as evaluated here has the potential to reduce the NOx emissions by 95% in exchange for approximately a 0.5% increase in fuel burn. The catalyst total frontal area required for this conversion is approximately 19 m2.
The deNOx at take-off conditions is approximately 75%. The lower NOx conversion efficiency at take-off is due to the higher pressures at sea-level (relative to cruise altitude) which decreases the effective diffusivity (Deff) of the reacting species by ∼60% relative to cruise conditions. The increased NO2 emission fraction at low thrust conditions (such as at idle conditions) does not affect the results because the conversion of NOx is limited by the bulk mass transfer and not the chemical kinetics (Da ≫ 1).
Reduction in the conversion efficiency while the catalyst warms up has not been accounted for. In addition, the impact of the NOx reduction across each flight segment, especially idle and taxi warrant further analysis with respect to local air quality.
The deNOx during cruise is higher (∼97%) which results in an effective deNOx of ∼95% over the full flight (a 1500 km range mission is assumed here). Furthermore, according to Yim et al.,71 cruise emissions account for three-quarters of the premature mortalities attributable to aviation PM2.5 and ozone. The design point of our catalyst is therefore chosen to be the cruise condition, however to ensure catalyst performance at off-design conditions, we calculate the temperature of the gas entering the catalyst at take-off and idle to be ∼480 and ∼250 °C respectively which fall well within the operating range (150–600 °C)17 of the zeolite class of substrates chosen in our analysis.
As the size of the catalyst is increased the pressure drop incurred can be reduced (decreasing fuel burn). However, this comes at the cost of additional weight (increasing fuel burn). This tradeoff is shown in the graph on the right in Fig. 4, as the frontal area of the catalyst is increased from approximately 5 m2 to 10 m2 the fuel burn penalty decreases. This is a consequence of the lower flow velocity and hence smaller pressure drop downstream of the LPT. Further increase in the flow through area results in an increase in fuel burn penalty. This is due to the catalyst mass, which affects the maximum landing mass of the aircraft and hence the fuel required to fly the same mission.
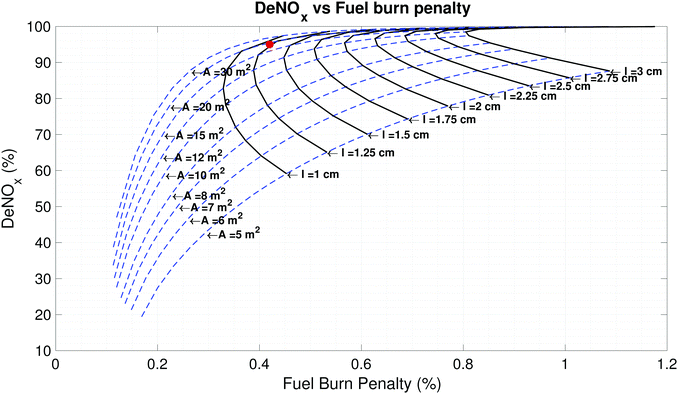
The dashed blue lines in Fig. 5 show that as the reacting length (l) is decreased for a fixed catalyst frontal area (A) the pressure drop and the catalyst volume (and hence catalyst mass) decrease. This causes the deNOx and fuel burn penalty to monotonically decrease. However, if l is held constant and A is increased, the pressure drop decreases but the catalyst mass increases. This causes the fuel burn penalty to first decrease and then increase as explained above. Higher lift to drag ratio airframes will mitigate the impact that this additional weight has on the fuel burn penalty, shifting the optimum. This is seen from the modified range equation (eqn (2)). Details of the SCR system at the chosen design point are outlined in Table 4.
| Parameter | Value |
|---|---|
| NOx reduction (deNOx) | 95% |
| Increase in fuel burn | 0.5% |
| Catalyst frontal area | 19 m2 |
| Reacting channel length | 1.25 cm |
| Catalyst porosity | 0.56 |
| Catalyst tortuosity factor | 2 |
| Catalyst mass (per engine) | 91 kg |
| Mass of reductant (for 1500 km mission) | 21 kg |
| Additional system mass (pumps, storage tanks, etc. per aircraft) | 128 kg |
| Pressure loss at cruise | 115 Pa |
| Packed volume of catalyst | 1.57 m3 |
3.4 Trade off between deNOx, ammonia slip, and fuel burn penalty
Emissions of unreacted ammonia, referred to as ammonia slip, can be quantified using the stoichiometric ratio of the SCR reaction. In some designs, a catalyst is introduced downstream of the SCR to oxidize any unreacted ammonia in the exhaust stream. Catalyst designs have also been proposed where the monolith substrate is coated in layers of different catalytic materials which minimizes ammonia slip. For a reacting length of 1.25 cm and a total frontal area of 19 m2 we calculate a 95% reduction in NOx emissions for approximately a 0.5% increase in fuel burn. Calculating the average ammonia slip over the mission in terms of an emission index gives an EI(NH3) of approximately 0.26 g NH3 per kg fuel.While ammonia slip at ground level results in the formation of PM2.5 which adversely affects human health,1 cruise altitude emissions of ammonia do not share the same risk, since neither the ammonia nor its products would reach population at ground level due to wet deposition and atmospheric transport phenomenon at cruise altitude. However, we do include these emissions. The impact of ammonia slip is captured by the GEOS-Chem simulations as presented in Section 3.7. As identified by Eastham et al.3 the transport of aviation attributable ozone from cruise altitude is the mechanism responsible for human exposure to both ozone and PM2.5. This is supported by the analysis presented in Section 3.7.
