Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A modelling study of OH, NO3 and H2SO4 in 2007–2018 at SMEAR II, Finland: analysis of long-term trends†
Dean
Chen
 a,
Carlton
Xavier
a,
Petri
Clusius
a,
Tuomo
Nieminen
a,
Carlton
Xavier
a,
Petri
Clusius
a,
Tuomo
Nieminen
 ab,
Pontus
Roldin
c,
Ximeng
Qi
d,
Lukas
Pichelstorfer
a,
Markku
Kulmala
ab,
Pontus
Roldin
c,
Ximeng
Qi
d,
Lukas
Pichelstorfer
a,
Markku
Kulmala
 ad,
Pekka
Rantala
a,
Juho
Aalto
a,
Nina
Sarnela
a,
Pasi
Kolari
a,
Petri
Keronen
a,
Matti P.
Rissanen
e,
Ditte
Taipale
a,
Benjamin
Foreback
a,
Metin
Baykara
ad,
Pekka
Rantala
a,
Juho
Aalto
a,
Nina
Sarnela
a,
Pasi
Kolari
a,
Petri
Keronen
a,
Matti P.
Rissanen
e,
Ditte
Taipale
a,
Benjamin
Foreback
a,
Metin
Baykara
 af,
Putian
Zhou
af,
Putian
Zhou
 *a and
Michael
Boy
*a and
Michael
Boy
 a
a
aInstitute for Atmospheric and Earth System Research/Physics, University of Helsinki, P.O. Box 64, 00014 Helsinki, Finland. E-mail: putian.zhou@helsinki.fi
bInstitute for Atmospheric and Earth System Research/Forest Sciences, University of Helsinki, P.O. Box 64, 00014 Helsinki, Finland
cDivision of Nuclear Physics, Department of Physics, Lund University, P.O. Box 118, SE-22100 Lund, Sweden
dJoint International Research Laboratory of Atmospheric and Earth System Sciences, School of Atmospheric Sciences, Nanjing University, Nanjing, 210023, China
eAerosol Physics Laboratory, Physics Unit, Faculty of Engineering and Natural Sciences, Tampere University, Tampere, Finland
fClimate and Marine Sciences Department, Eurasia Institute of Earth Sciences, Istanbul Technical University, Maslak 34469, Istanbul, Turkey
First published on 4th August 2021
Abstract
Major atmospheric oxidants (OH, O3 and NO3) dominate the atmospheric oxidation capacity, while H2SO4 is considered as a main driver for new particle formation. Although numerous studies have investigated the long-term trend of ozone in Europe, the trends of OH, NO3 and H2SO4 at specific sites are to a large extent unknown. The one-dimensional model SOSAA has been applied in several studies at the SMEAR II station and has been validated by measurements in several projects. Here, we applied the SOSAA model for the years 2007–2018 to simulate the atmospheric chemical components, especially the atmospheric oxidants OH and NO3, as well as H2SO4 at SMEAR II. The simulations were evaluated with observations from several shorter and longer campaigns at SMEAR II. Our results show that daily OH increased by 2.39% per year and NO3 decreased by 3.41% per year, with different trends of these oxidants during day and night. On the contrary, daytime sulfuric acid concentrations decreased by 2.78% per year, which correlated with the observed decreasing concentration of newly formed particles in the size range of 3–25 nm with 1.4% per year at SMEAR II during the years 1997–2012. Additionally, we compared our simulated OH, NO3 and H2SO4 concentrations with proxies, which are commonly applied in case a limited number of parameters are measured and no detailed model simulations are available.
Environmental significanceThe atmospheric oxidants (OH, O3 and NO3) are crucial in the oxidation processes of CH4, CO, SO2, etc., and play a significant role in climate forcing and environmental processes. By using a process-based model with comprehensive datasets at a boreal forest measurement station, this study provides a long-term trend analysis of these atmospheric oxidants, which gives an insight into (1) how the atmospheric oxidation capacity evolves in a boreal forest background and (2) what are the local changes in the environment. |
1. Introduction
Understanding the atmospheric oxidants (OH, O3 and NO3), their reactions and related processes is important as they are the main “cleaning protagonists” of the atmosphere. Many trace gases such as methane (CH4), volatile organic compounds (VOCs), nitrogen oxides (NOx = NO + NO2) and sulfur dioxide (SO2) are removed from the atmosphere by oxidation reactions. During the day, the hydroxyl radical (OH) is the dominant oxidant produced by photochemical processes in the troposphere.1 Since there is no sunlight at night, the nighttime concentration of OH is significantly lower and other oxidants dominate: ozone (O3), during the daytime, is formed by OH radical reactions with VOCs in the presence of NOx, and the nitrate radical (NO3) is generated mainly by the reaction of NO2 with O3.2–5 In general, OH is considered to contribute the most to the atmospheric oxidation capacity,6–10 while O3 and NO3 play a minor but nonetheless significant role.11 Oxidation of VOCs by OH, O3 and NO3 affects air quality and climate, as well as regional and global budgets of reactive nitrogen, ozone, and secondary organic aerosols (SOAs).12–15According to the latest global chemistry-transport model simulations, which used state-of-the-art new particle formation (NPF) parameterizations from the cloud chamber experiments in CERN,16,17 around 96% (ref. 18) or 100% (ref. 19) of the present-day NPF can be explained by H2SO4 clustering with ammonia, ions or organic compounds. Roldin et al. (2019)15 very recently observed NPF by considering sulfuric acid together with ammonia and/or ELVOCs during two periods in spring 2013 and 2014. Hence it is crucial to know the concentrations of H2SO4 for all NPF analyses and how they have changed in the past and will change in the future.
As biogenic sources dominate the global atmospheric VOC budget,20,21 it is important to understand the dynamics of biogenic emissions and their consequences to atmospheric processes. The boreal zone is the world's second largest forest region, after tropical forests,22,23 and boreal vegetation is dominated by evergreen coniferous trees that produce significant amounts of biogenic VOCs (BVOCs), mainly isoprene (C5H8), monoterpenes (C10H16) and sesquiterpenes (C15H24).24–26 Studies of the OH-reactivity in forest canopies have suggested large emissions of unknown reactive BVOCs.9,27,28
Studies on long-term trends of oxidants can provide an insight into how the atmospheric oxidation capacity evolves against the background of climate and local changes in the environment. Several studies have investigated the trends of atmospheric oxidants in Europe. In their studies, Wilson et al. (2012)29 and Yan et al. (2018)30 showed a general decreasing trend in ozone concentrations due to the decrease in NOx-emissions. Numerous studies have investigated global OH trends using chemical transport models or retrieval of remote sensing of methylchloroform (CH3CCl3, MCF).31–33 Prinn et al. (2001)32 predicted an overall global negative average OH trend of −0.64% per year between 1978 and 2000, whereas in a newer study, Montzka et al. (2011)34 found a small interannual OH variability, indicating that global OH is generally well buffered against perturbations. In situ long inter-annual OH measurements are relatively rare. However, one study by Rohrer et al. (2006)35 showed that there was no trend in the level of OH in the Hohenpeissenberg data set during the studied period 1999–2003 (estimated the annual trend to be less than ±2.5% per year) and that there was a positive correlation (r = 0.941) between OH and the photolysis frequency of ozone, J(O1D). Long term trends of NO3 are rarely studied, and only a few modelling studies on the long-term NO3 trend exist.36
A considerable number of field campaigns, in which OH concentrations were measured, have been compared to the results of modelling simulations.37–39 Most modelling studies reproduced the OH concentration within the uncertainty range of the OH measurements, including clean40–45 and urban areas.46–51 At the Station for Measuring Ecosystem – Atmosphere Relation (SMEAR II),52 located in Hyytiälä, Finland, OH concentrations were measured in two campaigns: European Integrated project on Aerosol, Cloud, Climate, and Air Quality Interactions (EUCAARI, 2007–2010)53 and Hyytiälä United Measurement of Photochemistry and Particles – Comprehensive Organic Particle and Environmental Chemistry (HUMPPA-COPEC).54 The results from these campaigns showed that modelled OH values were slightly overestimated compared to the measured values.39,55 During the HUMPPA-COPEC campaign in 2010 (ref. 54) and the IBAIRN (Influence of Biosphere – Atmosphere Interactions on the Reactive Nitrogen budget) campaign in 2016 (ref. 56), NO3 concentrations at SMEAR II were also measured, but most of the time the values were close to the limit of detection (LOD) of the instrument.56 However, due to the importance of the NO3 radical in the oxidation of BVOCs, Peräkylä et al. (2014)57 developed a NO3 proxy which was also used by Kontkanen et al. (2016)58 to derive the long-term trend of monoterpene concentrations at SMEAR II.
The aim of this study is to provide an insight into the long-term trend of the atmospheric oxidation capacity at the boreal forest in Finland from 2007 to 2018. Based on this, we estimate how the H2SO4 concentration has changed during this period, and how this could affect the frequency of new particle formation events at this site.
2. Methods
2.1. SMEAR II
The long-term measurements analyzed in this study were conducted at the SMEAR II station located in Hyytiälä (61°50′51′′N, 24°17′41′′E), Southern Finland.59 The station is surrounded by a 56 year old (in 2018) pine dominated forest that also contains Norway spruces and deciduous trees.60 SMEAR II is a unique field station with continuous measurements of physical, chemical and biological phenomena, processes and interactions between these elements. A detailed description of the site can be found on the SMEAR II website (https://www.atm.helsinki.fi/SMEAR/index.php/smear-ii).One of the major changes that affects the general trend of atmospheric composition is the growing vegetation. The mean height of dominant trees within 200 m from the measurement tower was 16.9 m in the year 2007 and increased to 20.5 m in 2018. The overstory canopy depth was 8 m in the year 2007 and increased to 8.63 m by 2014. Since the year 2014, it remained constant. The all-sided LAI (Leaf Area Index) of all trees in July increased from 5.5 m2 m−2 in 2007 to 5.9 m2 m−2 in 2015. After 2015, it was considered the same as in 2015. The dry biomass of the foliage in the year 2007 was 0.51 kg m−2 and reached 0.58 kg m−2 in 2018.
In this study, selected measurements during the period 2007 to 2018 from the SMEAR II station were used as input for the model simulations; partly to nudge the meteorological parameters to the observations (temperature, absolute humidity, wind speed and direction) or as continuous input for selected gases (O3, NOx, SO2, CO and CH4), solar irradiance (global short wave radiation and photosynthetically active radiation), soil properties (soil temperature, soil water content and soil heat flux) and particle condensation sink (calculated from the particle size distributions which are measured by DMPS and APS). The input of O3, NOx, SO2, CO and CH4 can be considered to account for the effects of local and regional transport or large scale variation of these species. The anthropogenic VOCs were not included in this study, but the anthropogenic influence at SMEAR II is low with the nearest significant city (about 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 inhabitants) located 60 km southwest.61 A detailed description of the station instrumentation (parameter, location, time resolution, method, and temporal coverage) is available on the SMEAR II website under “List of the measurements” https://www.atm.helsinki.fi/SMEAR/index.php/smear-ii/measurements.
000 inhabitants) located 60 km southwest.61 A detailed description of the station instrumentation (parameter, location, time resolution, method, and temporal coverage) is available on the SMEAR II website under “List of the measurements” https://www.atm.helsinki.fi/SMEAR/index.php/smear-ii/measurements.
Although SMEAR II already started its operational work in the late 90's, we decided to focus our long-term modelling activities from the year 2007 onwards. The reason was the NOx monitoring technique and the fact that the NOx concentrations have a large impact on the simulated OH concentrations. Until February 2007, a molybdenum converter was used to convert NO2 to NO. However, this technique also measures other nitrogen compounds (e.g., nitric acid, nitrous acid, and PAN) which are misinterpreted as NO and consequently the NO level is overestimated. Since March 2007, a photolytic blue light converter was used for only converting NO2 to NO, which enables more accurate data collection of NO (see Fig. S1 in the ESI†).
2.2. SOSAA
SOSAA (a model to simulate the concentrations of organic vapors, sulfuric Acid and aerosols) is a 1-D chemistry transport model used to study the atmospheric composition inside the planetary boundary layer. In the past, SOSAA has been applied to study the characteristics of oxidant reactivities,9,27,28 oxidation of trace gases,55 emission of BVOCs,62 and vertical exchange and dry deposition of ozone63 and BVOCs,64 respectively, as well as new particle formation and growth of sub-3 nm particles.65 SOSAA is written in Fortran and parallelized with MPI (Message Passing Interface). In this study, four different modules were used: (1) the meteorological module, which is derived from SCADIS;66–68 (2) the BVOC emission module, which is a modified version of MEGAN2.04 (Model of Emissions of Gases and Aerosols from Nature);21 (3) the chemistry module, which is created by KPP,69 with the chemical mechanism generated by MCM3.3.1 (see https://www.mcm.leeds.ac.uk/MCM);70–72 and (4) the gas dry deposition module, which is modified from MLC-CHEM.63,64,73 SOSAA describes the atmospheric boundary layer evolution and the vertical mixing of the chemical species in 51 vertical layers, from the surface up to 3 km. The simulation time step is 10 s for the meteorology module and 60 s for other modules.The meteorological module includes the prognostic equations for the horizontal wind vector, air temperature and absolute humidity. In this study, these prognostic variables at the upper boundary of the model domain were constrained with the ERA-Interim reanalysis data which were provided by the European Centre for Medium-Range Weather Forecast (ECMWF).74 In the lower part of the model domain from 4.2 m to 125 m above the ground, the air temperature, wind vector and absolute humidity were nudged to the vertically interpolated measurement data at SMEAR II with a nudging factor of 0.05, which represents the force of regional transport. The incoming short-wave and photosynthetically active radiation (PAR) at the canopy top, as well as the soil properties (soil temperature, soil water content and soil heat flux) were directly taken as input from SMEAR II measurements. The short-wave radiation was provided by the measurement data at SMEAR II, and the radiative transfer module from the ADCHEM model75 was used to split the observed radiation into the direct, diffuse, downward and upward radiation components. The radiative transfer module used the quadrature two-stream approximation scheme developed by Toon et al. (1989).76 All of the meteorological input data mentioned above were linearly interpolated to 10 s time resolution to match the simulation time step.
The standard emission potentials of the emitted BVOCs at SMEAR II, which were used to calculate the emission rates, refer to the values suggested in Zhou et al. (2017b).64 The chemistry scheme was derived from the one used in Zhou et al. (2017b)64 but with a newer MCM version 3.3.1. For the reactions of the stabilized Criegee intermediates (sCIs), we diverted from the MCM and instead used newer obtained reaction rates. For the sCIs from α-pinene, β-pinene and limonene, we have used the rates from Mauldin III et al. (2012)77 similarly to “Scenario C” in Boy et al. (2013).55 For the sCIs from isoprene, we used the rates from Welz et al. (2012)78 as done in “Scenario D” in Boy et al. (2013).55
The measured mixing ratios of CO, O3, NO, NO2 and SO2 at the height levels 4.2, 8.4, 16.8, 33.6, 50.4, 67.2, 101 and 125 m were averaged and then used as the input values for all the layers in the model. The LODs of SO2, NO, O3 and NO2 were set to 0.06 ppb, 0.05 ppb, 0.3 ppb and 0.1 ppb, respectively (Dr Pasi Kolari, personal discussion). However, for all these four species there exist several long periods when the measured values were below the LOD. In order to prevent the model from noise interference, which has too low values, all the values that are below the LOD are set to the LOD. We also did test runs by setting all values below the LOD to LOD/2. However, the model results showed a stepwise increase in the simulated OH, NO3 and H2SO4 concentrations at all times when the input data went from the LOD to LOD/2. So, we decided to use the LOD as a threshold in case the values are below the LOD for the four gaseous compounds discussed above. There are several other methods used in the literature to overcome this problem such as the “Uniform Fill-In” or the “Log Fill-In” methods discussed and tested by Cohen and Ryan (1998).79 However, as all the data below the LOD are unknown, no method predicts their distribution correctly which makes it difficult to choose a single technique that will be best at all times for various parameters. In Table S1 in the ESI,† we calculated the amount of data points for SO2 and NO above the LOD (the two parameters with the highest amount of data below the LOD) for different percentile ranges for each year to investigate if a trend in the below LOD data exists.
