Open Access Article
Open Access ArticleMonitoring spin-crossover phenomena via Re(I) luminescence in hybrid Fe(II) silica coated nanoparticles†
Ismael Francisco
Díaz-Ortega
ab,
Eva Luz
Fernández-Barbosa
a,
Silvia
Titos-Padilla
a,
Simon J. A.
Pope
 c,
Juan-Ramón
Jiménez
*a,
Enrique
Colacio
c,
Juan-Ramón
Jiménez
*a,
Enrique
Colacio
 *a and
Juan Manuel
Herrera
*a and
Juan Manuel
Herrera
 *a
*a
aDepartamento de Química Inorgánica, Facultad de Ciencias, Universidad de Granada and Unidad de Excelencia de Química (UEQ), Avda. Fuentenueva s/n, 18071, Granada, Spain. E-mail: jmherrera@ugr.es
bDepartamento de Química y Física-CIESOL, Universidad de Almería, Ctra. Sacramento s/n, 04120, Almería
cCardiff School of Chemistry, Cardiff University, Cardiff, CF10 3AT, UK
First published on 25th October 2021
Abstract
Bare (1) and silica coated (1@SiO2) spin crossover (SCO) nanoparticles based on the polymer {[Fe(NH2Trz)3](BF4)2}n have been prepared following a water-in-oil synthetic procedure. For 1, the critical temperatures of the spin transition are TC↓ = 214.6 K and TC↑ = 220.9 K. For 1@SiO2, the abruptness of the transition is enhanced and the critical temperatures are centred at room temperature (TC↓ = 292.1 K and TC↑ = 296.3 K). An inert Re(I) complex of formula [Re(phen)(CO)3(PETES)](PF6) (phen = 1, 10-phenanthroline; PETES = 2(4-pyridylethyl)triethoxysilane) (Re) was also synthesized yielding intense green emission centred at λem = 560 nm. The grafting of this complex on the silica shell of 1@SiO2 led to a bifunctional SCO-luminescence composite (1@SiO2/Re) whose luminescence properties were tuned by the spin state switching. Temperature-variable photophysical studies showed that luminescence and spin transition were synchronized through a radiative (trivial) energy transfer mechanism between the Re(I) and the Fe(II)-LS (LS, Low Spin) centres.
Introduction
In the last few years, many efforts have been devoted to the preparations of bifunctional nanomaterials combining spin crossover (SCO) behaviour and luminescence properties.1–4 These systems, in which the thermally induced spin transition tunes the luminescence signal are interesting from a fundamental point of view, and also for potential applications such as the development of photonic devices and thermal sensors. Indeed, in certain conditions, the detection of changes in the emission intensity can be preferable and more sensitive to the variation of the optical or magnetic properties of the material. Piguet et al. pave the way to prepare luminescence SCO compounds in the early 2000s. They synthesized a series of heterodimetallic SCO Fe(II)–Eu(III) complexes where the lanthanide emission was partially obscured through an intramolecular Eu(III) → Fe(II)-HS (High Spin) Förster energy transfer or quantitatively quenched due to spectral overlap between Eu(III)-based emission and Fe(II)-LS (Low Spin) absorptions.5–7 Since then, a significant number of compounds showing synergy between both properties have been reported. Most of these systems are based on Fe(II) SCO complexes and green-emitting luminophores. In the LS regime, the spectral overlap between the emission and the metal-to-ligand charge transfer (MLCT) and/or spin-allowed d–d (1A1 → 1T1, 1A1 → 1T2) absorptions of the Fe(II) centres, causes the luminescence quenching. When the LS → HS transition is thermally induced, weaker MLCT bands are found and the d–d transitions move to the near infrared region (ca. 900 nm), the emitter–acceptor spectral overlap vanish and the emission intensity increases. In order to combine the luminophore and the SCO moiety in the same material, two different approaches have mainly been considered. The first of them combines the SCO active moiety and the emitter in a single coordination compound. The emitter can be an organic fluorophore or luminescent complex which acts as: (i) ligand towards the Fe(II) centres;8–17 (ii) counterion;18 or (iii) guest molecules inserted within the cavities of three-dimensional SCO frameworks.19,20 In these cases, the location of both components can be studied through structural elucidation techniques allowing the accurate determination of the nature of the correlation between the SCO and the luminescence phenomena. A second strategy deals with the synthesis of SCO composites doped or decorated with luminescence species.21–25 In these cases, the location of both components is uncertain and to determine the interaction mechanism is challenging. However, as both units are almost chemically independent, it is possible to use different luminophores or modify their chemical nature without affecting significantly to the magnetic and optical properties of the SCO moiety. For example, Bousseksou and co-workers prepared a set of nanoparticles based on the Fe(II)-triazole family of coordination polymers doped with different organic fluorophores. Depending on the fluorophore