3.5 Effect of engine core size on post-combustion emissions control
The 2016 report by the National Academies of Sciences, Engineering and Medicine on reducing global aviation carbon dioxide emissions72 identifies small core engines as one of the high-priority research areas to reduce CO2 emissions from commercial aviation. The NASA N + 3 aircraft concept design and trade studies final report9 also outlines the interest in small core, high efficiency engines that are to be employed along with other configurations such as blended wing bodies, boundary layer ingestion and distributed propulsion.9,26,30 We evaluate the impact that a small core engine architecture would have with regards to the use of post-combustion emissions control as outlined in this work.Fig. 6 shows the results of evaluating the after-treatment methods on three different engine architectures. The conventional turbofan is representative of a modern mixed flow turbofan, the geared turbofan represents the state of the art low fan pressure ratio geared turbofans, and the small core engine is representative of an advanced engine architecture that was proposed to be used on the MIT D8 aircraft.26
We see from Fig. 6 that the performance of the post-combustion control system improves as the core size decreases. Considering the core size (expressed as the corrected mass flow at compressor exit), current generation engines have a core size of 3.18 kg s−1 (7 lb s−1), geared turbofans have a core size of 2.27 kg s−1 (5 lb s−1) and the next generation engines may have smaller core sizes of ∼0.68 kg s−1 (1.5 lb s−1).26 The thrust size for the conventional and geared turbofan engines is 110 kN (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lbf) and the small core engine has the above core size is 58 kN (13
000 lbf) and the small core engine has the above core size is 58 kN (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lbf). The small core engine has a lower thrust rating since the airframe envisioned by Lord et al.26 (the MIT D8 design) has a higher L/D of approximately 20.30
000 lbf). The small core engine has a lower thrust rating since the airframe envisioned by Lord et al.26 (the MIT D8 design) has a higher L/D of approximately 20.30
The authors envision the proposed post-combustion emissions control methods could be implemented with a small core architecture that could be housed within the body of the aircraft in a turbo-electric configuration or possibly with a decoupled propulsor such as in the D8 aircraft.26 This could allow installation of the catalyst in the fuselage of the aircraft. The core flow in such a design would thus contribute little or no thrust, although the design may be configured such that the core ingests the airframe boundary layer, providing scope for further improvement of the post-combustion emissions control performance.
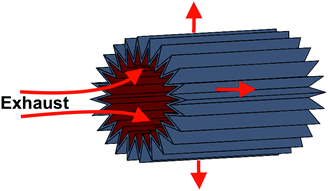
3.6 Packing constraints and maintenance
The packaging of this catalyst into the airframe may not be possible with a “flat” catalyst configuration as shown in Fig. 2. An air-filter like pleated design allows us to pack a large area catalyst into a small packing volume. A schematic is shown in Fig. 7, where the flow enters axially and leaves radially. As shown in the supplementary information, a pleated design with internal radius r, pleat depth h, N pleats, reacting length l, and total length L, surface area of the interior is given by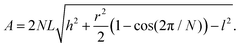 | (3) |
Applying eqn (3) shows that we can fit this area of catalyst into a cylinder of length 2.2 m and outer diameter of 1 m (using 24 pleats and a pleat depth of 18 cm). Detailed analysis concerning the packing and manufacturing of the catalyst design will be subject of future research.
The efficiency of the catalyst in removing NOx from the exhaust decreases over time. The typical life time of a catalyst used in ground-based power plants is ∼40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–60
000–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours.69 Assuming a similar life time for the catalyst used on board an aircraft and maintenance (C-check) intervals of ∼7500 hours,73 the catalyst will need to be replaced every 5–8 maintenance cycles.
000 hours.69 Assuming a similar life time for the catalyst used on board an aircraft and maintenance (C-check) intervals of ∼7500 hours,73 the catalyst will need to be replaced every 5–8 maintenance cycles.