The measured CH4 concentrations in 2014 were used as input in SOSAA for the year 2014. For other years, an annual global growth rate of 6 ppb per year was assumed, and the input time series of CH4 concentrations were thus added (after 2014) or subtracted (before 2014) a multiple times of 6 ppb from the time series in 2014 according to the year difference. The growth rate was chosen from the ‘NASA Earth Observatory’ website and represents the methane increase in 2007–2013 (https://www.earthobservatory.nasa.gov/images/87681/a-global-view-of-methane).
The condensation sinks (CS) for H2SO4 and HNO3 were provided as an input for the model. The CS was calculated based on the particle size distribution measured by using a DMPS (particles with diameters of 3–1000 nm) and an APS (particles with aerodynamic diameters of 0.5–20 μm) system,52,80 and the hygroscopic growth effect was corrected based on Laakso et al. (2001).81 Similarly, for the meteorological input data, the input mixing ratios and the CS were also linearly interpolated to 60 s time resolution to match the simulation time step of the emission and chemistry modules.
2.3. Statistical methods
The daily/daytime/nighttime trends of variables were calculated based on their daily/daytime/nighttime mean or median values. Whether to use mean or median for a variable is determined by its data value distribution. If the data are logarithmically distributed (O3, CO, CS, EM-MON, MON, OH, HO2, H2SO4, NO2, N2O5, and NO3), the median values are used. Here we should notice that although the data value distributions of SO2 and NO are also logarithmically distributed, we still used their mean values. The reason is that more than 50% of their measured concentrations lie below the LOD, which results in that their median values are equal to the LOD. For other variables (temperature, RH, and solar irradiance), the mean values were used. For the logarithmically distributed variables (besides the variables mentioned above, SO2 and NO are also included here), the daily/daytime/nighttime linear trend fittings were conducted on the logarithm with base 10 of their respective median or mean values. For other variables (temperature, RH, and solar irradiance), the linear trend fittings were performed directly on their respective mean values.Bootstrapping was used to estimate the confidence interval of the trend.82,83 We first fitted a linear trend to the time series and created a new data set by taking random samples from the original residuals (differences of the data values and the fitted linear trend) and adding these to the linear part. Then a new linear fit was made to this new data set. This procedure was repeated several times (typically 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 iterations). Here the idea was to test the monotonicity of the trend. The smaller the differences in the fitted trends were after many such iterations, more likely was the original trend to be monotonic. To obtain the confidence interval, we examined the 5th to 95th percentile range of the slopes obtained from bootstrapping iterations: if all of the slopes in this range were either positive or negative (thus not containing a zero trend), we concluded that the likelihood of the presence of a trend was higher than 95% (p < 0.05) and thus statistically significant.
000 iterations). Here the idea was to test the monotonicity of the trend. The smaller the differences in the fitted trends were after many such iterations, more likely was the original trend to be monotonic. To obtain the confidence interval, we examined the 5th to 95th percentile range of the slopes obtained from bootstrapping iterations: if all of the slopes in this range were either positive or negative (thus not containing a zero trend), we concluded that the likelihood of the presence of a trend was higher than 95% (p < 0.05) and thus statistically significant.
To get another estimate of the monotonicity of the trends, we also used the Mann–Kendall test for autocorrelated seasonal data,84,85 and the p-values are reported in Table 1 under PMK. The MK test is more conservative, but both our tests agree in the sense that a wider confidence interval or larger PMK value indicates larger yearly variation, and hence the prognostic capacity of the trend is smaller.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bootstrapping iterations, respectively) for the RLM values, and PMK values of MK numbers. The first, second and third rows for each parameter represent daily, daytime and nighttime values, respectively. Whether the median or mean values of different parameters were used to calculate the trends are also shown in the bracket after the parameter names. Except the emissions of the monoterpenes, which were averaged over the forest canopy height (18 m), all other parameters use the median or mean values from the height level of 20–40 m. The significant trends are marked in bold font. Detailed statistical methods are explained in Section 2.3
000 bootstrapping iterations, respectively) for the RLM values, and PMK values of MK numbers. The first, second and third rows for each parameter represent daily, daytime and nighttime values, respectively. Whether the median or mean values of different parameters were used to calculate the trends are also shown in the bracket after the parameter names. Except the emissions of the monoterpenes, which were averaged over the forest canopy height (18 m), all other parameters use the median or mean values from the height level of 20–40 m. The significant trends are marked in bold font. Detailed statistical methods are explained in Section 2.3
| Parameter | Time | Yearly trend | Seasonal trend | ||||
|---|---|---|---|---|---|---|---|
| RLM | MK | Winter | Spring | Summer | Autumn | ||
| Monoterpene emission rate (median) | Daily | +0.80 (−0.06, +1.90) | +0.72 (0.152) | +2.07 (−0.98, +5.87) | +0.53 (+0.06, +1.96) | +1.00 (−0.23, +2.30) | +0.40 (−1.04, +1.27) |
| Day | +0.73 (−0.14, +1.82) | +0.69 (0.166) | +1.88 (−1.30, +5.92) | +0.74 (+0.31, +2.13) | +0.80 (−0.41, +2.14) | +0.27 (−1.23, +1.16) | |
| Night | +0.76 (−0.17, +1.98) | +0.67 (0.230) | +2.06 (−0.83, +5.90) | +0.26 (−0.42, +1.62) | +1.14 (+0.05, +2.44) | +0.38 (−1.02, +1.39) | |
| Monoterpene concentration (median) | Daily | +3.43 (+1.95, +5.27) | +3.11 (0.010) | +4.24 (+2.00, +7.12) | +2.87 (+1.39, +5.22) | +3.06 (+0.32, +6.12) | +3.66 (+2.38, +4.56) |
| Day | +3.15 (+1.75, +4.98) | +2.82 (0.014) | +3.89 (+1.58, +7.03) | +2.78 (+1.18, +5.28) | +2.65 (+0.24, +5.40) | +3.36 (+2.23, +4.18) | |
| Night | +3.60 (+2.08, +5.43) | +3.46 (0.009) | +4.09 (+1.86, +6.99) | +2.74 (+1.36, +4.95) | +4.11 (+0.75, +7.76) | +3.71 (+2.32, +4.71) | |
| OH (median) | Daily | +2.39 (+0.95, +3.33) | +1.84 (0.036) | +9.65 (+3.67, +16.02) | +1.37 (+0.73, +2.93) | −0.85 (−2.24, +0.25) | +3.47 (+1.49, +4.83) |
| Day | +0.91 (−0.81, +2.10) | +1.10 (0.236) | +6.87 (+1.21, +11.41) | +0.68 (−0.46, +2.11) | −1.11 (−2.41, +0.04) | +1.14 (−1.04, +2.46) | |
| Night | +3.31 (+2.01, +4.62) | +2.40 (0.022) | +10.25 (+3.95, +17.32) | +2.25 (+1.29, +3.74) | +0.26 (−0.80, +1.32) | +4.54 (+2.39, +6.29) | |
| HO2 (median) | Daily | +3.34 (+2.00, +4.38) | +2.64 (0.013) | +10.75 (+3.97, +18.03) | +1.08 (+0.05, +3.03) | +0.78 (−0.68, +2.02) | +4.52 (+2.36, +5.97) |
| Day | +2.10 (+0.94, +3.10) | +1.48 (0.037) | +12.15 (+3.94, +21.27) | −0.05 (−1.26, +1.67) | +0.11 (−1.27, +1.43) | +2.33 (+0.26, +3.49) | |
| Night | +3.96 (+2.57, +5.32) | +3.19 (0.015) | +10.26 (+3.72, +17.74) | +2.10 (+0.95, +3.91) | +1.49 (−0.03, +3.04) | +5.39 (+3.05, +7.26) | |
| H2SO4 (median) | Daily | −1.54 (−4.60, +0.36) | −1.10 (0.521) | +2.96 (−1.64, +6.84) | −0.76 (−3.93, +2.84) | −5.02 (−9.06, −1.12) | +0.70 (−1.95, +3.06) |
| Day | −2.78 (−6.05, −0.63) | −2.62 (0.183) | −0.54 (−5.69, +2.85) | −0.66 (−4.32, +2.89) | −5.40 (−9.52, −1.07) | −1.12 (−3.76, +0.95) | |
| Night | −0.58 (−3.28, +1.49) | −0.34 (0.829) | +3.19 (−1.00, +7.11) | −0.77 (−4.39, +2.97) | −3.48 (−6.89, +0.20) | +1.87 (−0.92, +4.54) | |
| NO3 (median) | Daily | −3.41 (−4.98, −2.09) | −3.40 (0.014) | −2.99 (−4.50, −1.47) | −3.00 (−5.96, −0.26) | −3.38 (−5.54, −1.23) | −4.53 (−5.99, −2.73) |
| Day | −2.43 (−3.90, −0.88) | −2.54 (0.033) | −1.03 (−3.43, +2.24) | −2.58 (−5.20, −0.12) | −2.89 (−5.25, −0.64) | −3.65 (−4.74, −2.24) | |
| Night | −4.22 (−5.90, −2.86) | −4.11 (0.009) | −3.23 (−4.81, −1.90) | −3.80 (−6.55, −1.28) | −5.07 (−7.54, −2.68) | −5.27 (−6.92, −3.38) | |
| N2O5 (median) | Daily | −8.24 (−12.18, −5.96) | −7.84 (0.013) | −12.61 (−18.96, −7.00) | −5.36 (−11.06, −0.90) | −4.11 (−9.70, +1.76) | −9.09 (−12.05, −4.71) |
| Day | −6.90 (−10.39, −4.68) | −6.64 (0.018) | −10.62 (−15.71, −5.63) | −4.22 (−9.95, +0.26) | −2.52 (−8.36, +3.41) | −8.28 (−10.88, −4.16) | |
| Night | −9.16 (−13.18, −6.93) | −8.68 (0.010) | −12.69 (−19.48, −6.92) | −6.55 (−12.06, −2.14) | −6.68 (−12.16, −1.15) | −10.16 (−13.60, −5.46) | |
| SO2 (mean) | Daily | −5.43 (−8.10, −3.65) | −3.91 (0.013) | −10.18 (−17.00, −4.85) | −4.10 (−7.73, −0.53) | −4.57 (−7.75, −1.18) | −3.59 (−5.18, −1.98) |
| Day | −5.00 (−7.67, −3.22) | −2.65 (0.019) | −10.01 (−17.07, −4.08) | −3.20 (−6.81, +0.26) | −4.48 (−7.88, −0.88) | −2.76 (−4.03, −1.47) | |
| Night | −5.35 (−8.07, −3.55) | −3.07 (0.013) | −9.97 (−16.71, −4.73) | −4.54 (−8.29, −0.86) | −3.91 (−7.13, −0.59) | −3.54 (−5.04, −1.94) | |
| O3 (median) | Daily | −0.11 (−0.86, +0.05) | +0.16 (0.612) | +0.74 (−0.49, +1.45) | +0.13 (−0.51, +0.82) | −0.28 (−1.12, +0.48) | +0.04 (−0.93, +0.98) |
| Day | −0.18 (−0.88, −0.01) | +0.03 (0.884) | +0.89 (−0.31, +1.51) | +0.09 (−0.52, +0.75) | −0.37 (−1.16, +0.35) | −0.27 (−1.18, +0.60) | |
| Night | +0.04 (−0.76, +0.21) | +0.30 (0.330) | +0.67 (−0.59, +1.38) | +0.24 (−0.45, +0.95) | −0.02 (−0.89, +0.79) | +0.24 (−0.75, +1.27) | |
| CO (median) | Daily | −0.46 (−1.62, +0.38) | −0.42 (0.473) | −0.89 (−1.96, −0.28) | −0.96 (−1.72, −0.32) | −0.54 (−2.50, +1.41) | +0.75 (−0.89, +2.44) |
| Day | −0.47 (−1.63, +0.34) | −0.44 (0.456) | −0.98 (−2.01, −0.39) | −0.96 (−1.71, −0.31) | −0.54 (−2.47, +1.43) | +0.79 (−0.81, +2.45) | |
| Night | −0.43 (−1.63, +0.42) | −0.41 (0.499) | −0.85 (−1.93, −0.24) | −0.97 (−1.74, −0.30) | −0.53 (−2.41, +1.45) | +0.78 (−0.79, +2.48) | |
| NO (mean) | Daily | −0.37 (−1.13, +0.08) | −0.02 (0.907) | −2.61 (−4.96, −1.67) | +0.63 (−0.84, +1.95) | −0.02 (−0.50, +0.49) | −0.27 (−0.80, +0.20) |
| Day | −0.91 (−2.12, −0.19) | −0.13 (0.631) | −5.93 (−10.15, −3.86) | +0.80 (−1.18, +2.56) | −0.00 (−0.61, +0.68) | −0.65 (−1.61, +0.31) | |
| Night | +0.00 (−0.02, +0.02) | +0.00 (0.980) | −0.22 (−0.55, +0.10) | +0.01 (−0.01, +0.03) | −0.00 (−0.01, +0.00) | +0.02 (−0.03, +0.06) | |
| NO2 (median) | Daily | −3.80 (−5.99, −1.87) | −3.89 (0.031) | −8.49 (−12.49, −4.87) | −2.32 (−6.07, +0.99) | −1.34 (−4.36, +1.92) | −4.13 (−5.86, −1.96) |
| Day | −3.33 (−5.52, −1.43) | −3.31 (0.045) | −8.61 (−12.52, −5.05) | −1.48 (−5.21, +1.99) | −0.71 (−3.66, +2.45) | −3.67 (−5.28, −1.53) | |
| Night | −4.18 (−6.26, −2.32) | −4.21 (0.019) | −8.21 (−12.46, −4.53) | −3.08 (−6.18, −0.22) | −2.00 (−4.95, +1.04) | −4.64 (−6.66, −2.25) | |
| Global short wave radiation (mean, W m2 per year) | Daily | +0.24 (−0.85, +0.68) | +0.27 (0.110) | −0.20 (−0.68, −0.22) | +1.37 (−0.02, +3.43) | +0.85 (−1.25, +2.61) | +0.02 (−0.64, +0.30) |
| Day | +0.26 (−1.54, +1.05) | +0.47 (0.177) | −0.74 (−2.05, −0.80) | +2.42 (−0.01, +5.52) | +0.90 (−1.74, +3.34) | −0.06 (−1.40, +0.63) | |
| Night | +0.01 (−0.00, +0.03) | +0.01 (0.353) | +0.03 (+0.00, +0.07) | −0.01 (−0.02, +0.00) | −0.00 (−0.06, +0.05) | −0.00 (−0.01, +0.01) | |
| Temperature (mean, K per year) | Daily | +0.08 (+0.02, +0.24) | +0.03 (0.632) | +0.23 (−0.04, +0.68) | −0.02 (−0.10, +0.13) | −0.02 (−0.17, +0.14) | −0.03 (−0.14, +0.14) |
| Day | +0.08 (+0.02, +0.24) | +0.03 (0.569) | +0.23 (−0.06, +0.67) | −0.02 (−0.10, +0.13) | −0.02 (−0.18, +0.14) | −0.03 (−0.14, +0.13) | |
| Night | +0.08 (+0.03, +0.23) | +0.02 (0.714) | +0.24 (−0.04, +0.69) | −0.03 (−0.11, +0.12) | −0.00 (−0.14, +0.14) | −0.02 (−0.14, +0.14) | |
| Absolute humidity (mean, g m3 per year) | Daily | −0.11 (−0.47, +0.70) | −0.27 (0.343) | +0.21 (−1.23, +2.86) | −0.19 (−0.64, +0.51) | −0.26 (−0.67, +0.18) | −0.37 (−1.02, +0.11) |
| Day | −0.07 (−0.44, +0.76) | −0.24 (0.417) | +0.20 (−1.22, +2.88) | −0.13 (−0.58, +0.59) | −0.26 (−0.69, +0.19) | −0.31 (−1.04, +0.11) | |
| Night | −0.17 (−0.55, +0.65) | −0.33 (0.287) | +0.15 (−1.30, +2.73) | −0.29 (−0.76, +0.40) | −0.29 (−0.69, +0.14) | −0.36 (−0.98, +0.10) | |
| Condensation sink (median) | Daily | −1.59 (−3.49, −0.12) | −1.08 (0.356) | −4.76 (−7.84, −2.95) | −1.11 (−3.51, +1.51) | −0.04 (−1.64, +1.52) | −0.87 (−3.06, +1.07) |
| Day | −1.65 (−3.59, −0.20) | −1.10 (0.334) | −5.19 (−8.59, −3.36) | −1.22 (−3.73, +1.51) | −0.13 (−1.73, +1.48) | −0.68 (−3.01, +1.45) | |
| Night | −1.39 (−3.22, −0.02) | −0.89 (0.419) | −4.34 (−7.41, −2.64) | −1.14 (−3.42, +1.36) | +0.25 (−1.32, +1.85) | −0.81 (−2.98, +1.14) | |
Finally, the relative changes (and the 90% confidence interval from the bootstrapping test) which are shown in Table 1 are in the linear scale for all variables, describing the actual change in % per year. The trend lines shown in time series plots are obtained with a 1 year running median (window size of ±182 days); see Ma et al. (2016)86 for a detailed description.