nature, the emission was more or less efficiently modulated by the spin transition which remained almost identical compared to the non-doped material.21,22 Following a more elaborated strategy, our group prepared core SCO nanoparticles based on the {[Fe(HTrz)2(Trz)](BF4)}n polymer (Trz = 1,2,4-1H-triazole) embedded within a silica shell. In these hybrid Fe-Trz@SiO2 nanoparticles, the magnetic and optical bistability of the Fe(II) polymer was preserved. Dansyl fluorophores were covalently attached to their surface through alcoxysilane groups. In the low spin regime, the dansyl centered emission was quenched due to its spectral overlap with the absorption bands of the Fe(II) centres.23,24 However, due to the low thermal stability of the dansyl fluorophore, the emissive signal is progressively lost upon successive heating–cooling cycles. The use of more robust luminophores, such as Tb(III) complexes, allowed to confirm that the thermal variation of the emission was invariably synchronized with the SCO over several successive thermal cycles.25Thus, to avoid decomposition of the emissive moieties, the use of robust luminescence complexes seems a wise strategy. Another strategy deals with a decrease of the critical temperatures at which the spin transition takes place. For the Fe-triazole family of polymers, it is well known that the nature of the 4-substituent on the triazole ligand tunes the cooperativity and the spin transition over a wide range of temperatures.26 For example, compared to the polymers based on the 1,2,4-triazole ligand, the 4-substituted 4-amino-1,2,4-triazole (NH2-Trz) analogues show lower critical temperatures and narrower thermal hysteresis loops of ca. 10 K wide.27,28 In this work, both possibilities have been considered. First, we have prepared bare and silica coated nanoparticles based on the {[Fe(NH2-Trz)3](BF4)2}n polymer. Then, the surface of the SiO2 coated nanoparticles has been decorated with a robust green-emitting Re(I) complex. The structural, magnetic and optical properties of these new materials are presented.
Experimental section
Materials and chemicals
Solvents and the reactants 1,10-phenanthroline (phen), 2(4-pyridylethyl)triethoxysilane (PETES), tetraethyl orthosilicate (TEOS), rhenium pentacarbonyl chloride, 4-amino-1,2,4-triazole and iron(II) tetrafluoroborate were obtained from commercial sources and used as received.Instrumentation and characterization
Nanoparticles were characterized by transmission electron microscopy (TEM) using a LIBRA 120 PLUS Carl Zeiss electron microscope operating at 200 keV. 5 mg of the material was redispersed by sonication (30 min) in 1 mL of EtOH. Carbon reinforced copper grids (200 mesh) were submerged into suspension 50 times and then allowed to dry in air for at least 48 h. The size of the particles was determined by “manual counting” using ScionImage software (http://www.scioncorp.com). HAADF-STEM images and EDX analyses were recorded on a HAADF FEI TITAN G2 instrument working at an accelerating voltage of 200 kV in the scanning mode with a probe diameter of 0.5 nm. Elemental analyses were carried out on a Fisons-Carlo Erba analyser model EA 1108. Magnetic measurements were obtained with the use of a Quantum Design SQUID magnetometer MPMS-XL operating at a magnetic field of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 G in the 100–350 K temperature range. The heating–cooling cycles were performed with a rate of 10 K min−1. X-Ray powder diffraction data (Cu Kα, λ = 1.5418 Å) were collected at 25 °C on a Bruker D8 Discover vertical scan diffractometer equipped with a PILATUS3R 100K-A detector. The generator was operated at 50 kV and 1 mA. The powders were gently ground in an agate mortar and then deposited in the hollow of an aluminium holder equipped with a zero-background plate. The diffractograms were collected in the range of 2θ between 6°–55° at steps of 0.02° during 30 seconds per step. NMR characterizations were carried out on a 400 MHz (2 channels) BRUKER Nanobay Advance III. Reflectance spectra were recorded on a Varian Cary 5 UV-vis-NIR spectrophotometer equipped with a specially designed Praying Mantis diffuse reflection attachment. Emission and excitation spectra were measured on a UV-VIS-PTI QuantaMaster™ 8000 spectrofluorometer equipped with a Picosecond Photon Detector (230–850 nm, PPD-850, HORIBA Scientific) and a continuous Xenon Short Arc Lamp (190–2000 nm, USHIO). The temperature-ramp measurements (270 K–330 K) were recorded using an optical Closed Cycle Cryocooler (ARS DE-202PE) adapted for solid samples (45° angle with respect to the incoming excitation source). All the spectra (emission and excitation) were corrected with Real-time corrections function. TCSPC lifetime measurements were performed using a 375 nm excitation wavelength provided by a pulsed diode light source NanoLED 375L (<200 ps pulse, HORIBA Scientific).