3.7 Air quality impacts due to post-combustion emissions control
We use the GEOS-Chem global chemistry and transport model to estimate the air quality impacts of applying post-combustion emissions control to aviation.We find that the contribution of global aviation to NOx emissions while using post-combustion emissions control along with ULS fuel is approximately 0.11%, while the baseline contribution of aviation as outlined in Section 2.5 is ∼2.1%. Furthermore, the combination of PCEC with ULS fuel reduces aviation's contribution to NOx emissions in the free-troposphere of the Northern Hemisphere to 0.81% from a baseline of ∼20%. Additionally, baseline aviation emissions are responsible for ∼34% of the Northern Hemisphere NOx mixing ratios at typical cruise altitudes (10–12 km) (i.e. zonally mass averaged across cruise altitudes and the Northern Hemisphere). The use of PCEC along with ULS fuel reduces the aviation attributable NOx mixing ratio at Northern Hemisphere cruise altitudes to approximately 0.25% (see ESI† for further information).
| Species | Δozone [ppbv] | ΔPM2.5 [μg m−3] |
|---|---|---|
| Baseline aviation | 0.640 | 0.0702 |
| ULS | 0.641 | 0.0625 |
| PCEC with ULS | 0.0223 | 0.00911 |
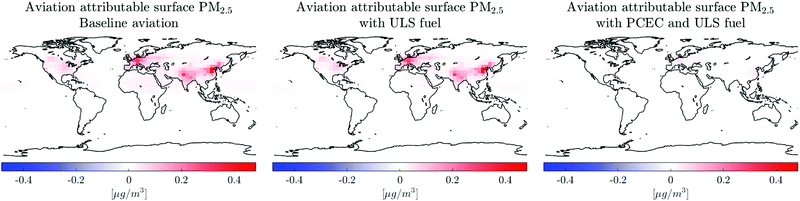
Post-combustion emissions control along with desulfurized jet fuel leads to a reduction (87% from the baseline as defined in Table 3) in population exposure to PM2.5 of which approximately 11% of the reduction in population exposure to PM2.5 is due to the use of ULS fuel and the rest is attributable to the removal of NOx. ULS fuel is required to prevent fouling of the catalyst as detailed in Section 1.2.4. The reduction in surface concentration of PM2.5 is therefore primarily attributable to the post-combustion reduction of NOx emissions. The global distribution of PM2.5 and the reduction due to PCEC is shown in Fig. 8.
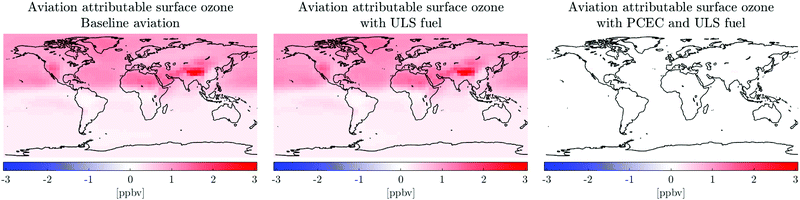
We find that using ULS fuel results in a reduction of sulfate aerosol in the lower stratosphere. This leads to a reduction in heterogeneous hydrolysis of N2O5 on sulfate aerosols and a subsequent reduction in ozone depletion by halogen catalysed cycles.74 This increases the ozone concentration in the lower stratosphere and in stratospheric air masses that enter the troposphere, thereby resulting in an increase in the surface concentration of ozone as seen in Table 5. This finding is consistent with the findings by Eastham et al.75
Furthermore, the identified pathway implies that this effect will reduce in future years as the concentration of halogens in the atmosphere decreases (since the adoption of the Montreal Protocol).
The average reduction in population exposure to ozone due to the use of post-combustion emissions control with ULS fuel is 97%. The reduction in surface ozone concentration is a consequence of the reduced NOx emissions due to post-combustion control through the mechanism described by Eastham et al.3
While reducing ground level ozone concentration has a health benefit, a reduction in column ozone can increase the risk of melanoma. However as estimated by Eastham et al.3 the avoided mortalities due to melanoma resulting from column ozone created by aviation is small (2.5%) compared to the PM2.5 and ozone related air quality impacts attributable to aviation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 globally [95% CI: 14
000 globally [95% CI: 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 34
000 to 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000]. Of this the premature mortalities due to aviation attributable PM2.5 is ∼15
000]. Of this the premature mortalities due to aviation attributable PM2.5 is ∼15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 [95% CI: 7300 to 22
000 [95% CI: 7300 to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000]. An additional 8900 [95% CI: 2900 to 15
000]. An additional 8900 [95% CI: 2900 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000] premature mortalities are due to an increased exposure to ozone.
000] premature mortalities are due to an increased exposure to ozone.
These baseline values are consistent with previous estimates of aviation attributable premature mortalities [3] when accounting for the addition of new emission inventories in GEOS-Chem and the increase in aviation fuel burn by ∼30% (188 Tg in the AEDT-2005 inventory vs. 240 Tg in the AEDT-2015 inventory). The PM2.5 and ozone attributable premature mortalities in each of the scenarios outlined in Table 3 are shown in Fig. 10.