2.4. Uncertainties
The uncertainties in this study are mainly related to the uncertainties of the input variables or the use of parametrizations inside the model. Concerning the input data, the aerosol condensation sink, which predicts in our study how rapidly sulfuric acid and nitric acid will condense on pre-existing aerosols, has the highest uncertainty. In these calculations, we applied measured particle size number concentrations from SMEAR II. However, the uncertainty of the predicted CS values due to potentially different hygroscopic growth behaviour depending on the chemical composition of the particles is difficult to estimate and could have an effect on the simulated acid concentrations.The second source of uncertainties is related to the meteorology module. It will be validated by comparison with measurements in the following section. The third uncertainty source is the large uncertainties of reaction rate coefficients in the applied chemistry schemes, and when the same reaction is studied by different groups with different techniques, the reaction rate coefficient may differ by a factor of 2 or even more.87 Moreover, for many reactions between OH and VOCs, no experimental data exist, so the reaction rate coefficients are only estimates, which increase the uncertainty even further.
The fourth uncertainty source is related to the emission module MEGAN. Guenther et al. (2012)88 estimated that the uncertainty associated with the annual global emissions of monoterpenes is a factor of three, and of methanol, acetone and acetaldehyde is a factor of two. However, in our simulations MEGAN was constrained by in situ measurement data, including the relevant meteorological variables and the standard emission factors, which decreased the uncertainty. Moreover, the uncertainty of the emission module can also be evaluated by comparing the measured and simulated monoterpene concentrations and fluxes in Section 3.3.
3. Results
The results will be presented in 6 sections: (1) validation of the meteorological module, (2) a short discussion of the trends of the measured parameters which are crucial for our model simulations, (3) BVOC model-measurement intercomparison and trends, (4) trends and campaign model-observation inter-comparisons of the hydroxyl and nitrate radicals, (5) trends and campaign model-observation inter-comparisons of sulfuric acid and (6) comparisons of proxies of OH, NO3 and H2SO4 with the model.Table 1 shows the trends calculated by the method described in Section 2.3 of all relevant model output parameters for the whole day (daily), daytime and nighttime, respectively, and also shows the seasonal trends calculated with the RLM method and the 90% confidence interval for the same parameters. Here the daytime is defined as the time period between sunrise and sunset; nighttime is defined between sunset and sunrise and the daily values are averaged for 24 hours. In the following sections we will discuss the values of single parameters in more detail.
3.1. Meteorological data analyses
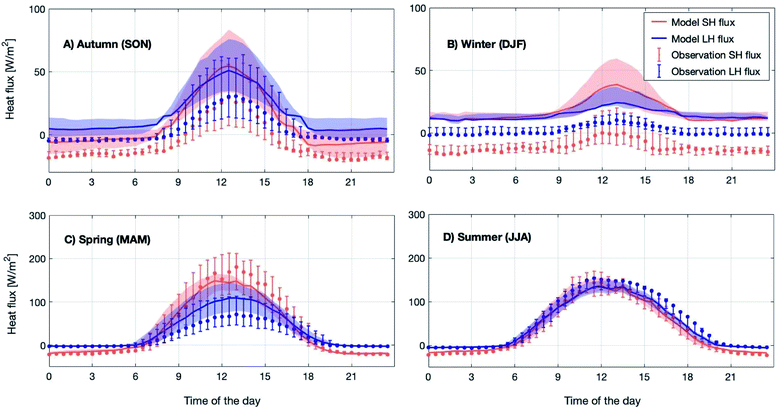
Meteorology is one of the major drivers for the change in atmospheric composition. We compared several measured meteorological parameters with the model outcome to validate the performance of the meteorological module in SOSAA. While temperature, water vapor and wind speed were nudged with the measurements, the heat fluxes and net radiation were simulated and their comparison with measurements provides an insight into the simulated energy balance above the forest canopy.Fig. 1 shows the modelled 12 year median diurnal cycles of sensible and latent heat fluxes for the four different seasons in 2007–2018. The comparison shows that the modelled sensible heat flux is within 25th–75th percentiles of the measured ones throughout the whole diurnal cycle in spring and summer and during daytime in autumn. However in winter and during autumn nighttime, the model always overestimated the measured sensible heat flux.
The modelled latent heat flux during spring daytime is about 20 W m−2 lower compared to the observations, which could be related to the melting of the snow cover on the ground. Note that the snow cover is not explicitly modelled in SOSAA. However, during summer, the model and measurements show good agreement. For the winter and autumn months, the simulated latent heat flux shows a similar overestimation to the sensible heat flux. For other seasons, they are most of the time within the 25th–75th percentile range. We want to point out that the measured fluxes in the winter and autumn months are very low (<10 W m−2) and an overall underestimation of heat fluxes during these periods is normal when applying the eddy covariance technique89 such as at SMEAR II; hence it is difficult to draw a conclusion on the accuracy of either the model or measurements during these periods.
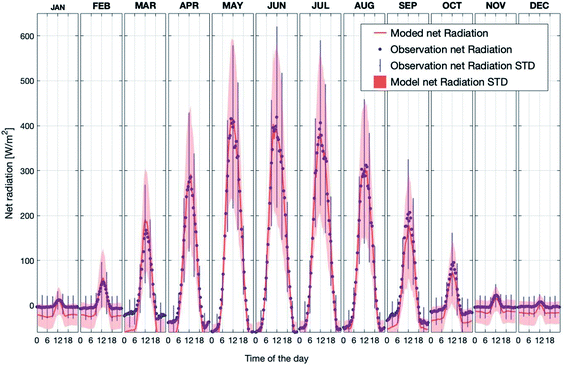
Fig. 2 shows the observed and simulated average diurnal cycle of net radiation at 125 m for each month in the period 2007–2018. Here, the net radiation is calculated as the total incoming short- and long-wave radiation minus the total outgoing short- and long-wave radiation at the canopy top. Thus, positive values represent more incoming than outgoing radiation and vice versa. The modelled daytime values agree well with the measurements for all months. By contrast, the modelled nighttime net radiation underestimated the measurements by about ∼10 W m−2 to ∼50 W m−2 from September to December and from January to March. In general, the model is consistent with the measurements, and is able to capture the diurnal pattern and seasonal trend of net radiation above the canopy. Therefore, considering the simulation results of SH and LH discussed above, the model can predict a reasonable energy balance inside and above the canopy.
 | ||
| Fig. 2 Observed and simulated average diurnal cycles of net radiation at SMEAR II for each month separately for the years 2007–2018 (STD = standard deviation). | ||
3.2. Trend of measured parameters
In Table 1 and Fig. S2,† the yearly and seasonal trends of selected measured parameters at SMEAR II are provided. Short wave global radiation, temperature, absolute humidity and condensation sink show no significant yearly trends. Only during the winter seasons, a clear daily decrease in the condensation sink by −3.96 (−7.48, −1.20) % per year is visible. This may partly be related to the reduction of primary aerosol emissions from traffic, industry and heating as pointed out by Nieminen et al. (2014).90The main inorganic gases (CO, O3, NO, NO2 and SO2) that serve as input to SOSAA reflect the influence of human impact on a regional scale. Carbon monoxide, for example, has a lifetime of approximately 1–3 months,91 and reveals the impact of large regional to hemispherical features. Nitrogen oxides and sulfur dioxide have lifetimes of days to weeks, respectively, and they are mainly related to local or regional changes. At a rural station such as SMEAR II, their concentrations are often below the LOD of the instruments. The 12-year concentrations of these five measured trace gases all show a negative trend reflecting the decreased anthropogenic impact on these gases in Europe during the last few decades.92 This trend in Europe was also confirmed by the latest EAA report (No 12/2018). Our trend analyses of daily values show that CO, NO and O3 concentrations only depict a marginal not significant decrease, while the concentrations of SO2 and NO2 drop by −5.43 (−8.10, +3.65) % per year and −3.80 (−5.99, −1.87) % per year, concerning the whole day, respectively.
However, as pointed out in Section 2.3, more than half of the SO2 and NO measurements are below the LOD of the instruments. Table S1 (in the ESI†) shows that a fraction of the NO and SO2 measurements is below the LOD in a year-wise fashion. The time period with SO2 below LOD within one year has increased from 2007 to 2018, which points to an even stronger decrease of SO2 concentrations than the 5.43% per year mentioned above. For NO, the amount of data measured below the LOD are much higher but without any visible trend. Note that in the evolution of the mean values and the 90th and 75th percentiles for NO, no trend is observed concerning the number of days with NO concentrations below the LOD (see also the discussion in Section 2.3).
3.3. BVOC's comparisons and trends
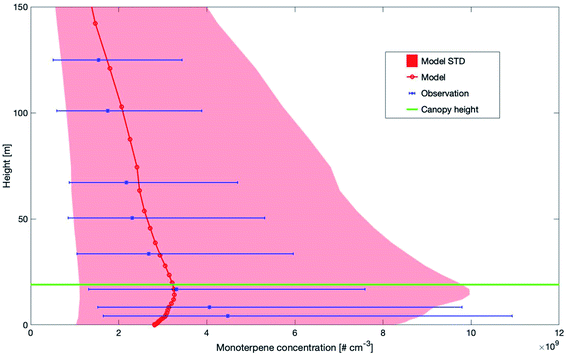
Fig. 3 shows the modelled versus the measured median monoterpene concentrations between 0 m and 150 m at SMEAR II. Both, the measurements and the simulations of monoterpene concentrations show an increase above the canopy. However, the peak from the measurements is at 4.2 m, while the model shows the highest values at 16.8 m. Modelled and measured values decrease at a similar rate above the canopy. The different height levels of the maximum could be related to the missing emission sources from understory vegetation and soil.93,94 Another reason for the decrease of the modelled concentrations inside the canopy could be related to the emission of monoterpenes from ground vegetation and soil as reported by Aaltonen et al. (2011).95 Currently, these sources of terpenes are not included in SOSAA and may explain the discrepancy in the lower canopy. For this reason, we compared the measured and modelled monoterpene concentrations for heights above the canopy.
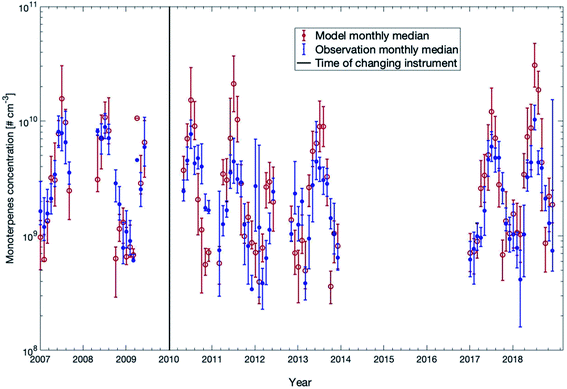
In Fig. 4, we compared the measured monoterpene concentrations against the model outcome between 32 m and 125 m for all years except years 2014–2016 (measured data were not available for this period). The model slightly underestimated the monoterpene concentrations in winter while overestimated the values in summer. In years when the summer was exceptionally warm (e.g., 2018), the model overestimated the monoterpene concentration by a factor of 2–3. The reason for this overshooting of the model during hot summers could be that the decrease of monoterpene emissions in the forest during drought is not accurately represented in the emission module MEGAN. In general, for more than 70% of the time, the modelled concentrations are within the range of 25th and 75th percentiles of the measured data points.
In order to compare the modelled and measured trends of monoterpene concentrations, we only consider the model data points when measurements were available to calculate the linear fittings or the trends. First, considering the measurement gap during 2014 to 2016, we can separate the datasets into two periods before and after the gap. One is from 2007 to 2013 and the other is from 2017 to 2018. The linear fittings show that the modelled trends of these two periods are −0.72 × 108 molecules per cm3 per year and 19 × 108 molecules per cm3 per year, and the measured trends are −6.8 × 108 molecules per cm3 per year and 6.5 × 108 molecules per cm3 per year, respectively (Fig. S3†). Therefore, the trends in the model and the measurements show both a decreasing trend from 2007 to 2013 and a similar change from 2017 to 2018. Secondly, the one-year moving averages of modelled and measured monoterpene concentrations also show consistent variations, both of which show a consecutive strong peak and dip from 2010 to 2014, and a sharp increase from 2017 to 2018 (Fig. S4†). The lower trends of measurement data may result from several very low measurement concentrations around the beginning of 2012, 2017 and 2018 which were caused by low temperature during these days (not shown here).