000 G in the 100–350 K temperature range. The heating–cooling cycles were performed with a rate of 10 K min−1. X-Ray powder diffraction data (Cu Kα, λ = 1.5418 Å) were collected at 25 °C on a Bruker D8 Discover vertical scan diffractometer equipped with a PILATUS3R 100K-A detector. The generator was operated at 50 kV and 1 mA. The powders were gently ground in an agate mortar and then deposited in the hollow of an aluminium holder equipped with a zero-background plate. The diffractograms were collected in the range of 2θ between 6°–55° at steps of 0.02° during 30 seconds per step. NMR characterizations were carried out on a 400 MHz (2 channels) BRUKER Nanobay Advance III. Reflectance spectra were recorded on a Varian Cary 5 UV-vis-NIR spectrophotometer equipped with a specially designed Praying Mantis diffuse reflection attachment. Emission and excitation spectra were measured on a UV-VIS-PTI QuantaMaster™ 8000 spectrofluorometer equipped with a Picosecond Photon Detector (230–850 nm, PPD-850, HORIBA Scientific) and a continuous Xenon Short Arc Lamp (190–2000 nm, USHIO). The temperature-ramp measurements (270 K–330 K) were recorded using an optical Closed Cycle Cryocooler (ARS DE-202PE) adapted for solid samples (45° angle with respect to the incoming excitation source). All the spectra (emission and excitation) were corrected with Real-time corrections function. TCSPC lifetime measurements were performed using a 375 nm excitation wavelength provided by a pulsed diode light source NanoLED 375L (<200 ps pulse, HORIBA Scientific).
All these techniques are available at the Centro de Instrumentación Científica (CIC) of the University of Granada.
Syntheses
Results and discussion
Synthesis and structural characterization of SCO nanoparticles 1 and 1@SiO2
Based on a classical water-in-oil synthetic approach, bare [Fe(NH2Trz)3](BF4)2 (1) and core/shell [Fe(NH2Trz)3](BF4)2@SiO2 (1@SiO2) nanoparticles were prepared following the procedure described previously by our research group.23 Two microemulsions containing Fe(BF4)2·6H2O or 4-amino-1,2,4-triazole (NH2Trz) ligand and appropriate amounts of surfactants, co-surfactants, an oil phase a (for 1@SiO2 the silica precursor tetraethylorthosilicate, TEOS, was additionally added to both microemulsions), were prepared separately and then mixed and stirred vigorously for 24 h at room temperature. After this time, acetone was added to the colourless microemulsion causing the nanoparticles precipitation. In the case of 1@SiO2, a change of colour to deep violet was observed. This colour change, associated with the HS → LS transition in the Fe-NH2Trz polymer, demonstrates that such a transition takes place at room-temperature and can be induced by small stimuli, such as for example, a change in the polarity of the solvent around the Fe(II) polymer. The white colour of 1 did not change after the addition of acetone, which indicates that in this case, the HS → LS transition takes place at lower temperatures. HR-TEM images of 1 and 1@SiO2 are shown in Fig. 1. In both cases the nanoparticles display a long and thin rod-like shape. For 1, the width of the nanoparticles is regular with a mean value of 35.28 ± 5.95 nm. Conversely, the length is quite heterogeneous, with sizes that vary between 100 nm and 650 nm. Statistically, the calculated mean length is 288.70 ± 133.12 nm. 1@SiO2 nanoparticles show a homogeneous mean length of 317.9 ± 75.0 nm and a mean width of 45.3 ± 7.3 nm. In both cases, the nanoparticles are monodisperse and well defined.The X-ray powder diffraction patterns of both samples match well with that shown by the bulk Fe-NH2-Trz polymer (Fig. S1†). A lower degree of crystallinity is observed for 1@SiO2, which is attributed to the amorphous nature of the silica shell. The elemental analyses (Table S1†) reveal the analogy between these two samples and the bulk material. For 1@SiO2, an estimated molar ratio Fe/SiO2 of 1/1.5 was found.