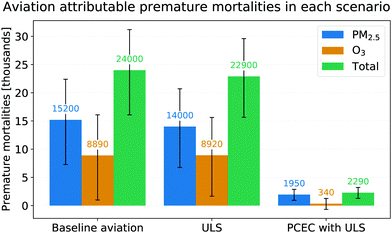 | ||
| Fig. 10 Premature mortalities attributable to aviation under the scenarios considered. The error bars shown are the 95% confidence intervals from the Monte Carlo runs. | ||
Post-combustion emissions control used with ULS jet fuel (PCEC-ULS), decreases the population exposure to PM2.5 and ozone by reducing NOx and SOx emissions. Converting exposure to mortality using the concentration response functions described earlier, we estimate that ∼13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 premature mortalities (95% CI: 6300 to 19
000 premature mortalities (95% CI: 6300 to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) due to exposure to PM2.5 and ∼8500 premature mortalities (95% CI: 2800 to 14
000) due to exposure to PM2.5 and ∼8500 premature mortalities (95% CI: 2800 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) due to exposure to ozone are avoided by using PCEC and ULS fuel. Furthermore, ∼12
000) due to exposure to ozone are avoided by using PCEC and ULS fuel. Furthermore, ∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (95% CI: 5900 to 18
000 (95% CI: 5900 to 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) of the ∼13
000) of the ∼13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 avoided premature mortalities due to decreased exposure to PM2.5 are attributable to the post-combustion removal of NOx emissions while the remaining avoided premature mortalities are attributable to reduced PM2.5 from the use of ULS fuel (see Section 2.5).
000 avoided premature mortalities due to decreased exposure to PM2.5 are attributable to the post-combustion removal of NOx emissions while the remaining avoided premature mortalities are attributable to reduced PM2.5 from the use of ULS fuel (see Section 2.5).
Therefore ∼22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 [95% CI: 13
000 [95% CI: 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 31
000 to 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000] total premature mortalities are avoided due to the use of PCEC with ULS fuel annually. This is approximately 92% of all premature mortalities attributable to aviation as calculated in this study. The air quality benefits of using PCEC-ULS are monetized as described in Section 2.5.2. The benefit associated with the averted premature mortalities by using PCEC (with ULS fuel), amounts to approximately 77 billion USD (2015) annually [95% CI: 45 to 110 billion USD], or $320 per tonne of fuel burned.
000] total premature mortalities are avoided due to the use of PCEC with ULS fuel annually. This is approximately 92% of all premature mortalities attributable to aviation as calculated in this study. The air quality benefits of using PCEC-ULS are monetized as described in Section 2.5.2. The benefit associated with the averted premature mortalities by using PCEC (with ULS fuel), amounts to approximately 77 billion USD (2015) annually [95% CI: 45 to 110 billion USD], or $320 per tonne of fuel burned.
3.8 Climate impacts due to post-combustion emissions control
The change in RF due to aviation emissions as estimated by RRTMG is shown in Table 6. We use RRTMG to estimate RF for tropospheric nitrates, sulfates and black carbon. Ozone RF is quantified in APMT, using an approach which accounts for both short-term and long-term ozone responses.| Baseline aviation | ULS | PCEC with ULS | |
|---|---|---|---|
| Sulfates | −6.21 | −1.41 | −0.114 |
| Nitrates | −0.667 | −1.83 | −0.387 |
| Black carbon | 0.537 | 0.534 | 0.568 |
The lower sulfate concentration when ULS fuel is used, reduces competition for available ammonium resulting in an increase in nitrate formation and therefore an increased cooling effect from nitrates in the ULS scenario as seen in Table 6. The changes in black carbon RF are negligible. The values of radiative forcing from Table 6 are used in APMT-IC to estimate the climate damages due to aviation.
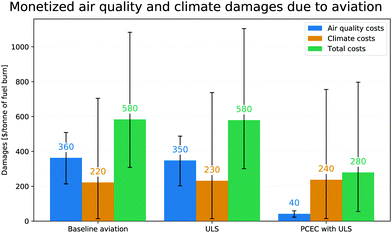
The total climate damages associated with ULS fuel and post-combustion emissions control is estimated using APMT-IC to be approximately 57 billion USD (or $238 per tonne of fuel burned) compared to a baseline climate damage of 53 billion USD (or $222 per tonne of fuel burned) due to global aviation without post-combustion emissions control. These damages include the life cycle emissions of CO2 as detailed in Table 7. Therefore the use of PCEC with ULS fuel results in a ∼7.5% increase in climate damages from all aviation. As seen in Table 7 the dominant contribution is from the decreased cooling effect due to a lower sulfate aerosol concentration when ULS fuel is used. The increase in climate damages due to an increase in fuel burn (∼0.5%) as a result of the additional weight and pressure losses introduced by the PCEC system is partially offset by the lower combustion CO2 emissions from the ULS fuel used22,38 as seen in Table 7.