3.4. Long term trends of the oxidants
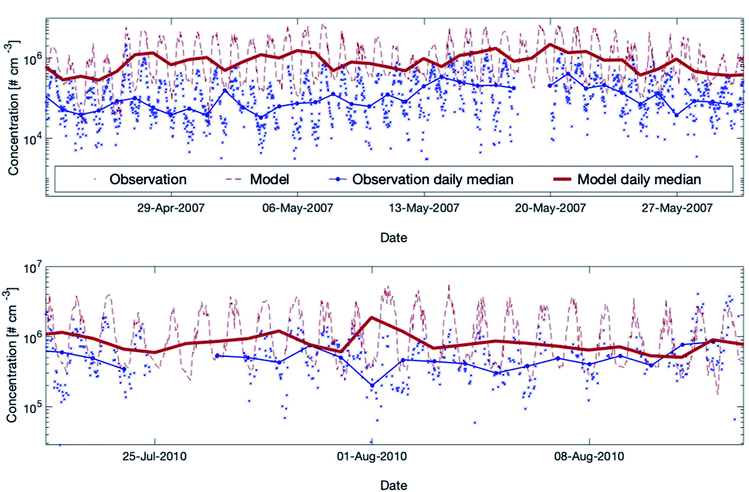 | ||
| Fig. 5 Measured versus modelled hydroxyl radial concentrations for two periods during the EUCAARI (upper plot) and the HUMPPA-COPEC (lower plot) campaigns. For EUCAARI, the exact measurement height is unknown, and Petäjä et al. (2009)39 mentioned that it was close to the ground level in a forest clearing. For HUMPPA-COPEC, the OH concentrations measured by CIMS (Chemical Ionization Mass Spectrometry) are used here and the measurement height was at the ground level in a different forest clearing at SMEAR II.96 For both campaigns, we applied here the model height level at 32.8 m assuming that the two measurement places were in the forest clearing instead of inside the forest canopy. | ||
During the campaign in 2007, the model overpredicted the OH concentrations substantially but the model performance showed better agreement in 2010. The main cause for the discrepancies between the two measured data sets is related to the two different seasons (May and August) during which the campaigns were conducted. The reason why the model agrees satisfactorily with the measurements in August and rather poorly in May is more complex. Additional studies comparing measured and modelled OH-reactivity at the same location9,27,28 showed a high missing OH-reactivity while including all measured gaseous compounds in SOSAA. These discrepancies indicate the existence of unknown compounds during springtime and early summer, which are not included in SOSAA. Preferred reactions of these species with the hydroxyl radical might explain the simulated, strong overestimation of the OH concentration at SMEAR II. Note that the uncertainty of point measurements is considerable for the boreal forest environment, as already mentioned in Section 3.3.1. The overall conclusion is that SOSAA is able to simulate the OH concentrations at SMEAR II in a sufficient way during summer but overestimates OH in spring. There are no measurements available for other seasons for comparison.
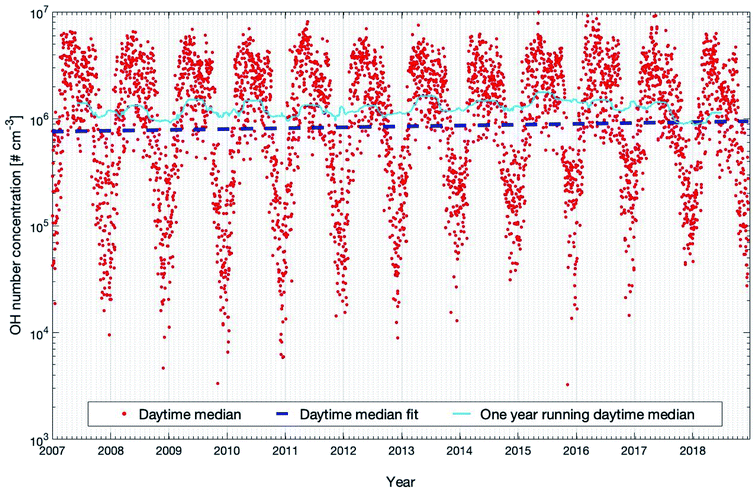
Fig. 6 shows the daytime time-series of modelled OH concentrations from 2007 to 2018 as OH production is related to photochemical reactions and peaks during this time of the day.9 The concentration shows a clear seasonal cycle with peaks in spring and late summer, which partly result from the patterns of ozone (peak in spring, see Fig. S2†) and the solar irradiance (peak in summer, see Fig. S2†), both required to produce an excited oxygen atom (O(1D)) which then reacts with one water molecule to form two hydroxyl radicals. Table 1 shows that OH shows significant increases in daily and nighttime trends which are +2.39 (+0.95, +3.33) % per year and 3.31 (+2.01, +4.62) % per year, respectively, with both PMK values below 0.04. However, during daytime, the OH concentration only shows a marginal increase of +0.91 (−0.81, +2.10) % per year with a high statistical uncertainty and the PMK value is 0.24. The trend of OH shown here is mainly related to its sink terms instead of the source terms because the main source terms of OH, e.g., O3 and global short-wave radiation, do not show any significant increasing trends (Table 1). Among all the sink terms of OH, CO plays a major role and accounts for about 30–40% of the annual removal of OH at SMEAR II.28,97Table 1 shows a decreasing trend of CO which is consistent with previous studies.98,99 Moreover, the time series of the mixing ratio of CO in winter also shows an opposite trend to the OH concentration (Fig. S6†). However, all the decreasing daily, daytime and nighttime trends of CO are not significant (Table 1), which indicates that in order to explain the OH trend, other sink terms and their seasonal trends should be taken into account.
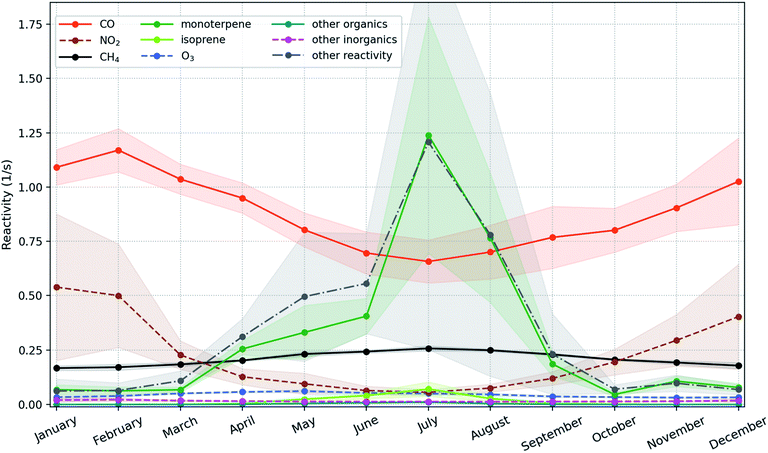
Since the daytime length is shortest in winter and autumn while longest in summer and spring, the nighttime increasing trend is dominated by the trends of winter and autumn that are +10.25 (+3.95, +17.32) % per year and +4.54 (+2.39, +6.29) % per year, respectively. Fig. 7 shows that the strong increasing trends of OH in these two seasons are caused by the combined effects of decreasing CO and NO2. The deceasing trend of NO2 is much higher than that of CO with ∼9.7 times in winter and ∼5.9 times in autumn, but the OH reactivity due to NO2 is only about half of that due to CO (Fig. 7). The seasonal inter-annual trends of OH reactivities also show an apparent drop of ROH,CO (OH reactivity due to CO) and ROH,NO2 (OH reactivity due to NO2) during winter with ROH,NO2 being a factor of 2–3 lower compared to ROH,CO (Fig. S7†). Therefore, as a dominant sink of OH in winter, CO is the main factor to explain the long-term modelled OH trend, and NO2 also contributes a comparable portion. Moreover, monoterpenes can also produce OH via ozonolysis reactions, which are a main source of OH under dark conditions.100–102 So the increasing trend of monoterpenes could enhance the increasing nighttime trend of OH.
In spring and summer, the monoterpenes and the compounds produced from the second or higher order reactions start to be competitive with or dominant over CO among the OH reactivity contributors (Fig. 7 and S7†). The forest stands near SMEAR II are dominated by Scots pine, which produces relatively low isoprene (e.g., Rinne et al. (2009)26 and references therein). For example, during summer months between 2010 and 2013 at SMEAR II, the measured flux of isoprene + MBO (2-methyl-3-buten-2-ol) was usually around one order of magnitude smaller than that of monoterpenes.103 This indicates that the compounds produced from the second or higher order reactions are mainly the second or higher order oxidized products of monoterpenes. Therefore, the increasing trend of monoterpenes can offset the effect of decreasing CO, leading to the insignificant daytime trends in spring and summer. And finally, a strong increasing nighttime trend and an insignificant daytime trend together lead to a significant but weaker daily trend of OH.
In the future, assuming decreasing emissions of CO and NO2 (change in energy production and lower nitrogen compounds of traffic emissions) and increasing monoterpene emissions in the boreal region due to climate warming,104 we would expect an increase of OH during winter and autumn months in central south Finland. During spring and summer, other sink terms are more relevant and OH should be buffered and remain quite constant.
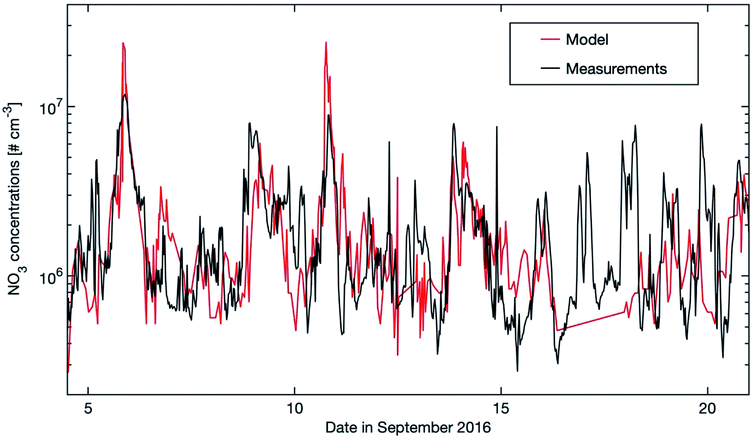 | ||
| Fig. 8 Modelled NO3 concentrations (20–40 m) and stationary state NO3 mixing ratios calculated from the production term (k[NO2][O3]) and using kOTG + k[NO] + JNO3 as the loss term with kOTG representing the measured reactive loss of NO3 to organic trace gases (Fig. 10 in Liebmann et al., 2018).56 Measurements were obtained from the common inlet at a height of 8.5 m apart from the NO3 photolysis rate (taken from a height of 35 m on an adjacent tower), wind direction (WD) and wind speed (WS) (both at 16.5 m on the 128 m tower). | ||
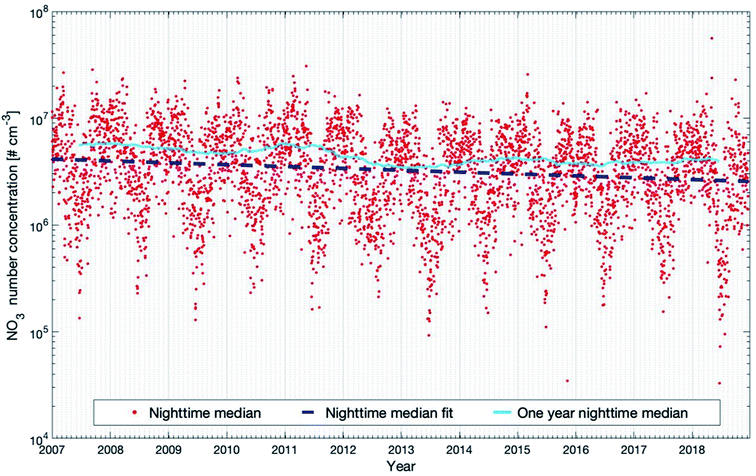
Fig. 9 shows the nighttime median NO3 concentration and the trends for the selected 12 years. The seasonal cycle shows a double-peak in late autumn and early spring, respectively. The reason is that the NO2 concentration is highest during these periods (see Fig. S2 in the ESI†), which may result from the high NO emissions from the microbial processes in the forest soil105,106 when the organic litter mass was high. As for the trend, the daytime and nighttime inter-annual trends are quite alike. The decreasing trend at night-time (−4.22 (−5.90 and −2.86) % per year) is slightly higher than the decreasing trend at daytime (−2.43 (−3.90 and −0.90) % per year). The 12 year trend of nighttime NO3 can be explained by the decrease of nighttime NO2, since the main source of NO3 comes from the oxidation of NO2 by O3. In Table 1, we can see that nighttime O3 shows no trend for the 12 years. However, nighttime NO2 shows a significant negative trend of −4.18 (−6.27 and −2.32) % per year, being consistent with the decreasing trend of nighttime NO3. The one-year running median of the nighttime NO3 concentration also shows an oscillation of 3–3.5 years during the years 2007–2018, but since this period is relatively short, it is hard to conclude on the reasons (Fig. 9).
3.5. Sulfuric acid model comparison and long-term trends
Sulfuric acid was measured at SMEAR II during the last few years for several periods. In Fig. 10, we provide a comparison with the outcome of our model simulations for the years 2016 to 2018 (scatter plot and daily distributions for these data sets are provided in Fig. S8 in the ESI†). A detailed description of the instrument used in this study is provided in Jokinen et al. (2012).107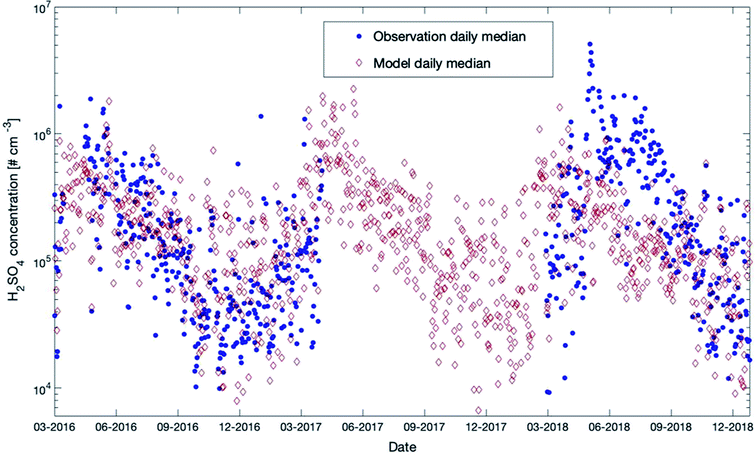 | ||
| Fig. 10 Measured (35 m) and modelled (32.8 m) daily median sulfuric acid concentrations at SMEAR II for the years 2016–2018. | ||
During 2016–2017, the model and measurements agreed at most times in autumn and winter. In summer 2018, the model tended to underestimate the measured concentrations but showed a very good agreement for the same year during autumn. During winter nighttime, the observations were partly below the model results and reached values down to a couple of hundreds of molecules per cm3. However, the LOD of the instrument was 4 × 104 molecules cm−3 (ref. 107) and most of the measurements during that period were below the LOD. This could explain why the daily median values in winter were lower compared to the simulated values.