Synthesis and photophysical characterization of [Re(phen)(CO)3(PETES)]PF6 (Re)
The Re-alcoxysilane conjugate [Re(phen)(CO)3(PETES)]PF6 (Re) was obtained following the synthetic procedure depicted in Scheme 1a. In a first step, the reaction of equimolar amounts of Re(CO)5Cl and 1,10-phenanthroline in toluene afforded the well-known complex [Re(phen)(CO)3Cl].29 Further reaction with AgPF6 in CH3CN gave the cationic complex [Re(phen)(CO)3(CH3CN)]PF6, which is highly soluble in common organic solvents. The coordinated acetonitrile molecule can be easily displaced by others ligands such as pyridine derivates.30 Reaction of this complex with the alcoxysilane derivate 2(4-pyridilethyl)triethoxysilane (PETES) provided the desired Re-alcoxysilane conjugate Re in a very good yield and with a high degree of purity (Fig. S2†).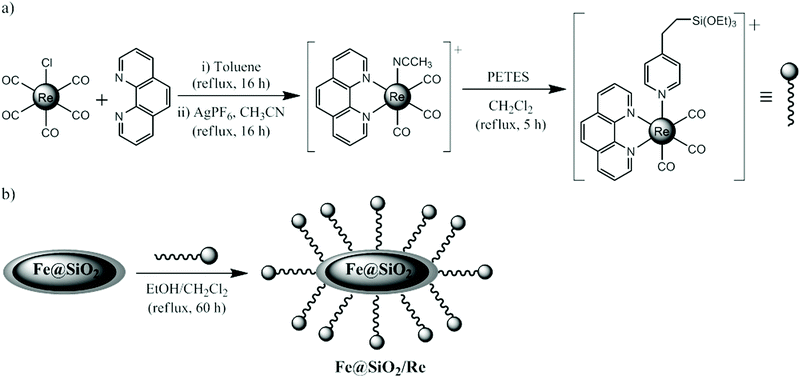 | ||
| Scheme 1 Syntheses of the Re-alcoxisilane conjugate [Re(phen)(CO)3(PETES)]PF6 (Re) (a) and the hybrid nanoparticles 1@SiO2/Re (b). | ||
The absorption and emission spectra of this complex recorded in CH2Cl2 solutions are shown in Fig. 2a. The absorption spectrum shows two main peaks at 220 nm and 274 nm which correspond to the π ← π* transitions of the PETES and 1,10-phenanthroline respectively. A band of lower intensity located at 365 nm corresponds to the π*(phen) ← dπ(Re) 1MLCT transition. Excitation at 365 nm yields an intense and unstructured emission band centred at 560 nm. This emission band is ascribed to the radiative deexcitation from a 3MLCT level. In the solid state, the emission is slightly blue shifted due to rigidochromic effect, with the maximum located at 525 nm. To establish the dependence of the emission with the temperature, solid state emission spectra were recorded between 275 K and 330 K (Fig. 2b). A small decrease of the emission intensity of ca. 5% is observed. This behaviour indicates that the thermal quenching of the Re luminescence is not significant in this range of temperature.
 | ||
| Fig. 2 (a) Room temperature UV-Vis (blue) and emission (green) spectra of complex Re in dichloromethane. (b) Solid state emission spectra of complex Re measured in the temperature range 275 K–330 K. | ||
Solid-state emission lifetime measurements were performed at T = 273 K and 330 K and the kinetic decay fitted to a double-exponential (Fig. S4†). The mean excited-state lifetimes show a decrease by a factor of 0.82 (2875 ns at 273 K and 2346 ns at 330 K) upon increasing the temperature (Table S2†).
This new heteroleptic Re complex can be easily embedded and/or grafted to the surface of silica nanoparticles. At the best of our knowledge, no examples of hybrid Re(I)-silica nanoparticles have been previously reported.
Synthesis and structural characterization of bifunctional SCO/luminescence nanoparticles 1@SiO2/Re
To functionalize the surface of 1@SiO2 with the Re conjugate, both species were suspended in a mixture of CH2Cl2/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and stirred at reflux for 60 h (Scheme 1b). After several washing steps to remove unreacted species, hybrid 1@SiO2/Re nanoparticles were isolated as a pale yellow powder. HAADF-STEM and EDX-compositional mapping images are shown in Fig. 3. The images confirm that the Re(I) complex is grafted to the silica shell and randomly distributed across the whole surface of the nanoparticle. A mean atomic Fe/Re ratio of 20/1 was found. Elemental analysis (Table S1†) did not provide a precise formula for this sample, due to the complexity of the material and the fact that the Re complex is randomly distributed on the nanoparticles (it can be attached on their surface through the total or partial hydrolysis of the three ethoxy groups). Nevertheless, the increase in the C and H percentages found compared to 1@SiO2 indicates the effective graft of the Re complex onto the nanoparticles.
1, v/v) and stirred at reflux for 60 h (Scheme 1b). After several washing steps to remove unreacted species, hybrid 1@SiO2/Re nanoparticles were isolated as a pale yellow powder. HAADF-STEM and EDX-compositional mapping images are shown in Fig. 3. The images confirm that the Re(I) complex is grafted to the silica shell and randomly distributed across the whole surface of the nanoparticle. A mean atomic Fe/Re ratio of 20/1 was found. Elemental analysis (Table S1†) did not provide a precise formula for this sample, due to the complexity of the material and the fact that the Re complex is randomly distributed on the nanoparticles (it can be attached on their surface through the total or partial hydrolysis of the three ethoxy groups). Nevertheless, the increase in the C and H percentages found compared to 1@SiO2 indicates the effective graft of the Re complex onto the nanoparticles.