| Baseline | ULS | PCEC with ULS | |
|---|---|---|---|
| a Note that the total cost includes life cycle CO2 and consists of other short-lived forcers that are not shown in the table. | |||
| Life cycle CO2 (of which combustion CO2) | 40.4 (33.7) | 41.0 (33.5) | 41.3 (33.7) |
| NOx | −1.02 | −1.43 | −0.175 |
| Sulfates | −2.19 | −0.0120 | −0.00100 |
| Black carbon | 0.190 | 0.189 | 0.201 |
| Total costa | 53.2 | 55.6 | 57.2 |
The net benefit (i.e. the monetized benefit due to avoided premature mortalities less the increase in climate damages) is therefore approximately 73 billion USD annually [95% CI: 40 to 100 billion USD] or a mean value of $304 per ton of jet fuel burnt. The environmental costs normalized by fuel burn (from degraded air quality and climate related damages) are shown in Fig. 11. The baseline costs are consistent with recent work by Grobler et al.76
4 Conclusions
This work is the first proposal and assessment of post-combustion emissions control techniques for aircraft gas turbine engines and evaluates the case for the use of selective catalytic reduction for NOx control in the aviation sector. The analytical approach, developed based on prior work done in SCR applications for diesel engines shows that a 95% reduction in NOx emissions can be achieved for approximately a 0.5% increase in fuel burn. The sensitivity of the fuel burn to catalyst mass and catalyst induced pressure drop show that the performance of the emissions control system improves for future designs where smaller core sizes, higher engine efficiency and higher L/D airframes are expected. Furthermore optimization and improvements in catalyst technology will further improve the performance of post-combustion emissions control.The current work quantifies the impact that a fleet-wide adoption of post-combustion emissions control will have on air quality and climate. However, the size requirements of the SCR system, particularly of the catalyst, imply that they will have to be housed within the aircraft fuselage, making this unsuitable for certain classes of aircraft. Post-combustion emissions control systems might be better suited for a hybrid- or turbo-electric design with small core engines. A NASA N + 3 aircraft design such as the D8 with small core engines, and turbo-electric designs, may offer further potential for optimization. Additionally using post-combustion emissions control to reduce NOx could result in combustor design space benefits that improve combustor efficiency. Further analysis is required to quantify the performance of such an integrated aircraft system. The spatial distribution of aviation hubs and missions flown by aircraft where PCEC is feasible might result in spatial variations of the impacts, which also need to be quantified. Since the implementation cost of post-combustion emissions control technology is dependent on the aircraft configuration and specific design concepts, we do not include the cost of implementation in this analysis. However we estimate that the increase in annual fleet-wide operating cost due to the increased fuel burn (of ∼1.30 Tg per year) is approximately 875 million USD based on average price of $86 per bbl for Jet-A.77
Using GEOS-Chem it is estimated that approximately 87% of surface PM2.5 concentration and 97% of ozone concentration due aviation emissions is averted with the use of post-combustion emissions control with desulfurized jet fuel (as is required for PCEC) for a fleet-wide implementation (as a hypothetical analysis scenario). An analysis based on epidemiological studies shows that ∼22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 premature mortalities are avoided (∼92% of all premature mortalities attributable to aviation) due to exposure to PM2.5 and ozone, if post-combustion emissions control is used along with ULS jet fuel. The mean monetized air quality benefits due to this is estimated to be $77 billion annually. The increase climate damages associated with the use of post-combustion emissions control is estimated using APMT-IC to be $4 billion annually. An environmental cost-benefit analysis, therefore, indicates that the net benefit of post-combustion emissions control is approximately $73 billion annually or $304 per ton of jet fuel burned.
000 premature mortalities are avoided (∼92% of all premature mortalities attributable to aviation) due to exposure to PM2.5 and ozone, if post-combustion emissions control is used along with ULS jet fuel. The mean monetized air quality benefits due to this is estimated to be $77 billion annually. The increase climate damages associated with the use of post-combustion emissions control is estimated using APMT-IC to be $4 billion annually. An environmental cost-benefit analysis, therefore, indicates that the net benefit of post-combustion emissions control is approximately $73 billion annually or $304 per ton of jet fuel burned.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the informative discussions with Professor Wai Cheng from the Sloan Automotive Laboratory and Dr Justin Kamp from the Department of Chemical Engineering at the Massachusetts Institute of Technology. We would also like to thank Pratt and Whitney for providing representative engine models to perform this study.References
- D. W. Dockery, C. A. Pope, X. Xu, J. D. Spengler, J. H. Ware, M. E. Fay, B. G. Ferris and F. E. Speizer, N. Engl. J. Med., 1993, 329, 1753–1759 CrossRef CAS.
- M. Kampa and E. Castanas, Environ. Pollut., 2008, 151, 362–367 CrossRef CAS.
- S. D. Eastham and S. R. H. Barrett, Atmos. Environ., 2016, 144, 17–23 CrossRef CAS.
- Boeing Commercial Airplanes Market Analysis, Current Market Outlook 2016–2035, 2016, http://www.boeing.com/cmo.
- C. S. Dorbian, P. J. Wolfe and I. A. Waitz, Atmos. Environ., 2011, 45, 2750–2759 CrossRef CAS.
- H. Cohen, G. F. C. Rogers and H. I. H. Saravanamuttoo, Gas Turbine Theory, Longman Pub Group, 1996 Search PubMed.
- A. H. Lefebvre, Int. J. Turbo Jet-Engines, 1986, 21, 231–243 Search PubMed.
- T. C. Lieuwen and V. Yang, Gas Turbine Emissions, Cambridge University Press, 2013 Search PubMed.