Both the model and the observations present an interesting pattern for the three years: a peak in early spring and then a continuous decline of concentrations for the rest of the year. There is a smaller second peak in summers visible in the model data set, but this peak is weaker compared to the spring peak, as can also be seen in Fig. 11, which provides the modelled 12-year daytime median concentrations of sulfuric acid. The reason for this pattern, which is visible in the model outcomes for all years, is a combination of mainly three effects: SO2, one of the two main precursors of H2SO4, peaks in late winter and early spring and OH reaches its yearly maximum in spring. Additionally, the condensation sink, representing the rate of how fast sulfuric acid molecules will condense on the existing particles, has a clear maximum in summer (Fig. S2 in the ESI†). These three parameters are mainly responsible for the sulfuric acid pattern. Note that a similar pattern has been observed for the occurrence of NPF events at SMEAR II for several years.90
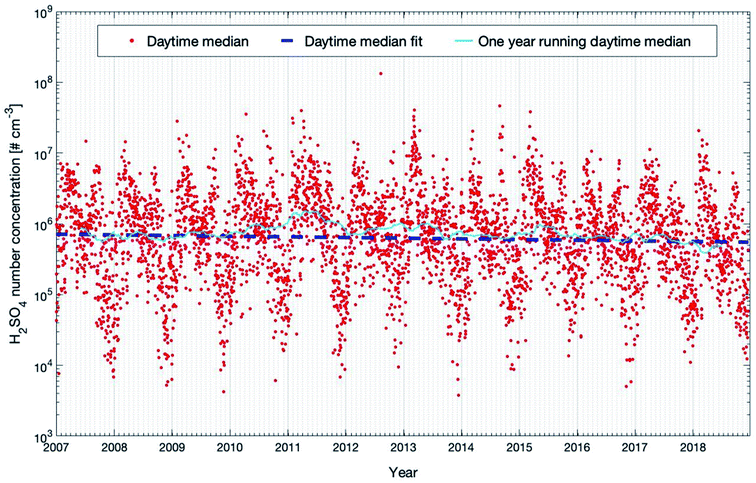
Nieminen et al. (2014)90 predicted the trend of sulfuric acid based on a proxy calculation (see the next section) with −1.3% on NPF days and −0.3% on non-NPF days per year for the years 1997–2012. In our study, we applied SOSAA simulations for the years 2007–2018 for the same location. Our model results predict a stronger decrease of daytime H2SO4 of −2.78 (−6.05 and −0.63) % per year (see Table 1). However, the confidence interval of this trend is quite broad and the PMK value is 0.18, which suggests that caution should be taken to interpret this trend too far to the future. The trend in the studied time span is greatly influenced by the large yearly variation. With decreasing emission of SO2 in the future (related to improved filter systems), we can expect a decreasing trend of H2SO4 in central southern Finland which could have a significant impact on the amount of newly formed particles and consequently the number concentrations of cloud condensation nuclei.
3.6. Proxy comparison for OH, H2SO4 and NO3
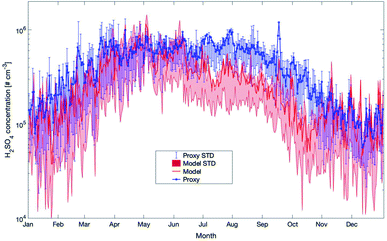
During the last few years, several proxies have been developed for compounds such as the hydroxyl or nitrate radicals due to the absence or sparse long-term observations for these parameters. In this section, we will compare some of these proxies with the outcome of our model simulations for SMEAR II. This, however, should not be seen as a validation of the proxies but rather to investigate how well the simulations agree with them. The proxies compared were developed based on datasets from SMEAR II.The first proxy we compare is for the OH radical. It is based on Petäjä et al. (2009)39 and Nieminen et al. (2014).90 The results of the proxy, together with the outcome of the model simulations, are presented in Fig. 12. In this proxy, the hydroxyl radical is calculated as
| [OH] = ((8.4 × 10−7/8.6 × 10−10) × UVB0.32)1.92 | (1) |
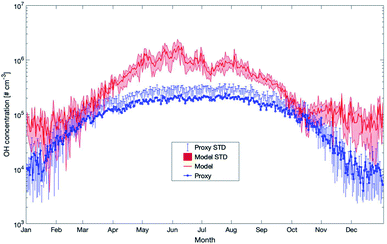
Later in the year, from October to February, the proxy showed very low OH values related to the main and only source by UVB. In reality, the ozonolysis of terpenes will contribute to the OH-concentration at this time and the model data seems to be more realistic during periods with low photolysis rates. However, as long as these unknown compounds are not identified and no long-term measurements of OH at SMEAR II exist, any final conclusion about whether the proxy or the model is more correct can only be speculation. However, based on our previous OH-reactivity studies, we are confident that certain crucial reactions are still missing. For this reason, the simplified relation between UVB and OH could provide a more realistic picture on the hydroxyl radical concentrations during spring and early summer.
The next proxy we compare with our model simulations is for sulfuric acid. It is based on a new parameterization method from Dada et al. (2020).108 This proxy is calculated as follows:
| [H2SO4] = −CS/2 × k3 + ((CS/2 × k3)2 + [SO2]/k3 × (k2 × GloRad + k2 × [O3] × [alkene]))0.5. | (2) |
As already pointed out in the previous section, the modelled sulfuric acid concentrations show a clear peak in early spring and then a nearly continuous decrease for the rest of the year (see Fig. 13). The proxy follows this pattern throughout the year but exceeds the modelled data by a factor of 2 from the end of June to the end of October and during some shorter periods in winter. As the measured values for these periods seem to agree well with the model results, we conclude that the proxy in summer and autumn overestimates the H2SO4 concentrations and we assume that for this parameter, the model provides a more realistic picture of the sulfuric acid concentrations.
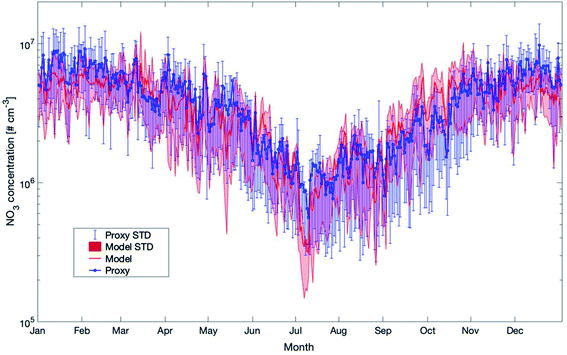
The last proxy we want to compare against the model results is for the nitrate radical. It is based on Peräkylä et al. (2014)57 and Kontkanen et al. (2016).58 The concentration of NO3 is calculated based on the following equation:
| [NO3] = kO3+NO2 × [O3] × [NO2] × τNO3 | (3) |
Previous studies assumed a steady state between the production of NO3 from the reaction between O3 and NO2 and the removal of NO3.57,58 This gap could be filled by our study. A comparison between the proxy and the model data shows that the long-term trend from both methods is in very good agreement (Fig. 14). As these two methods applied here agree very well, it is likely that the predicted values for NO3 are reliable and could be applied in further studies.
4. Summary and perspectives
In this study, we investigated the trends of selected atmospherically crucial gaseous compounds at the SMEAR II station in southern Finland for the period 2007–2018. The main focus was on the hydroxyl and nitrate radicals as well as on sulfuric acid, as no long-term measurements of these compounds exist. To validate the SOSAA model, we first compared the OH, H2SO4 and NO3 simulations with the existing measurements from several campaigns. For H2SO4, the model underestimated the measured values in summer 2018 but reproduced the measurements for the same period in 2016. For all other seasons, the model and the measurements agree satisfactorily. The OH radical was only measured during two short campaigns in May 2007 (ref. 53) and August 2010.54 The comparison between the observed and modelled OH yielded different results in the campaigns of May 2007 and August 2010. In 2007 the model predicted about twice as high values as measured whereas in 2010 the model agreed quite well with the measurements, reflecting the existence of an unknown sink(s) for OH in spring and early summer. In the case of the nitrate radical, no direct measurements at SMEAR II exist. However, Liebmann et al. (2018)56 measured the NO3 reactivity based on which they estimated the NO3 mixing ratios. The comparison of the indirect NO3 measurements with our model results showed a good agreement for the selected short period in September 2016.The long-term trends (12 years) of the two important oxidants OH and NO3 were investigated. Our results indicate that the daily OH concentration increases during this period with a rate of +2.38 (+0.95, +3.33) % per year, which relates to a significant increase of OH during winter nighttime (+10.66 (+5.04, 17.37) % per year). The main reason is likely a drop in carbon monoxide concentrations (∼0.5% per year) during winter representing the main sink term for OH during this period. This result was surprising as the monoterpenes, the main biogenic VOC at SMEAR II, increased by about +3.40 (+1.23, +6.02) % per year and reacted strongly with OH. Therefore, the predicted OH trend shows that the climatic temperature increases (∼0.8 K in 12 years at SMEAR II) and the following rise in BVOC emissions is buffered by a decline of carbon monoxide in winter months. In case the current negative trend in CO continues – mostly related to improved combustion techniques – OH will slightly rise if monoterpene concentrations do not increase more significantly. This would cause a positive impact on the atmospheric oxidation capacity. Vice versa is the situation for NO3, showing a nighttime decrease by −4.22 (−5.90, −2.86) % per year which is caused by the drop in nitrogen dioxide. As all anthropogenic NOx emissions in Europe have decreased significantly during the last few decades (EAA report No 12/2018) and are predicted to decrease further, we expect that the nitrate radical will continue to drop in the future. As pointed out by Mogensen et al. (2015),9 NO3 is the strongest oxidant during nighttime and can have an aerosol yield when reacting with monoterpenes up to 65%.110 A continuous drop in NO3 in the boreal forest could implicate a negative impact on the growth of SOA during nighttime and decrease in the CCN concentrations.
Sulfuric acid was investigated as it is one of the most important precursors of new particle formation (NPF). The outcome of our study indicates that the sulfuric acid concentration decreases by −2.78 (−6.05 and −0.63) % per year during daytime, which likely is related to the reduction in the emissions of sulfur dioxide in Europe during the last few decades (EAA report No 12/2018). In case the negative trend of sulfuric acid (steered by SO2) will continue in the next few decades, it could significantly affect the amount of NPF events in the boreal region. However, whether or not this will have a positive or negative impact on our future climate is currently unclear. In the past, it was typically assumed that NPF events will provide more CCN, followed by more cloud droplets, leading to an increased albedo through “brighter” clouds.111 In this way, NPF would cool the planet and counteract the effect of greenhouse gases. However, recently the research results of Roldin et al. (2019)15 counteract this assumption. Their results showed that under some meteorological conditions a high number of newly formed particles increase in size at the expense of the larger aerosol particles over the boreal forest – and it is only the larger aerosol particles that have a cooling effect on the planet. Facing the controversial discussion on this topic in the scientific community, it is difficult to state whether the decrease of sulfuric acid should be seen as positive or negative. However, it is certain that H2SO4 has decreased in the last few decades and, most likely, will continue to drop in the future.
Proxies are commonly applied in case a limited number of parameters are measured and no detailed model simulations are available. We compared the concentrations of OH, NO3 and H2SO4 calculated from proxies39,57,58,90,108 with our model outcomes. Our comparisons showed that the proxies for OH and H2SO4, at certain times of the year, agree very well with the model results but also differ significantly during other periods. For the nitrate radical, the model and proxy results are in good agreement.
Special issue statement
This article is part of the special issue “Pan-Eurasian Experiment (PEEX)”. It is not associated with a conference.Data availability
All data shown in the figures, tables and additional raw data are available upon request from the corresponding author (PZ).Author contributions
M. Boy and PZ conceived the idea and coordinated the work. DC performed the model simulations, wrote main parts of the manuscript and plotted Fig. 1 to 6, 8 to 14, S1, S2, S5 and S8.† CX, PC and TN implemented the statistical analysis of the data and plotted Fig. 7, S3, S4, S6 and S7.† PZ, M. Boy, P. Roldin and XQ were the main model developers and assisted in setting up the model for the simulations. P. Rantala, JA, NS, P. Kolari and P. Keronen provided the input data for the model runs and for the model evaluation. LP, MK, MPR, DT, BF and M. Baykara contributed significantly to the data interpretation of the model. All co-authors commented on the paper and supported the finalization of the manuscript. PZ oversaw the responses to the reviewer comments.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The work was supported by the ACCC Flagship, funded by the Academy of Finland grant number 337549. We also acknowledge the support by the Academy of Finland (Center of Excellence in Atmospheric Sciences (grant no. 4100104)) and the computational resources from CSC – IT Center for Science, Finland. Dean Chen acknowledges the China Scholarship Council (201604910541). Putian Zhou acknowledges the University of Helsinki Three Year Grant (“AGES”: 2018–2020). Lukas Pichelstorfer acknowledges the Austrian Science Funds (FWF) under grant (J-4241 Schrödinger Programme). Pontus Roldin acknowledges the Swedish Research Council FORMAS (project no. 2014-1445, 2015-749, and 2018-01745), the Swedish Research Council VR (project. no. 2019-05006) and the Swedish Strategic Research Program MERGE. Ximeng Qi acknowledges the National Natural Science Foundation of China (41805101).References
- S. Gligorovski, R. Strekowski, S. Barbati and D. Vione, Environmental Implications of Hydroxyl Radicals (˙OH), Chem. Rev., 2015, 115(24), 13051–13092, DOI:10.1021/cr500310b.
- I. Bey, B. Aumont and G. Toupance, A modeling study of the nighttime radical chemistry in the lower continental troposphere: 1. Development of a detailed chemical mechanism including nighttime chemistry, J. Geophys. Res., D: Atmos., 2001, 106(9), 9959–9990, DOI:10.1029/2000jd900347.
- B. J. Allan, J. M. C. Plane, H. Coe and J. Shillito, Observations of NO3 concentration profiles in the troposphere, J. Geophys. Res., 2002, 107(D21), 4588, DOI:10.1029/2002jd002112.
- S. S. Brown, H. Stark, T. B. Ryerson, E. J. Williams, D. K. Nicks, M. Trainer, F. C. Fehsenfeld and A. R. Ravishankara, Nitrogen oxides in the nocturnal boundary layer: simultaneous in situ measurements of NO3, N2O5, NO2, NO, and O3, J. Geophys. Res., D: Atmos., 2003, 108(9), 4299, DOI:10.1029/2002jd002917.
- J. N. Crowley, G. Schuster, N. Pouvesle, U. Parchatka, H. Fischer, B. Bonn, H. Bingemer and J. Lelieveld, Nocturnal nitrogen oxides at a rural mountain-site in south-western Germany, Atmos. Chem. Phys., 2010, 10(6), 2795–2812, DOI:10.5194/acp-10-2795-2010.
- Y. F. Elshorbany, R. Kurtenbach, P. Wiesen, E. Lissi, M. Rubio, G. Villena, E. Gramsch, A. R. Rickard, M. J. Pilling and J. Kleffmann, Oxidation capacity of the city air of Santiago, Chile, Atmos. Chem. Phys., 2009, 9(6), 2257–2273, DOI:10.5194/acp-9-2257-2009.
- R. Volkamer, P. Sheehy, L. T. Molina and M. J. Molina, Oxidative capacity of the Mexico City atmosphere – Part 1: a radical source perspective, Atmos. Chem. Phys., 2010, 10(14), 6969–6991, DOI:10.5194/acp-10-6969-2010.