 | ||
Fig. 3 HAADF-STEM image (left) and EDX compositional maps (left) of Fe, Si and Re (top, middle and bottom respectively) of sample 1@SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re. Re. | ||
Magnetic properties
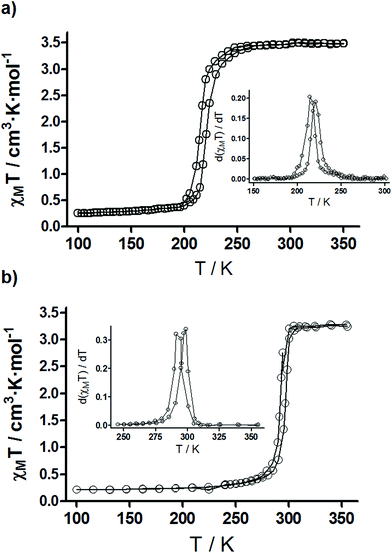
The magnetic properties of 1 and 1@SiO2 in the form of χMT vs. T are represented in Fig. 4. For 1, the χMT value at 350 K is equal to 3.49 cm3 K mol−1, close to the expected value for an high spin Fe(II) ion. Upon cooling, the χMT product decreases sharply at TC↓ = 214.6 K to reach a value of 0.257 cm3 K mol−1 at 100 K, which indicates that approximately 7.4% of the Fe(II) centres remain in HS configuration at this temperature. On the warming cycle, the LS → HS transition takes place at TC↑ = 220.9 K, thus displaying a thermal hysteresis loop of 6.3 K wide. Compared to the bulk sample,26 the critical temperatures of the spin transition in 1 decrease noticeably about 30 K and the hysteresis loop slightly narrows. This behaviour is usually observed when the size of the particles decreases from the micro- to the nanoscale.31–34 For 1@SiO2 the spin transition moves to higher temperatures and appears centred at room temperature (TC↓ = 292.1 K, TC↑ = 296.3 K, ΔTC = 4.2 K). To quantify the abruptness of the transition, the ΔT80 parameter was determined (Table 1).35 This parameter corresponds to the difference of temperatures at which 80% and 20% of the Fe(II) ions switch their electronic configuration. When the nanostructured Fe(II) polymer is embedded within a silica shell, the transition is more abrupt as indicated by a higher ΔT80 parameter (Table 1). The changes observed in the physical parameters of the LS ↔ HS transition for 1 and 1@SiO2 are due to the well-known effects that the rigid silica matrix embedding the nanoparticles exerts on the magnetic properties of these materials.36–39 Finally, for 1@SiO2/Re, the thermal variation of χMT is quite similar to 1@SiO2 (Fig. S6† and Table 1) revealing that the LS ↔ HS transition is barely affected by the surface functionalization of the silica nanoparticles.| T C↓a (K) | ΔT80↓ | T C↑a (K) | ΔT80↑ | ΔT (K) | % HS150 Kb | |
|---|---|---|---|---|---|---|
| a T C↓ and TC↑ are the critical temperature for the HS–LS and LS–HS transitions in the cooling and warming modes respectively. b %HS150 K represents the percentage of residual Fe(II) ions that remain in the HS state below TC↓. | ||||||
| 1 | 214.6 | 13.1 | 220.9 | 13.6 | 6.3 | 8.4 |
| 1@SiO2 | 292.1 | 19.1 | 296.3 | 19.4 | 4.2 | 6.8 |
| 1@SiO2/Re | 290.2 | 20.1 | 297.3 | 20.2 | 7.1 | 10.3 |
Thermal dependence of the luminescence properties of 1@SiO2/Re
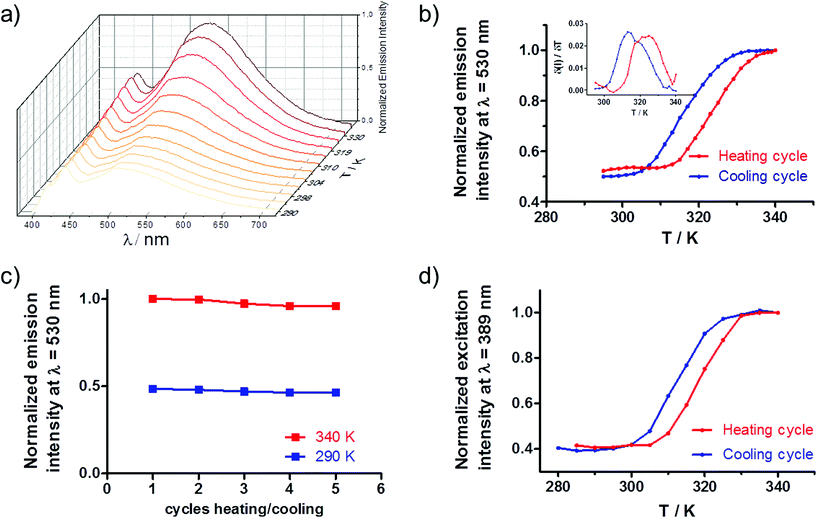
To determine if the emission properties of the rhenium complex are correlated with the spin transition of the Fe(II) centres in 1@SiO2/Re, variable-temperature emission spectra have been recorded in the range between 290 K and 340 K. As shown in Fig. 5a, at 290 K the emission is almost identical to the isolated Re complex. As the temperature rises (heating cycle), the emission intensity grows continuously to reach a 2.2-fold increase at 340 K. When the temperature falls back to 290 K (cooling cycle), the intensity of the emission also decreases gradually up to 290 K, with both spectra at this temperature, before and after the thermal cycle, being virtually identical. The thermal variation of the luminescence intensity for the heating/cooling cycle (Fig. 5b) describes a hysteresis cycle 8 K wide (TC↑ = 323 K and TC↓ = 315 K), very similar to that observed for the thermal variation of the magnetic properties (Fig. S6†). Although a deviation of ca. 20 K exists between the critical temperatures for the thermal variation of the χMT product (TC↑ = 290 K and TC↓ = 297 K) and the luminescence, which is probably due to instrumental limitations (emissive and magnetic experiments were carried out at different temperature-sweep speeds), it is evident that emission intensity and SCO transition are synchronized. To determine the thermal stability of the rhenium complex in this system, the sample was exposed to successive heating/cooling cycles between 290 K and 340 K (Fig. 5c). After five cycles, the emissions at high and low temperature are alike, confirming the stability of the rhenium complex in this range of temperature. Additionally, variable-temperature excitation spectra were also recorded (Fig. S7†). A broad excitation band centred at λ = 389 nm is observed whose intensity increases as the temperature rises, reaching