- E. M. Greitzer, P. A. Bonnefoy, E. D. L. R. Blanco, C. S. Dorbian, M. Drela, D. K. Hall, R. J. Hansman, J. I. Hileman, R. H. Liebeck, J. Lovegren, P. Mody, J. A. Pertuze, S. Sato, Z. S. Spakovszky, C. S. Tan and J. S. Hollman, N + 3 Aircraft Concept Designs and Trade Studies, Final Report Volume 1, 2010, https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20100042401.pdf.
- A. Lefebvre, Gas Turbine Combustion, Taylor & Francis, 1983 Search PubMed.
- ed. H. Jääskeläinen and M. K. Khair, DieselNet Technology Guide: Diesel Engines, 2013, https://www.dieselnet.com/tech/diesel_engines.php Search PubMed.
- T. V. Johnson, Urea-SCR Technology for deNOx After Treatment of Diesel Exhausts, Springer, New York, 2014, pp. 3–31 Search PubMed.
- M. Koebel, M. Elsener and M. Kleemann, Catal. Today, 2000, 59, 335–345 CrossRef CAS.
- E. Tronconi and A. Beretta, Catal. Today, 1999, 52, 249–258 CrossRef CAS.
- M. J. Moore, Proc. Inst. Mech. Eng., Part A, 1997, 211, 43–52 CrossRef.
- M. T. Timko, S. C. Herndon, E. C. Wood, T. B. Onasch, M. J. Northway, J. T. Jayne, M. R. Canagaratna, R. C. Miake-Lye and W. B. Knighton, J. Eng. Gas Turbines Power, 2010, 132, 061504 CrossRef.
- W. A. Majewski, DieselNet Technology Guide: Diesel Catalysts, 2005, http://www.dieselnet.com/tech/cat_scr.php.
- W. A. Majewski and H. E. Jääskeläinen, DieselNet Technology Guide: Catalytic Coating & Materials, 2005, https://www.dieselnet.com/tech/cat_mat.php.
- J. Wiehl and C. D. Vogt, MTZ Worldwide, 2003, 64, 8–11 CrossRef.
- G. Fulks, G. B. Fisher, K. Rahmoeller, M.-C. Wu, E. D'Herde and J. Tan, SAE Technical Paper, 2009.
- Environmental Protection Agency Control of Air Pollution From New Motor Vehicles: Heavy-Duty Engine and Vehicle Standards and Highway Diesel Fuel Sulfur Control Requirements, 12, 2001.
- S. R. H. Barrett, S. H. L. Yim, C. K. Gilmore, L. T. Murray, S. R. Kuhn, A. P. K. Tai, R. M. Yantosca, D. W. Byun, F. Ngan, X. Li, J. I. Levy, A. Ashok, J. Koo, H. M. Wong, O. Dessens, S. Balasubramanian, G. G. Fleming, M. N. Pearlson, C. Wollersheim, R. Malina, S. Arunachalam, F. S. Binkowski, E. M. Leibensperger, D. J. Jacob, J. I. Hileman and I. A. Waitz, Environ. Sci. Technol., 2012, 46, 4275–4282 CrossRef CAS.
- A. F. El-Sayed, Aircraft Propulsion and Gas Turbine Engines, CRC Press, 2nd edn, 2017 Search PubMed.
- Paccar MX13 Engine Specifications, https://paccarpowertrain.com/MX-13-Spec-Sheet.pdf.
- M. P. Harold and P. Metkar, Urea-SCR Technology for deNOx After Treatment of Diesel Exhausts, Springer, New York, 2014, pp. 311–356 Search PubMed.
- W. K. Lord, G. Suciu, J. Chandler and K. Hasel, 53rd AIAA Aerospace Sciences Meeting, 2015.
- W. A. Majewski, DieselNet Technology Guide: Catalyst Fundamentals, 2000, https://www.dieselnet.com/tech/cat_fund.php.
- E. Tronconi and P. Forzatti, AIChE J., 1992, 38, 201–210 CrossRef CAS.
- W. A. Majewski and H. E. Jääskeläinen, DieselNet Technology Guide: Cellular Monolith Substrates, 1998, https://www.dieselnet.com/tech/cat_substrate.php.
- M. Drela, 29th AIAA Applied Aerodynamics Conference, 2011, 27–30.
- J. Anderson, Introduction to Flight, McGraw-Hill Higher Education, 2015 Search PubMed.
- I. Bey, D. J. Jacob, R. M. Yantosca, J. A. Logan, B. D. Field, A. M. Fiore, Q. Li, H. Y. Liu, L. J. Mickley and M. G. Schultz, J. Geophys. Res.: Atmos., 2001, 106, 23073–23095 CrossRef CAS.
- S. D. Eastham, D. K. Weisenstein and S. R. Barrett, Atmos. Environ., 2014, 89, 52–63 CrossRef CAS.