- J. Mao, X. Ren, S. Chen, W. H. Brune, Z. Chen, M. Martinez, H. Harder, B. Lefer, B. Rappenglück, J. Flynn and M. Leuchner, Atmospheric oxidation capacity in the summer of Houston 2006: comparison with summer measurements in other metropolitan studies, Atmos. Environ., 2010, 44(33), 4107–4115, DOI:10.1016/j.atmosenv.2009.01.013 , ISSN 1352-2310..
- D. Mogensen, R. Gierens, J. N. Crowley, P. Keronen, S. Smolander, A. Sogachev, A. C. Nölscher, L. Zhou, M. Kulmala, M. J. Tang, J. Williams and M. Boy, Simulations of atmospheric OH, O3 and NO3 reactivities within and above the boreal forest, Atmos. Chem. Phys., 2015, 15(7), 3909–3932, DOI:10.5194/acp-15-3909-2015.
- P. A. Feiner, W. H. Brune, D. O. Miller, L. Zhang, R. C. Cohen, P. S. Romer, A. H. Goldstein, F. N. Keutsch, K. M. Skog, P. O. Wennberg, T. B. Nguyen, A. P. Teng, J. DeGouw, A. Koss, R. J. Wild, S. S. Brown, A. Guenther, E. Edgerton, K. Baumann and J. L. Fry, Testing Atmospheric Oxidation in an Alabama Forest, J. Atmos. Sci., 2016, 73(12), 4699–4710, DOI:10.1175/jas-d-16-0044.1.
- D. Stone, M. J. Evans, H. Walker, T. Ingham, S. Vaughan, B. Ouyang, O. J. Kennedy, M. W. McLeod, R. L. Jones, J. Hopkins, S. Punjabi, R. Lidster, J. F. Hamilton, J. D. Lee, A. C. Lewis, L. J. Carpenter, G. Forster, D. E. Oram, C. E. Reeves, S. Bauguitte, W. Morgan, H. Coe, E. Aruffo, C. Dari-Salisburgo, F. Giammaria, P. Di Carlo and D. E. Heard, Radical chemistry at night: comparisons between observed and modelled HOx, NO3 and N2O5 during the RONOCO project, Atmos. Chem. Phys., 2014, 14(3), 1299–1321, DOI:10.5194/acp-14-1299-2014.
- B. Bonn, R. von Kuhlmann and M. G. Lawrence, High contribution of biogenic hydroperoxides to secondary organic aerosol formation, Geophys. Res. Lett., 2004, 31(10), L10108, DOI:10.1029/2003gl019172.
- M. Claeys, B. Graham, G. Vas, W. Wang, R. Vermeylen, V. Pashynska, J. Cafmeyer, P. Guyon, M. O. Andreae, P. Artaxo and W. Maenhaut, Formation of secondary organic aerosols through photooxidation of isoprene, Science, 2004, 303, 1173–1176, DOI:10.1126/science.1092805.
- M. Hallquist, J. C. Wenger, U. Baltensperger, Y. Rudich, D. Simpson, M. Claeys, J. Dommen, N. M. Donahue, C. George, A. H. Goldstein, J. F. Hamilton, H. Herrmann, T. Hoffmann, Y. Iinuma, M. Jang, M. E. Jenkin, J. L. Jimenez, A. Kiendler-Scharr, W. Maenhaut, G. McFiggans, T. F. Mentel, A. Monod, A. S. H. Prévôt, J. H. Seinfeld, J. D. Surratt, R. Szmigielski and J. Wildt, The formation, properties and impact of secondary organic aerosol: current and emerging issues, Atmos. Chem. Phys., 2009, 9, 5155–5236, DOI:10.5194/acp-9-5155-2009.
- P. Roldin, M. Ehn, T. Kurtén, T. Olenius, M. P. Rissanen, N. Sarnela, J. Elm, P. Rantala, L. Hao, N. Hyttinen, L. Heikkinen, D. R. Worsnop, L. Pichelstorfer, C. Xavier, P. Clusius, E. Öström, T. Petäjä, M. Kulmala, H. Vehkamäki, A. Virtanen, I. Riipinen and M. Boy, The role of highly oxygenated organic molecules in the Boreal aerosol-cloud-climate system, Nat. Commun., 2019, 10(1), 4370, DOI:10.1038/s41467-019-12338-8.
- F. Riccobono, S. Schobesberger, C. E. Scott, J. Dommen, I. K. Ortega, L. Rondo, J. Almeida, A. Amorim, F. Bianchi, M. Breitenlechner, A. David, A. Downard, E. M. Dunne, J. Duplissy, S. Ehrhart, R. C. Flagan, A. Franchin, A. Hansel, H. Junninen, M. Kajos, H. Keskinen, A. Kupc, A. Kurten, A. N. Kvashin, A. Laaksonen, K. Lehtipalo, V. Makhmutov, S. Mathot, T. Nieminen, A. Onnela, T. Petäjä, A. P. Praplan, F. D. Santos, S. Schallhart, J. H. Seinfeld, M. Sipila, D. V. Spracklen, Y. Stozhkov, F. Stratmann, A. Tome, G. Tsagkogeorgas, P. Vaattovaara, Y. Viisanen, A. Vrtala, P. E. Wagner, E. Weingartner, H. Wex, D. Wimmer, K. S. Carslaw, J. Curtius, N. M. Donahue, J. Kirkby, M. Kulmala, D. R. Worsnop and U. Baltensperger, Oxidation Products of Biogenic Emissions Contribute to Nucleation of Atmospheric Particles, Science, 2014, 344(6185), 717–721, DOI:10.1126/science.1243527.
- J. Kirkby, J. Duplissy, K. Sengupta, C. Frege, H. Gordon, C. Williamson, M. Heinritzi, M. Simon, C. Yan, J. Almeida, J. Tröstl, T. Nieminen, I. K. Ortega, R. Wagner, A. Adamov, A. Amorim, A.-K. Bernhammer, F. Bianchi, M. Breitenlechner, S. Brilke, X. Chen, J. Craven, A. Dias, S. Ehrhart, R. C. Flagan, A. Franchin, C. Fuchs, R. Guida, J. Hakala, C. R. Hoyle, T. Jokinen, H. Junninen, J. Kangasluoma, J. Kim, M. Krapf, A. Kürten, A. Laaksonen, K. Lehtipalo, V. Makhmutov, S. Mathot, U. Molteni, A. Onnela, O. Peräkylä, F. Piel, T. Petäjä, A. P. Praplan, K. Pringle, A. Rap, N. A. D. Richards, I. Riipinen, M. P. Rissanen, L. Rondo, N. Sarnela, S. Schobesberger, C. E. Scott, J. H. Seinfeld, M. Sipilä, G. Steiner, Y. Stozhkov, F. Stratmann, A. Tomé, A. Virtanen, A. L. Vogel, A. C. Wagner, P. E. Wagner, E. Weingartner, D. Wimmer, P. M. Winkler, P. Ye, X. Zhang, A. Hansel, J. Dommen, N. M. Donahue, D. R. Worsnop, U. Baltensperger, M. Kulmala, K. S. Carslaw and J. Curtius, Ion-induced nucleation of pure biogenic particles, Nature, 2016, 533, 521–526, DOI:10.1038/nature17953.
- H. Gordon, J. Kirkby, U. Baltensperger, F. Bianchi, M. Breitenlechner, J. Curtius, A. Dias, J. Dommen, N. M. Donahue, E. M. Dunne, J. Duplissy, S. Ehrhart, R. C. Flagan, C. Frege, C. Fuchs, A. Hansel, C. R. Hoyle, M. Kulmala, A. Kürten, K. Lehtipalo, V. Makhmutov, U. Molteni, M. P. Rissanen, Y. Stozkhov, J. Tröstl, G. Tsagkogeorgas, R. Wagner, C. Williamson, D. Wimmer, P. M. Winkler, C. Yan and K. S. Carslaw, Causes and importance of new particle formation in the present-day and preindustrial atmospheres, J. Geophys. Res.: Atmos., 2017, 122, 8739–8760, DOI:10.1002/2017jd026844.
- E. M. Dunne, H. Gordon, A. Kürten, J. Almeida, J. Duplissy, C. Williamson, I. K. Ortega, K. J. Pringle, A. Adamov, U. Baltensperger, P. Barmet, F. Benduhn, F. Bianchi, M. Breitenlechner, A. Clarke, J. Curtius, J. Dommen, N. M. Donahue, S. Ehrhart, R. C. Flagan, A. Franchin, R. Guida, J. Hakala, A. Hansel, M. Heinritzi, T. Jokinen, J. Kangasluoma, J. Kirkby, M. Kulmala, A. Kupc, M. J. Lawler, K. Lehtipalo, V. Makhmutov, G. Mann, S. Mathot, J. Merikanto, P. Miettinen, A. Nenes, A. Onnela, A. Rap, C. L. S. Reddington, F. Riccobono, N. A. D. Richards, M. P. Rissanen, L. Rondo, N. Sarnela, S. Schobesberger, K. Sengupta, M. Simon, M. Sipilä, J. N. Smith, Y. Stozkhov, A. Tomé, J. Tröstl, P. E. Wagner, D. Wimmer, P. M. Winkler, D. R. Worsnop and K. S. Carslaw, Global atmospheric particle formation from CERN CLOUD measurements, Science, 2016, 354(6316), 1119–1124, DOI:10.1126/science.aaf2649.
- A. B. Guenther, C. Hewitt, D. Erickson, R. Fall, C. Geron, T. Graedel, P. Harley, L. Klinger, M. Lerdau, W. McKay, T. Pierce, R. Scholes, R. Steinbrecher, R. Tallamraju, J. Taylor and P. Zimmerman, A global model of natural volatile organic compound emissions, J. Geophys. Res., 1995, 100, 8873–8892 CrossRef CAS.
- A. B. Guenther, T. Karl, P. Harley, C. Wiedinmyer, P. I. Palmer and C. Geron, Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature), Atmos. Chem. Phys., 2006, 6, 3181–3210 CrossRef CAS.
- G. B. Bonan, Forests and Climate Change: Forcings, Feedbacks and the Climate Benefits of Forests, Science, 2008, 320(5882), 1444–1449, DOI:10.1126/science.1155121.
- FAO, Global Forest Resources Assessment 2015, UN Food and Agriculture Organization, Rome, 2015 Search PubMed.
- H. Hakola, J. Rinne and T. Laurila, The hydrocarbon emission rates of tea-leafed willow (Salix phylicifolia), silver birch (Betula pendula) and European aspen (Populus tremula), Atmos. Environ., 1998, 32(10), 1825–1833 CrossRef CAS.
- H. Hakola, V. Tarvainen, J. Bäck, H. Ranta, B. Bonn, J. Rinne and M. Kulmala, Seasonal variation of mono- and sesquiterpene emission rates of Scots pine, Biogeosciences, 2006, 3(1), 93–101 CrossRef CAS.
- J. Rinne, J. Bäck and H. Hakola, Biogenic volatile organic compound emissions from the Eurasian taiga: current knowledge and future directions, Boreal Environ. Res., 2009, 14, 807–826 CAS.
- D. Mogensen, S. Smolander, A. Sogachev, L. Zhou, V. Sinha, A. Guenther, J. Williams, T. Nieminen, M. K. Kajos, J. Rinne, M. Kulmala and M. Boy, Modelling atmospheric OH-reactivity in a boreal forest ecosystem, Atmos. Chem. Phys., 2011, 11, 9709–9719 CrossRef CAS.
- A. P. Praplan, T. Tykkä, D. Chen, M. Boy, D. Taipale, V. Vakkari, P. Zhou, T. Petäjä and H. Hellén, Long-term total OH reactivity measurements in a boreal forest, Atmos. Chem. Phys., 2019, 19(23), 14431–14453 CrossRef CAS.
- R. C. Wilson, Z. L. Fleming, P. S. Monks, G. Clain, S. Henne, I. B. Konovalov, S. Szopa and L. Menut, Have primary emission reduction measures reduced ozone across Europe? An analysis of European rural background ozone trends 1996–2005, Atmos. Chem. Phys., 2012, 12(1), 437–454 CrossRef CAS.
- Y. Yan, A. Pozzer, N. Ojha, J. Lin and J. Lelieveld, Analysis of European ozone trends in the period 1995–2014, Atmos. Chem. Phys., 2018, 18(8), 5589–5605 CrossRef CAS.
- S. A. Montzka, C. M. Spivakovsky, J. H. Butler, J. W. Elkins, L. T. Lock and D. J. Mondeel, New observational constraints for atmospheric hydroxyl on global and hemispheric scales, Science, 2000, 288(5465), 500–503 CrossRef CAS PubMed.
- R. G. Prinn, J. Huang, R. F. Weiss, D. M. Cunnold, P. J. Fraser, P. G. Simmonds, A. McCulloch, C. Harth, P. Salameh, S. O'Doherty, R. H. J. Wang, L. Porter and B. R. Miller, Evidence for substantial variations of atmospheric hydroxyl radicals in the past two decades, Science, 2001, 292(5523), 1882–1888 CrossRef CAS PubMed.
- S. Kirschke, P. Bousquet, P. Ciais, M. Saunois, J. G. Canadell, E. J. Dlugokencky, P. Bergamaschi, D. Bergmann, D. R. Blake, L. Bruhwiler, P. Cameron-Smith, S. Castaldi, F. Chevallier, L. Feng, A. Fraser, M. Heimann, E. L. Hodson, S. Houweling, B. Josse, P. J. Fraser, P. B. Krummel, J.-F. Lamarque, R. L. Langenfelds, C. Le Quéré, V. Naik, S. O'Doherty, P. I. Palmer, I. Pison, D. Plummer, B. Poulter, R. G. Prinn, M. Rigby, B. Ringeval, M. Santini, M. Schmidt, D. T. Shindell, I. J. Simpson, R. Spahni, L. P. Steele, S. A. Strode, K. Sudo, S. Szopa, G. R. van der Werf, A. Voulgarakis, M. van Weele, R. F. Weiss, J. E. Williams and G. Zeng, Three decades of global methane sources and sinks, Nat. Geosci., 2013, 6, 813–823 CrossRef CAS.
- S. A. Montzka, M. Krol, E. Dlugokencky, B. Hall, P. Jöckel and J. Lelieveld, Small interannual variability of global atmospheric hydroxyl, Science, 2011, 331(6013), 67–69 CrossRef CAS PubMed.
- F. Rohrer and H. Berresheim, Strong correlation between levels of tropospheric hydroxyl radicals and solar ultraviolet radiation, Nature, 2006, 442(7099), 184–187, DOI:10.1038/nature04924.
- F. Heintz, U. Platt, H. Flentje and R. Dubois, Long-term observation of nitrate radicals at the Tor Station, Kap Arkona (Rügen), J. Geophys. Res., D: Atmos., 1996, 101(17), 22891–22910, DOI:10.1029/96JD01549.
- F. L. Eisele and D. J. Tanner, Ion-assisted tropospheric OH measurements, J. Geophys. Res., 1991, 96(5), 9295–9308, DOI:10.1029/91jd00198.
- F. Holland, M. Hessling and A. Hofzumahaus, In Situ Measurement of Tropospheric OH Radicals by Laser-Induced Fluorescence – A Description of the KFA Instrument, J. Atmos. Sci., 1991, 52(19), 3393–3401 CrossRef.