a maximum at 340 K and returning to the initial value when the temperature drops back to 280 K (cooling cycle). The thermal variation of the excitation and emission intensity maxima display identical hysteresis cycles (Fig. 5d and b respectively). These experiments demonstrate that changes in the Re(I) emission intensity and thermally induced spin transition in the Fe(II) centres are synchronized.These results constitute a new example of bifunctional SCO-Luminescent system where the emission properties of the luminophore are regulated by the electronic configuration of the Fe(II) centres through an energy transfer mechanism. Full spectral overlap between the Re3MLCT emission and d–d absorption bands of the Fe(II)-LS ions exists (Fig. S3†), which is a necessary condition for energy transfer (ET) to occur. To elucidate the nature of the energy transfer, it is necessary to determine if the emission lifetime of the Re(I) moieties are affected by the spin transition. For a radiative (trivial) ET process, donor and acceptor are independent and the photons emitted by the Re(I) complex are simply reabsorbed by the Fe(II)-LS centres. In this case, the emission lifetime of the luminophore remains unchanged, regardless of the electronic configuration of the Fe(II) ions. Conversely, for a non-radiative Förster resonance energy transfer (FRET), the emission lifetime in the presence of acceptor Fe(II)-LS ions will decrease according to the efficiency of the process, as the presence of acceptors constitutes an additional pathway for the deactivation of the emitter's excited state. Solid state lifetime measurements were performed at 273 K and 323 K (Fig. S5†). The kinetic decays were fitted to a three-exponential function which indicates the existence of different chemical environments around the Re(I) centres (Table S2†). The average lifetimes found were 39 ns (T = 273 K) and 33 ns (T = 330 K), which represents a decrease of the emission lifetime by a factor of ca. 0.84. For the free Re complex the average lifetimes were significantly longer (see above), which suggest the existence of dynamic quenching between the Re(I) and the Fe(II) moieties. However, the decrease in the lifetime induced by the rise of temperature in Re was about 18%, i.e. a factor of 0.82, quite similar to that observed for 1@SiO2/Re. Thus, the decrease of the emission lifetimes observed in 1@SiO2/Re between 273 K and 330 K is mainly due to the rise of temperature and/or instrumental error and independent of the Fe(II) spin state. These results suggest that trivial energy transfer occurs between the Re complex and the SCO compound and rules out the existence of significant FRET.
Conclusions
In summary, bare and silica coated nanocomposites have been prepared based on the SCO Fe(II) polymer {[Fe(NH2Trz)3](BF4)2}n and characterized from a structural and magnetic point of view. Compared to the bulk, bare Fe-NH2Trz nanoparticles exhibit a spin transition with a similar cooperativity, although the critical temperatures of the transition decrease significantly. The cooperativity increases when the nanoparticles are embedded within a silica shell as well as the critical temperatures which appear in this case centred at room temperature.A robust luminescent Re(I) complex was grafted onto the silica shell of the nanoparticles affording a bifunctional SCO-luminescence material highly stable towards photo-bleaching and temperature. Temperature-variable photo-physical studies revealed that emissive properties and Fe(II) spin state are synchronized. In the LS regime, the Re(I)-based 3MLCT emission is obscured due to the existence of a radiative Re(I) → Fe(II)-LS energy transfer where the emitted photons are reabsorbed by the d–d absorption bands of the Fe(II)-LS centres. The viability of 5d6 luminescence complexes to prepare highly stable bifunctional SCO-luminescence materials has been demonstrated.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from Projects CTQ2014-56312-P and PGC2018-102052-B-C21 financed by MCIN/AEI/10.13039/501100011033/FEDER “Una manera de hacer Europa”, the Junta de Andalucía (FQM-195), Feder project A-FQM-172-UGR18 and the University of Granada is gratefully acknowledged. I.-F. Díaz-Ortega and J.-R Jiménez are also thankful to the Junta de Andalucía for Postdoctoral research fellowships.References
- C. M. Quintero, G. Félix, I. Suleimanov, J. S. Costa, G. Molnár, L. Salmon, W. Nicolazzi and A. Bousseksou, Beilstein J. Nanotechnol., 2014, 5, 2230–2239 CrossRef PubMed.