- R. Gelaro, W. McCarty, M. J. Suárez, R. Todling, A. Molod, L. Takacs, C. A. Randles, A. Darmenov, M. G. Bosilovich, R. Reichle, K. Wargan, L. Coy, R. Cullather, C. Draper, S. Akella, V. Buchard, A. Conaty, A. M. da Silva, W. Gu, G.-K. Kim, R. Koster, R. Lucchesi, D. Merkova, J. E. Nielsen, G. Partyka, S. Pawson, W. Putman, M. Rienecker, S. D. Schubert, M. Sienkiewicz and B. Zhao, J. Clim., 2017, 30, 5419–5454 CrossRef.
- C. K. Gilmore, S. R. Barrett, J. Koo and Q. Wang, Environ. Res. Lett., 2013, 8(3), 034027 CrossRef.
- M. Woody, B. Haeng Baek, Z. Adelman, M. Omary, Y. Fat Lam, J. Jason West and S. Arunachalam, Atmos. Environ., 2011, 45, 3424–3433 CrossRef CAS.
- K. Dahlmann, V. Grewe, M. Ponater and S. Matthes, Atmos. Environ., 2011, 45, 2860–2868 CrossRef CAS.
- R. Stratton, H. Wong and J. Hileman, Life cycle greenhouse gas emissions from alternative jet fuels, 2010 Search PubMed.
- Y. Bicer, I. Dincer, C. Zamfierescu, G. Vezina and F. Raso, J. Cleaner Prod., 2016, 135, 1379–1395 CrossRef CAS.
- European Commission, Joint Research Centre (JRC)/Netherlands Environmental Assessment Agency (PBL), Emission Database for Global Atmospheric Research (EDGAR), 2011, http://edgar.jrc.ec.europa.eu, Release version 4.2.
- T. C. Bond, E. Bhardwaj, R. Dong, R. Jogani, S. Jung, C. Roden, D. G. Streets and N. M. Trautmann, Global Biogeochem. Cycles, 2007, 21(2) DOI:10.1029/2006GB002840.
- M. G. Schultz, A. Heil, J. J. Hoelzemann, A. Spessa, K. Thonicke, J. G. Goldammer, A. C. Held, J. M. C. Pereira and M. van het Bolscher, Global Biogeochem. Cycles, 2008, 22(2) DOI:10.1029/2007GB003031.
- C. Wang, J. J. Corbett and J. Firestone, Environ. Sci. Technol., 2008, 42, 193–199 CrossRef CAS.
- G. C. M. Vinken, K. F. Boersma, D. J. Jacob and E. W. Meijer, Atmos. Chem. Phys., 2011, 11, 11707–11722 CrossRef CAS.
- Z. A. Tzompa-Sosa, E. Mahieu, B. Franco, C. A. Keller, A. J. Turner, D. Helmig, A. Fried, D. Richter, P. Weibring, J. Walega, T. I. Yacovitch, S. C. Herndon, D. R. Blake, F. Hase, J. W. Hannigan, S. Conway, K. Strong, M. Schneider and E. V. Fischer, J. Geophys. Res.: Atmos., 2017, 122, 2493–2512 CAS.
- D. B. Millet, A. Guenther, D. A. Siegel, N. B. Nelson, H. B. Singh, J. A. de Gouw, C. Warneke, J. Williams, G. Eerdekens, V. Sinha, T. Karl, F. Flocke, E. Apel, D. D. Riemer, P. I. Palmer and M. Barkley, Atmos. Chem. Phys., 2010, 10, 3405–3425 CrossRef CAS.
- M. Li, Q. Zhang, J. Kurokawa, J.-H. Woo, K. B. He, Z. Lu, T. Ohara, Y. Song, D. G. Streets, G. R. Carmichael, Y. F. Cheng, C. P. Hong, H. Huo, X. J. Jiang, S. C. Kang, F. Liu, H. Su and B. Zheng, Atmos. Chem. Phys. Discuss., 2015, 15, 34813–34869 CrossRef.
- H. Simon, L. Beck, P. V. Bhave, F. Divita, Y. Hsu, D. Luecken, J. D. Mobley, G. A. Pouliot, A. Reff, G. Sarwar and M. Strum, Atmos. Pollut. Res., 2010, 1, 196–206 CrossRef CAS.
- Air Pollutant Emission Inventory (APEI) Historical Trends – Open Government Portal, https://open.canada.ca/data/en/dataset/fa1c88a8-bf78-4fcb-9c1e-2a5534b92131, (Accessed on 01/29/2019).
- H. Kuhns, M. Green and V. Etyemezian, BRAVO Technical Steering Committee, 2001.
- M. Auvray and I. Bey, J. Geophys. Res.: Atmos., 2005, 110, D11303 CrossRef.
- L. T. Murray, D. J. Jacob, J. A. Logan, R. C. Hudman and W. J. Koshak, J. Geophys. Res.: Atmos., 2012, 117, D20307 CrossRef.
- R. C. Hudman, N. E. Moore, A. K. Mebust, R. V. Martin, A. R. Russell, L. C. Valin and R. C. Cohen, Atmos. Chem. Phys., 2012, 12, 7779–7795 CrossRef CAS.