- T. Petäjä, R. L. Mauldin III, E. Kosciuch, J. McGrath, T. Nieminen, P. Paasonen, M. Boy, A. Adamov, T. Kotiaho and M. Kulmala, Sulfuric acid and OH concentrations in a boreal forest site, Atmos. Chem. Phys., 2009, 9(19), 7435–7448 CrossRef.
- D. Tan, I. Faloona, J. Simpas, W. Brune, P. Shepson, T. L. Couch, A. Sumner, M. Carroll, T. Thornberry, E. Apel, D. Riemer and W. Stockwell, HOx budgets in a deciduous forest: results from the PROPHET summer 1998 campaign, J. Geophys. Res.: Atmos., 2001, 106, 24407–24427 CrossRef CAS.
- X. Ren, W. Brune, C. Cantrell, G. Edwards, T. Shirley, A. Metcalf and R. Lesher, Hydroxyl and peroxy radical chemistry in a rural area of central pennsylvania: observations and model comparisons, J. Atmos. Chem., 2005, 52, 231–257 CrossRef CAS.
- D. Kubistin, H. Harder, M. Martinez, M. Rudolf, R. Sander, H. Bozem, G. Eerdekens, H. Fischer, C. Gurk, T. Klüpfel, R. Königstedt, U. Parchatka, C. L. Schiller, A. Stickler, D. Taraborrelli, J. Williams and J. Lelieveld, Hydroxyl radicals in the tropical troposphere over the Suriname rainforest: comparison of measurements with the box model MECCA, Atmos. Chem. Phys., 2010, 10(19), 9705–9728 CrossRef CAS.
- R. Dlugi, M. Berger, M. Zelger, A. Hofzumahaus, M. Siese, F. Holland, A. Wisthaler, W. Grabmer, A. Hansel, R. Koppmann, G. Kramm, M. Möllmann-Coers and A. Knaps, Turbulent exchange and segregation of HOx radicals and volatile organic compounds above a deciduous forest, Atmos. Chem. Phys., 2010, 10(13), 6215–6235 CrossRef CAS.
- Y. Kanaya, A. Hofzumahaus, H.-P. Dorn, T. Brauers, H. Fuchs, F. Holland, F. Rohrer, B. Bohn, R. Tillmann, R. Wegener, A. Wahner, Y. Kajii, K. Miyamoto, S. Nishida, K. Watanabe, A. Yoshino, D. Kubistin, M. Martinez, M. Rudolf, H. Harder, H. Berresheim, T. Elste, C. PlassDülmer, G. Stange, J. Kleffmann, Y. Elshorbany and U. Schurath, Comparisons of observed and modeled OH and HO2 concentrations during the ambient measurement period of the HOx Comp field campaign, Atmos. Chem. Phys., 2012, 12(5), 2567–2585 CrossRef CAS.
- E. Regelin, H. Harder, M. Martinez, D. Kubistin, C. Tatum Ernest, H. Bozem, T. Klippel, Z. Hosaynali-Beygi, H. Fischer, R. Sander, P. Jöckel, R. Königstedt and J. Lelieveld, HOx measurements in the summertime upper troposphere over Europe: a comparison of observations to a box model and a 3-D model, Atmos. Chem. Phys., 2013, 13(21), 10703–10720 CrossRef.
- D. E. Heard, L. J. Carpenter, D. J. Creasey, J. R. Hopkins, J. D. Lee, A. C. Lewis, M. J. Pilling, P. W. Seakins, N. Carslaw and K. M. Emmerson, High levels of the hydroxyl radical in the winter urban troposphere, Geophys. Res. Lett., 2004, 31(18), L18112 CrossRef.
- K. Emmerson, N. Carslaw, L. Carpenter, D. Heard, J. D. Lee and M. Pilling, Urban Atmospheric Chemistry During the PUMA Campaign 1: Comparison of Modelled OH and HO2 Concentrations with Measurements, J. Atmos. Chem., 2005, 52, 143–164 CrossRef CAS.
- T. R. Shirley, W. H. Brune, X. Ren, J. Mao, R. Lesher, B. Cardenas, R. Volkamer, L. T. Molina, M. J. Molina, B. Lamb, E. Velasco, T. Jobson and M. Alexander, Atmospheric oxidation in the Mexico City Metropolitan Area (MCMA) during April 2003, Atmos. Chem. Phys., 2006, 6(9), 2753–2765 CrossRef CAS.
- M. Martinez, H. Harder, T. A. Kovacs, J. B. Simpas, J. Bassis, R. Lesher, W. H. Brune, G. J. Frost, E. J. Williams, C. A. Stroud, B. T. Jobson, J. M. Roberts, S. R. Hall, R. E. Shetter, B. Wert, A. Fried, B. Alicke, J. Stutz, V. L. Young, A. B. White and R. J. Zamora, OH and HO2 concentrations, production and loss rates during the Southern Oxidant Study in Nashville, TN, summer 1999, J. Geophys. Res., 2003, 108(19), 4617, DOI:10.1029/2003jd003551.
- K. M. Emmerson, N. Carslaw, D. C. Carslaw, J. D. Lee, G. McFiggans, W. J. Bloss, T. Gravestock, D. E. Heard, J. Hopkins, T. Ingham, M. J. Pilling, S. C. Smith, M. Jacob and P. S. Monks, Free radical modelling studies during the UK TORCH campaign in summer 2003, Atmos. Chem. Phys., 2007, 7(1), 167–181 CrossRef CAS.
- S. M. Griffith, R. F. Hansen, S. Dusanter, V. Michoud, J. B. Gilman, W. C. Kuster, P. R. Veres, M. Graus, J. A. de Gouw, J. Roberts, C. Young, R. Washenfelder, S. S. Brown, R. Thalman, E. Waxman, R. Volkamer, C. Tsai, J. Stutz, J. H. Flynn, N. Grossberg, B. Lefer, S. L. Alvarez, B. Rappenglueck, L. H. Mielke, H. D. Osthoff and P. S. Stevens, Measurements of hydroxyl and hydroperoxy radicals during CalNex-LA: model comparisons and radical budgets, J. Geophys. Res.: Atmos., 2016, 121(8), 4211–4232 CrossRef CAS.
- M. Kulmala, K. Hämeri, P. P. Aalto, J. M. Mäkelä, L. Pirjola, E. D. Nilsson, G. Buzorius, Ü. Rannik, M. D. Maso, W. Seidl, T. Hoffman, R. Janson, H.-C. Hansson, Y. Viisanen, A. Laaksonen and C. D. O'dowd, Overview of the international project on biogenic aerosol formation in the boreal forest (BIOFOR), Tellus B, 2001, 53(4), 324–343 CrossRef.
- M. Kulmala, A. Asmi, H. K. Lappalainen, U. Baltensperger, J.-L. Brenguier, M. C. Facchini, H.-C. Hansson, Ø. Hov, C. D. O'Dowd, U. Pöschl, A. Wiedensohler, R. Boers, O. Boucher, G. de Leeuw, H. A. C. Denier van der Gon, J. Feichter, R. Krejci, P. Laj, H. Lihavainen, U. Lohmann, G. McFiggans, T. Mentel, C. Pilinis, I. Riipinen, M. Schulz, A. Stohl, E. Swietlicki, E. Vignati, C. Alves, M. Amann, M. Ammann, S. Arabas, P. Artaxo, H. Baars, D. C. S. Beddows, R. Bergström, J. P. Beukes, M. Bilde, J. F. Burkhart, F. Canonaco, S. L. Clegg, H. Coe, S. Crumeyrolle, B. D'Anna, S. Decesari, S. Gilardoni, M. Fischer, A. M. Fjaeraa, C. Fountoukis, C. George, L. Gomes, P. Halloran, T. Hamburger, R. M. Harrison, H. Herrmann, T. Hoffmann, C. Hoose, M. Hu, A. Hyvärinen, U. Hõrrak, Y. Iinuma, T. Iversen, M. Josipovic, M. Kanakidou, A. Kiendler-Scharr, A. Kirkevåg, G. Kiss, Z. Klimont, P. Kolmonen, M. Komppula, J.-E. Kristjánsson, L. Laakso, A. Laaksonen, L. Labonnote, V. A. Lanz, K. E. J. Lehtinen, L. V. Rizzo, R. Makkonen, H. E. Manninen, G. McMeeking, J. Merikanto, A. Minikin, S. Mirme, W. T. Morgan, E. Nemitz, D. O'Donnell, T. S. Panwar, H. Pawlowska, A. Petzold, J. J. Pienaar, C. Pio, C. Plass-Duelmer, A. S. H. Prévôt, S. Pryor, C. L. Reddington, G. Roberts, D. Rosenfeld, J. Schwarz, Ø. Seland, K. Sellegri, X. J. Shen, M. Shiraiwa, H. Siebert, B. Sierau, D. Simpson, J. Y. Sun, D. Topping, P. Tunved, P. Vaattovaara, V. Vakkari, J. P. Veefkind, A. Visschedijk, H. Vuollekoski, R. Vuolo, B. Wehner, J. Wildt, S. Woodward, D. R. Worsnop, G.-J. van Zadelhoff, A. A. Zardini, K. Zhang, P. G. van Zyl, V.-M. Kerminen, K. S. Carslaw and S. N. Pandis, General overview: European Integrated project on Aerosol Cloud Climate and Air Quality interactions (EUCAARI) integrating aerosol research from nano to global scales, Atmos. Chem. Phys., 2011, 11(24), 13061–13143 CrossRef CAS.
- J. Williams, J. Crowley, H. Fischer, H. Harder, M. Martinez, T. Petäjä, J. Rinne, J. Bäck, M. Boy, M. Dal Maso, J. Hakala, M. Kajos, P. Keronen, P. Rantala, J. Aalto, H. Aaltonen, J. Paatero, T. Vesala, H. Hakola, J. Levula, T. Pohja, F. Herrmann, J. Auld, E. Mesarchaki, W. Song, N. Yassaa, A. Nölscher, A. M. Johnson, T. Custer, V. Sinha, J. Thieser, N. Pouvesle, D. Taraborrelli, M. J. Tang, H. Bozem, Z. Hosaynali-Beygi, R. Axinte, R. Oswald, A. Novelli, D. Kubistin, K. Hens, U. Javed, K. Trawny, C. Breitenberger, P. J. Hidalgo, C. J. Ebben, F. M. Geiger, A. L. Corrigan, L. M. Russell, H. G. Ouwersloot, J. Vilà-Guerau de Arellano, L. Ganzeveld, A. Vogel, M. Beck, A. Bayerle, C. J. Kampf, M. Bertelmann, F. Köllner, T. Hoffmann, J. Valverde, D. González, M.-L. Riekkola, M. Kulmala and J. Lelieveld, The summertime Boreal forest field measurement intensive (HUMPPA-COPEC-2010): an overview of meteorological and chemical influences, Atmos. Chem. Phys., 2011, 11(20), 10599–10618 CrossRef CAS.
- M. Boy, D. Mogensen, S. Smolander, L. Zhou, T. Nieminen, P. Paasonen, C. Plass-Dülmer, M. Sipilä, T. Petäjä, L. Mauldin, H. Berresheim and M. Kulmala, Oxidation of SO2 by stabilized Criegee intermediate (sCI) radicals as a crucial source for atmospheric sulfuric acid concentrations, Atmos. Chem. Phys., 2013, 13(7), 3865–3879 CrossRef.
- J. Liebmann, E. Karu, N. Sobanski, J. Schuladen, M. Ehn, S. Schallhart, L. Quéléver, H. Hellen, H. Hakola, T. Hoffmann, J. Williams, H. Fischer, J. Lelieveld and J. N. Crowley, Direct measurement of NO3 radical reactivity in a boreal forest, Atmos. Chem. Phys., 2018, 18(5), 3799–3815 CrossRef CAS.
- O. Peräkylä, M. Vogt, O.-P. Tikkanen, T. Laurila, M. K. Kajos, P. A. Rantala, J. Patokoski, J. Aalto, T. Yli-Juuti, M. Ehn, M. Sipilä, P. Paasonen, M. Rissanen, T. Nieminen, R. Taipale, P. Keronen, H. K. Lappalainen, T. M. Ruuskanen, J. Rinne, V.-M. Kerminen, M. Kulmala, J. Bäck and T. Petäjä, Monoterpenes' oxidation capacity and rate over a boreal forest: temporal variation and connection to growth of newly formed particles, Boreal Environ. Res., 2014, 19, 293–310 Search PubMed.
- J. Kontkanen, P. Paasonen, J. Aalto, J. Bäck, P. Rantala, T. Petäjä and M. Kulmala, Simple proxies for estimating the concentrations of monoterpenes and their oxidation products at a boreal forest site, Atmos. Chem. Phys., 2016, 16(20), 13291–13307, DOI:10.5194/acp-16-13291-2016.
- P. Hari and M. Kulmala, Station for Measuring Ecosystem-Atmosphere Relations (SMEAR II), Boreal Environ. Res., 2005, 10(5), 315–322 CAS.
- J. Bäck, J. Aalto, M. Henriksson, H. Hakola, Q. He and M. Boy, Chemodiversity of a Scots pine stand and implications for terpene air concentrations, Biogeosciences, 2012, 9(2), 689–702, DOI:10.5194/bg-9-689-2012.
- H. Hellén, A. P. Praplan, T. Tykkä, I. Ylivinkka, V. Vakkari, J. Bäck, T. Petäjä, M. Kulmala and H. Hakola, Long-term measurements of volatile organic compounds highlight the importance of sesquiterpenes for the atmospheric chemistry of a boreal forest, Atmos. Chem. Phys., 2018, 18(19), 13839–13863, DOI:10.5194/acp-18-13839-2018.
- S. Smolander, Q. He, D. Mogensen, L. Zhou, J. Bäck, T. Ruuskanen, S. Noe, A. Guenther, H. Aaltonen, M. Kulmala and M. Boy, Comparing three vegetation monoterpene emission models to measured gas concentrations with a model of meteorology, air chemistry and chemical transport, Biogeosciences, 2014, 11(19), 5425–5443, DOI:10.5194/bg-11-5425-2014.
- P. Zhou, L. Ganzeveld, Ü. Rannik, L. Zhou, R. Gierens, D. Taipale, I. Mammarella and M. Boy, Simulating ozone dry deposition at a boreal forest with a multi-layer canopy deposition model, Atmos. Chem. Phys., 2017, 17, 1361–1379 CrossRef CAS.
- P. Zhou, L. Ganzeveld, D. Taipale, Ü. Rannik, P. Rantala, M. P. Rissanen, D. Chen and M. Boy, Boreal forest BVOC exchange: emissions versus in-canopy sinks, Atmos. Chem. Phys., 2017, 17, 14309–14332 CrossRef CAS.
- L. Zhou, T. Nieminen, D. Mogensen, S. Smolander, A. Rusanen, M. Kulmala and M. Boy, SOSAA—a new model to simulate the concentrations of organic vapours, sulphuric acid and aerosols inside the ABL—Part 2: aerosol dynamics and one case study at a boreal forest site, Boreal Environ. Res., 2014, 19, 237–256 Search PubMed , https://www.helda.helsinki.fi/handle/10138/165201.
- A. Sogachev, G. V. Menzhulin, M. Heimann and J. Lloyd, A simple three-dimensional canopy – planetary boundary layer simulation model for scalar concentrations and fluxes, Tellus B, 2002, 54(5), 784–819, DOI:10.1034/j.1600-0889.2002.201353.x.