- H. J. Shepherd, C. M. Quintero, G. Molnár, L. Salmon and A. Bousseksou, in Spin-Crossover Materials: Properties and Applications, John Wiley & Sons Ltd, Oxford, UK, 2013, pp. 347–373 Search PubMed.
- K. Senthil Kumar and M. Ruben, Coord. Chem. Rev., 2017, 346, 176–205 CrossRef CAS.
- H. J. Shepherd, G. Molnár, W. Nicolazzi, L. Salmon and A. Bousseksou, Eur. J. Inorg. Chem., 2013, 2013, 653–661 CrossRef CAS.
- C. Edder, C. Piguet, G. Bernardinelli, J. Mareda, C. G. Bochet, J. C. G. Bunzli and G. Hopfgartner, Inorg. Chem., 2000, 39, 5059–5073 CrossRef CAS PubMed.
- C. Edder, C. Piguet, J. C. G. Bünzli and G. Hopfgartner, Chem. – Eur. J., 2001, 7, 3014–3024 CrossRef CAS.
- T. Lathion, A. Fürstenberg, C. Besnard, A. Hauser, A. Bousseksou and C. Piguet, Inorg. Chem., 2020, 59, 1091–1103 CrossRef CAS PubMed.
- M. Hasegawa, F. Renz, T. Hara, Y. Kikuchi, Y. Fukuda, J. Okubo, T. Hoshi and W. Linert, Chem. Phys., 2002, 277, 21–30 CrossRef CAS.
- Y. Garcia, F. Robert, A. D. Naik, G. Zhou, B. Tinant, K. Robeyns, S. Michotte and L. Piraux, J. Am. Chem. Soc., 2011, 133, 15850–15853 CrossRef CAS PubMed.
- C. F. Wang, G. Y. Yang, Z. S. Yao and J. Tao, Chem. – Eur. J., 2018, 24, 3218–3224 CrossRef CAS PubMed.
- J. L. Wang, Q. Liu, Y. S. Meng, X. Liu, H. Zheng, Q. Shi, C. Y. Duan and T. Liu, Chem. Sci., 2018, 9, 2892–2897 RSC.
- C. Lochenie, K. Schötz, F. Panzer, H. Kurz, B. Maier, F. Puchtler, S. Agarwal, A. Köhler and B. Weber, J. Am. Chem. Soc., 2018, 140, 700–709 CrossRef CAS PubMed.
- J. Yuan, S. Q. Wu, M. J. Liu, O. Sato and H. Z. Kou, J. Am. Chem. Soc., 2018, 140, 9426–9433 CrossRef CAS PubMed.
- B. Benaicha, K. Van Do, A. Yangui, N. Pittala, A. Lusson, M. Sy, G. Bouchez, H. Fourati, C. J. Gómez-Garciá, S. Triki and K. Boukheddaden, Chem. Sci., 2019, 10, 6791–6798 RSC.
- J. Y. Ge, Z. Chen, L. Zhang, X. Liang, J. Su, M. Kurmoo and J. L. Zuo, Angew. Chem., Int. Ed., 2019, 58, 8789–8793 CrossRef CAS PubMed.
- S. Ghosh, S. Kamilya, T. Pramanik, M. Rouzières, R. Herchel, S. Mehta and A. Mondal, Inorg. Chem., 2020, 59, 13009–13013 CrossRef CAS PubMed.
- B. Schäfer, T. Bauer, I. Faus, J. A. Wolny, F. Dahms, O. Fuhr, S. Lebedkin, H. C. Wille, K. Schlage, K. Chevalier, F. Rupp, R. Diller, V. Schünemann, M. M. Kappes and M. Ruben, Dalton Trans., 2017, 46, 2289–2302 RSC.
- H. Matsukizono, K. Kuroiwa and N. Kimizuka, Chem. Lett., 2008, 37, 446–447 CrossRef CAS.
- T. Delgado, M. Meneses-Sánchez, L. Piñeiro-López, C. Bartual-Murgui, M. C. Muñoz and J. A. Real, Chem. Sci., 2018, 9, 8446–8452 RSC.