- Q. Liang, R. S. Stolarski, S. R. Kawa, J. E. Nielsen, A. R. Douglass, J. M. Rodriguez, D. R. Blake, E. L. Atlas and L. E. Ott, Atmos. Chem. Phys., 2010, 10, 2269–2286 CrossRef CAS.
- C. Ordóñez, J.-F. Lamarque, S. Tilmes, D. E. Kinnison, E. L. Atlas, D. R. Blake, G. S. Santos, G. Brasseur and A. Saiz-Lopez, Atmos. Chem. Phys., 2012, 12, 1423–1447 CrossRef.
- A. B. Guenther, X. Jiang, C. L. Heald, T. Sakulyanontvittaya, T. Duhl, L. K. Emmons and X. Wang, Geosci. Model Dev., 2012, 5, 1471–1492 CrossRef.
- K. Marais, S. P. Lukachko, M. Jun, A. Mahashabde and I. A. Waitz, Meteorol. Z., 2008, 17, 157–172 CrossRef.
- M. Jerrett, R. T. Burnett, C. A. Pope, K. Ito, G. Thurston, D. Krewski, Y. Shi, E. Calle and M. Thun, N. Engl. J. Med., 2009, 360, 1085–1095 CrossRef CAS.
- G. Hoek, R. M. Krishnan, R. Beelen, A. Peters, B. Ostro, B. Brunekreef and J. D. Kaufman, Environ. Health, 2013, 12, 43 CrossRef CAS.
- T. Cameron and B. Ostro, Washington, DC, Science Advisory Board, 2004.
- Guidelines for Preparing Economic Analysis (2010, revised 2014), 2014, https://www.epa.gov/environmental-economics/guidelines-preparing-economic-analysis-2010-revised-2014, (Accessed on 01/29/2019).
- L. A. Robinson and J. K. Hammitt, The effect of income on the value of mortality and morbidity risk reductions. Recommended Income Elasticity and Income Growth Estimates: Technical Memorandum, 2016, https://yosemite.epa.gov/sab/SABPRODUCT.NSF/81e39f4c09954fcb85256ead006be86e/0CA9E925C9A702F285257F380050C842/File/Income+Elasticity+Technical+Memorandum_final_2_5_16_docx.pdf, (Accessed on 01/29/2019).
- E. Tronconi, Catal. Today, 1997, 34, 421–427 CrossRef CAS.
- M. J. Tang, R. A. Cox and M. Kalberer, Atmos. Chem. Phys., 2014, 14, 9233–9247 CrossRef.
- J. W. Beeckman, Ind. Eng. Chem. Res., 1991, 30, 428–430 CrossRef CAS.
- C. N. Satterfield, Mass Transfer Heterogeneous Catalysis, MIT Press, Cambridge, MA and London, England, 1970 Search PubMed.
- ICAO Aircraft Engine Emissions Databank, 2017, http://www.easa.europa.eu/document-library/icao-aircraft-engine-emissions-databank, Version 24.
- Storage and handling of anhydrous ammonia, 2011, https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.111.
- Cost Reports and Guidance for Air Pollution Regulations, 2019, https://www.epa.gov/economic-and-cost-analysis-air-pollution-regulations/cost-reports-and-guidance-air-pollution.
- Blackmer NH3/LPG Pump, 3′′-Dultmeier Sales, https://www.dultmeier.com/products/0.851.2042/11668#.
- S. H. L. Yim, G. L. Lee, I. H. Lee, F. Allroggen, A. Ashok, F. Caiazzo, S. D. Eastham, R. Malina and S. R. H. Barrett, Environ. Res. Lett., 2015, 10, 034001 CrossRef.
- Committee on Propulsion and Energy Systems to Reduce Commercial Aviation Carbon Emissions, Commercial Aircraft Propulsion and Energy Systems Research, National Academies Press, Washington, DC, 2016 Search PubMed.
- MRO Management, Extension Lead, 2017, http://www.mromanagement.com/feature/airbus-to-publish-a320neo-maintenance-tasks.
- J. H. Seinfeld and P. N. Spyros, Atmospheric chemistry and physics: from air pollution to climate change, John Wiley & Sons, Hoboken, New Jersey, 1998 Search PubMed.
- S. D. Eastham, D. K. Weisenstein, D. W. Keith and S. R. Barrett, Atmos. Environ., 2018, 187, 424–434 CrossRef CAS.
- C. Grobler, P. J. Wolfe, K. Dasadhikari, I. C. Dedoussi, F. Allroggen, R. L. Speth, S. D. Eastham, A. Agarwal, M. D. Staples, J. Sabnis and S. R. H. Barrett, Environ. Res. Lett., 2019, 14, 114031 CrossRef CAS.
- Jet Fuel Price Monitor, https://www.iata.org/publications/economics/fuel-monitor/Pages/index.aspx, (Accessed on 01/29/2019).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ee02362k |
| This journal is © The Royal Society of Chemistry 2021 |