- A. Sogachev, O. Panferov, G. Gravenhorst and T. Vesala, Numerical analysis of flux footprints for different landscapes, Theor. Appl. Climatol., 2005, 80(2–4), 169–185, DOI:10.1007/s00704-004-0098-8.
- A. Sogachev and O. Panferov, Modification of Two-Equation Models to Account for Plant Drag, Boundary-Layer Meteorology, 2006, 121(2), 229–266, DOI:10.1007/s10546-006-9073-5.
- V. Damian, A. Sandu, M. Damian, F. Potra and G. R. Carmichael, The kinetic preprocessor KPP-a software environment for solving chemical kinetics, Comput. Chem. Eng., 2002, 26(11), 1567–1579, DOI:10.1016/s0098-1354(02)00128-x.
- M. E. Jenkin, S. M. Saunders and M. J. Pilling, The tropospheric degradation of volatile organic compounds: a protocol for mechanism development, Atmos. Environ., 1997, 31(1), 81–104, DOI:10.1016/s1352-2310(96)00105-7.
- S. M. Saunders, M. E. Jenkin, R. G. Derwent and M. J. Pilling, Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): tropospheric degradation of non-aromatic volatile organic compounds, Atmos. Chem. Phys., 2003, 3(1), 161–180, DOI:10.5194/acp-3-161-2003.
- M. E. Jenkin, K. P. Wyche, C. J. Evans, T. Carr, P. S. Monks, M. R. Alfarra, M. H. Barley, G. B. McFiggans, J. C. Young and A. R. Rickard, Development and chamber evaluation of the MCM v3.2 degradation scheme for β-caryophyllene, Atmos. Chem. Phys., 2012, 12(11), 5275–5308, DOI:10.5194/acp-12-5275-2012.
- L. N. Ganzeveld, J. Lelieveld, F. J. Dentener, M. C. Krol and G. J. Roelofs, Atmosphere-biosphere trace gas exchanges simulated with a single-column model, J. Geophys. Res.: Atmos., 2002, 107, ACH 8-1–ACH 8-21 Search PubMed.
- D. P. Dee, S. M. Uppala, A. J. Simmons, P. Berrisford, P. Poli, S. Kobayashi, U. Andrae, M. A. Balmaseda, G. Balsamo, P. Bauer, P. Bechtold, A. C. M. Beljaars, L. van de Berg, J. Bidlot, N. Bormann, C. Delsol, R. Dragani, M. Fuentes, A. J. Geer, L. Haimberger, S. B. Healy, H. Hersbach, E. V. Hólm, L. Isaksen, P. Kållberg, M. Köhler, M. Matricardi, A. P. McNally, B. M. Monge-Sanz, J.-J. Morcrette, B.-K. Park, C. Peubey, P. de Rosnay, C. Tavolato, J.-N. Thépaut and F. Vitart, The era-interim reanalysis: configuration and performance of the data assimilation system, Q. J. R. Metereol. Soc., 2011, 137(656), 553–597 CrossRef.
- P. Roldin, E. Swietlicki, A. Massling, A. Kristensson, J. Löndahl, A. Eriksson, J. Pagels and S. Gustafsson, Aerosol ageing in an urban plume – implication for climate, Atmos. Chem. Phys., 2011, 11(12), 5897–5915, DOI:10.5194/acp-11-5897-2011.
- O. B. Toon, C. P. McKay, T. P. Ackerman and K. Santhanam, Rapid calculation of radiative heating rates and photodissociation rates in inhomogeneous multiple scattering atmospheres, J. Geophys. Res., D: Atmos., 1989, 94(13), 16287, DOI:10.1029/jd094id13p16287.
- R. L. Mauldin III, T. Berndt, M. Sipilä, P. Paasonen, T. Petäjä, S. Kim, T. Kurtén, F. Stratmann, V.-M. Kerminen and M. Kulmala, A new atmospherically relevant oxidant, Nature, 2012, 488, 193–196 CrossRef PubMed.
- O. Welz, J. D. Savee, D. L. Osborn, S. S. Vasu, C. J. Percival, D. E. Shallcross and C. A. Taatjes, Direct kinetic measure-ments of Criegee Intermediate (CH2OO) formed by reaction of CH2I with O2, Science, 2012, 335, 204–207 CrossRef CAS PubMed.
- M. A. Cohen and P. B. Ryan, Observations Less than the Analytical Limit of Detection: A New Approach, JAPCA, 1989, 39(3), 328–329, DOI:10.1080/08940630.1989.10466534.
- L. Pirjola, M. Kulmala, A. Bischoff, M. Wilck and F. Stratmann, Effects of aerosol dynamics on the formation of sulphuric acid aerosols, J. Aerosol Sci., 1998, 29, S825–S826, DOI:10.1016/s0021-8502(98)90595-x.
- L. Laakso, M. Kulmala and K. E. J. Lehtinen, Effect of condensation rate enhancement factor on 3-nm (diameter) particle formation in binary ion-induced and homogeneous nucleation, J. Geophys. Res., D: Atmos., 2003, 108(18), 4574, DOI:10.1029/2003jd003432.
- D. S. Wilks, Resampling Hypothesis Tests for Autocorrelated Fields, J. Clim., 1997, 10(1), 65–82, DOI:10.1175/1520-0442.
- A. Asmi, M. Collaud Coen, J. A. Ogren, E. Andrews, P. Sheridan, A. Jefferson, E. Weingartner, U. Baltensperger, N. Bukowiecki, H. Lihavainen, N. Kivekäs, E. Asmi, P. P. Aalto, M. Kulmala, A. Wiedensohler, W. Birmili, A. Hamed, C. O'Dowd, S. G. Jennings, R. Weller, H. Flentje, A. M. Fjaeraa, M. Fiebig, C. L. Myhre, A. G. Hallar, E. Swietlicki, A. Kristensson and P. Laj, Aerosol decadal trends – Part 2: in situ aerosol particle number concentrations at GAW and ACTRIS stations, Atmos. Chem. Phys., 2013, 13(2), 895–916 CrossRef.
- K. W. Hipel and A. I. McLeod, Time Series Modelling of Water Resources and Environmental Systems, Elsevier, Amsterdam, 1994, vol. 45 Search PubMed.
- M. Hussain and I. Mahmud, pyMannKendall: a python package for non parametric Mann Kendall family of trend tests, J. Open Source Softw., 2019, 4(39), 1556, DOI:10.21105/joss.01556.
- Z. Ma, J. Xu, W. Quan, Z. Zhang, W. Lin and X. Xu, Significant increase of surface ozone at a rural site, north of eastern China, Atmos. Chem. Phys., 2016, 16(6), 3969–3977, DOI:10.5194/acp-16-3969-2016.
- R. Atkinson, S. M. Aschmann, J. Arey and B. Shorees, Formation of OH radicals in the gas phase reactions of O3 with a series of terpenes, J. Geophys. Res.: Atmos., 1992, 97, 6065–6073 CrossRef CAS.
- A. B. Guenther, X. Jiang, C. L. Heald, T. Sakulyanontvittaya, T. Duhl, L. K. Emmons and X. Wang, The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions, Geosci. Model Dev., 2012, 5, 1471–1492, DOI:10.5194/gmd-5-1471-2012.
- T. Foken, The energy balance closure problem: an overview, Ecol. Appl., 2008, 18(6), 1351–1367 CrossRef PubMed.
- T. Nieminen, A. Asmi, M. Dal Maso, P. P. Aalto, P. Keronen, T. Petäjä, M. Kulmala and V.-M. Kerminen, Trends in atmospheric new-particle formation: 16 years of observations in a boreal-forest environment, Boreal Environ. Res., 2014, 19, 191–214 Search PubMed.
- J. H. Seinfeld and S. N. Pandis, Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, John Wiley & Sons, New York, 2nd edn, 2006 Search PubMed.
- R. M. Hoesly, S. J. Smith, L. Feng, Z. Klimont, G. Janssens-Maenhout, T. Pitkanen, J. J. Seibert, L. Vu, R. J. Andres, R. M. Bolt, T. C. Bond, L. Dawidowski, N. Kholod, J.-I. Kurokawa, M. Li, L. Liu, Z. Lu, M. C. P. Moura, P. R. O'Rourke and Q. Zhang, Historical (1750–2014) anthropogenic emissions of reactive gases and aerosols from the Community Emissions Data System (CEDS), Geosci. Model Dev., 2018, 11(1), 369–408 CrossRef CAS.
- M. Mäki, J. Heinonsalo, H. Hellén and J. Bäck, Contribution of understorey vegetation and soil processes to boreal forest isoprenoid exchange, Biogeosciences, 2017, 14, 1055–1073, DOI:10.5194/bg-14-1055-2017.
- M. Mäki, J. Aalto, H. Hellén, M. Pihlatie and J. Bäck, Interannual and Seasonal Dynamics of Volatile Organic Compound Fluxes From the Boreal Forest Floor, Front. Plant Sci., 2019, 10, 191, DOI:10.3389/fpls.2019.00191.
- H. Aaltonen, J. Pumpanen, M. Pihlatie, H. Hakola, H. Hellén, L. Kulmala, T. Vesala and J. Bäck, Boreal pine forest floor biogenic volatile organic compound emissions peak in early summer and autumn, Agricultural and Forest Meteorology, 2011, 151(6), 682–691 CrossRef.
- K. Hens, A. Novelli, M. Martinez, J. Auld, R. Axinte, B. Bohn, H. Fischer, P. Keronen, D. Kubistin, A. C. Nölscher, R. Oswald, P. Paasonen, T. Petäjä, E. Regelin, R. Sander, V. Sinha, M. Sipilä, D. Taraborrelli, C. Tatum Ernest, J. Williams, J. Lelieveld and H. Harder, Observation and modelling of HOx radicals in a boreal forest, Atmos. Chem. Phys., 2014, 14, 8723–8747 CrossRef.
- M. Boy, O. Hellmuth, H. Korhonen, E. D. Nilsson, D. ReVelle, A. Turnipseed, F. Arnold and M. Kulmala, MALTE-model to predict new aerosol formation in the lower troposphere, Atmos. Chem. Phys., 2006, 6, 4499–4517 CrossRef CAS.
- V. V. Petrenko, P. Martinerie, P. Novelli, D. M. Etheridge, I. Levin, Z. Wang, T. Blunier, J. Chappellaz, J. Kaiser, P. Lang, L. P. Steele, S. Hammer, J. Mak, R. L. Langenfelds, J. Schwander, J. P. Severinghaus, E. Witrant, G. Petron, M. O. Battle, G. Forster, W. T. Sturges, J.-F. Lamarque, K. Steffen and J. W. C. White, A 60 year record of atmospheric carbon monoxide reconstructed from greenland firn air, Atmos. Chem. Phys., 2013, 13(15), 7567–7585 CrossRef.
- Z. Jiang, J. R. Worden, H. Worden, M. Deeter, D. B. A. Jones, A. F. Arellano and D. K. Henze, A 15-year record of CO emissions constrained by MOPITT CO observations, Atmos. Chem. Phys., 2017, 17, 4565–4583, DOI:10.5194/acp-17-4565-2017.
- D. Zhang and R. Zhang, Ozonolysis of α-pinene and β-pinene: kinetics and mechanism, J. Chem. Phys., 2005, 122, 114308, DOI:10.1063/1.1862616.
- T. L. Nguyen, J. Peeters and L. Vereecken, Theoretical study of the gas-phase ozonolysis of β-pinene (C10H16), Phys. Chem. Chem. Phys., 2009, 11, 5643–5656 RSC.
- M. S. Alam, A. R. Rickard, M. Camredon, K. P. Wyche, T. Carr, K. E. Hornsby, P. S. Monks and W. J. Bloss, Radical Product Yields from the Ozonolysis of Short Chain Alkenes under Atmospheric Boundary Layer Conditions, J. Phys. Chem. A, 2013, 117, 12468–12483 CrossRef CAS PubMed.
- P. Rantala, J. Aalto, R. Taipale, T. M. Ruuskanen and J. Rinne, Annual cycle of volatile organic compound exchange between a boreal pine forest and the atmosphere, Biogeosciences, 2015, 12, 5753–5770, DOI:10.5194/bg-12-5753-2015.
- M. K. Sporre, S. M. Blichner, I. H. H. Karset, R. Makkonen and T. K. Berntsen, BVOC–aerosol–climate feedbacks investigated using NorESM, Atmos. Chem. Phys., 2019, 19, 4763–4782, DOI:10.5194/acp-19-4763-2019.
- R. Conrad, Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO), Microbiol. Rev., 1996, 60(4), 609–640, DOI:10.1128/mr.60.4.609-640.1996.
- M. Kesik, P. Ambus, R. Baritz, N. Brüggemann, K. Butterbach-Bahl, M. Damm, J. Duyzer, L. Horváth, R. Kiese, B. Kitzler, A. Leip, C. Li, M. Pihlatie, K. Pilegaard, S. Seufert, D. Simpson, U. Skiba, G. Smiatek, T. Vesala and S. Zechmeister-Boltenstern, Inventories of N2O and NO emissions from European forest soils, Biogeosciences, 2005, 2(4), 353–375 CrossRef CAS.
- T. Jokinen, M. Sipilä, H. Junninen, M. Ehn, G. Lönn, J. Hakala, T. Petäjä, R. L. Mauldin III, M. Kulmala and D. R. Worsnop, Atmospheric sulphuric acid and neutral cluster measurements using CI-APi-TOF, Atmos. Chem. Phys., 2012, 12(9), 4117–4125 CrossRef CAS.
- L. Dada, I. Ylivinkka, R. Baalbaki, C. Li, Y. Guo, C. Yan, L. Yao, N. Sarnela, T. Jokinen, K. R. Daellenbach, R. Yin, C. Deng, B. Chu, T. Nieminen, Y. Wang, Z. Lin, R. C. Thakur, J. Kontkanen, D. Stolzenburg, M. Sipilä, T. Hussein, P. Paasonen, F. Bianchi, I. Salma, T. Weidinger, M. Pikridas, J. Sciare, J. Jiang, Y. Liu, T. Petäjä, V.-M. Kerminen and M. Kulmala, Sources and sinks driving sulfuric acid concentrations in contrasting environments: implications on proxy calculations, Atmos. Chem. Phys., 2020, 20(20), 11747–11766 CrossRef CAS.
- R. Atkinson and J. Arey, Atmospheric Degradation of Volatile Organic Compounds, Chem. Rev., 2003, 103(12), 4605–4638, DOI:10.1021/cr0206420.
- J. L. Fry, D. C. Draper, K. C. Barsanti, J. N. Smith, J. Ortega, P. M. Winkler, M. J. Lawler, S. S. Brown, P. M. Edwards, R. C. Cohen and L. Lee, Secondary Organic Aerosol Formation and Organic Nitrate Yield from NO3 Oxidation of Biogenic Hydrocarbons, Environ. Sci. Technol., 2014, 48(20), 11944–11953 CrossRef CAS PubMed.
- R. Makkonen, A. Asmi, V.-M. Kerminen, M. Boy, A. Arneth, P. Hari and M. Kulmala, Air pollution control and decreasing new particle formation lead to strong climate warming, Atmos. Chem. Phys., 2012, 12, 1515–1524 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ea00020a |
| This journal is © The Royal Society of Chemistry 2021 |