- M. Meneses-Sánchez, L. Piñeiro-López, T. Delgado, C. Bartual-Murgui, M. C. Muñoz, P. Chakraborty and J. A. Real, J. Mater. Chem. C, 2020, 8, 1623–1633 RSC.
- L. Salmon, G. Molnár, D. Zitouni, C. Quintero, C. Bergaud, J. C. Micheau and A. Bousseksou, J. Mater. Chem., 2010, 20, 5499–5503 RSC.
- C. M. Quintero, I. A. Gural'Skiy, L. Salmon, G. Molnár, C. Bergaud and A. Bousseksou, J. Mater. Chem., 2012, 22, 3745–3751 RSC.
- S. Titos-Padilla, J. M. Herrera, X.-W. Chen, J. J. Delgado and E. Colacio, Angew. Chem., Int. Ed., 2011, 50, 3290–3293 CrossRef CAS PubMed.
- J. M. Herrera, S. Titos, S. Pope, I. Berlanga, F. Zamora, J. J. Delgado, K. Kamenev, X. Wang, A. Prescimone, E. K. Brechin and E. Colacio, J. Mater. Chem. C, 2015, 7819–7829 RSC.
- I. Suleimanov, O. Kraieva, G. Molnár, L. Salmon and A. Bousseksou, Chem. Commun., 2015, 51, 15098–15101 RSC.
- O. Roubeau, Chem. – Eur. J., 2012, 18, 15230–15244 CrossRef CAS PubMed.
- M. B. Bushuev, L. G. Lavrenova, V. N. Ikorskii, Y. G. Shvedenkov, V. A. Varnek, L. A. Sheludyakova and S. V. Larionov, Russ. J. Coord. Chem. Khim., 2004, 30, 284–290 CrossRef CAS.
- V. A. Varnek and L. G. Lavrenova, J. Struct. Chem., 1995, 36, 104–111 CrossRef.
- M. Wrighton and D. L. Morse, J. Am. Chem. Soc., 1974, 96, 998–1003 CrossRef CAS.
- Photochemistry and Photophysics of Coordination Compounds II, ed. V. Balzani and S. Campagna, Springer Berlin Heidelberg, Berlin, Heidelberg, 2007, vol. 281 Search PubMed.
- I. Boldog, A. B. Gaspar, V. Martínez, P. Pardo-Ibañez, V. Ksenofontov, A. Bhattacharjee, P. Gütlich and J. A. Real, Angew. Chem., Int. Ed., 2008, 47, 6433–6437 CrossRef CAS PubMed.
- T. Forestier, S. Mornet, N. Daro, T. Nishihara, S. I. Mouri, K. Tanaka, O. Fouché, E. Freysz and J. F. Létard, Chem. Commun., 2008, 4327–4329 RSC.
- T. Forestier, A. Kaiba, S. Pechev, D. Denux, P. Guionneau, C. Etrillard, N. Daro, E. Freysz and J. F. Létard, Chem. – Eur. J., 2009, 15, 6122–6130 CrossRef CAS PubMed.
- F. Volatron, L. Catala, E. Rivière, A. Gloter, O. Stéphan and T. Mallah, Inorg. Chem., 2008, 47, 6584–6586 CrossRef CAS PubMed.
- K. Jonas, A. Jean-Paul, C. Renée, C. Epiphane, K. Olivier, J. G. Haasnoot, G. Françoise, J. Charlotte, A. Bousseksou, L. Jorge, V. François and G. V. Anne, Chem. Mater., 1994, 6, 1404–1412 CrossRef.
- G. Chastanet, C. a. Tovee, G. Hyett, M. a. Halcrow and J.-F. Létard, Dalton Trans., 2012, 41, 4896–4902 RSC.
- M. P. Cuéllar, A. Lapresta-Fernández, J. M. Herrera, A. Salinas-Castillo, M. D. C. Pegalajar, S. Titos-Padilla, E. Colacio and L. F. Capitán-Vallvey, Sens. Actuators, B, 2015, 208, 180–187 CrossRef.
- A. Lapresta-Fernández, M. P. Cuéllar, J. M. Herrera, A. Salinas-Castillo, M. D. C. Pegalajar, S. Titos-Padilla, E. Colacio and L. F. Capitán-Vallvey, J. Mater. Chem. C, 2014, 2, 7292–7303 RSC.
- P. Durand, S. Pillet, E. E. Bendeif, C. Carteret, M. Bouazaoui, H. El Hamzaoui, B. Capoen, L. Salmon, S. Hébert, J. Ghanbaja, L. Aranda and D. Schaniel, J. Mater. Chem. C, 2013, 1, 1933–1942 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray powder diffraction diagrams, elemental analyses, 1H-NMR spectrum of Re, additional photophysical experiments (reflectance spectra, excited state decay profiles and excitation spectra measured at different temperatures) and magnetic properties of 1@SiO2/Re. See DOI: 10.1039/d1dt03334d |
| This journal is © The Royal Society of Chemistry 2021 |