Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation of a hydride containing amido-zincate using pinacolborane†
Marina
Uzelac
,
Kang
Yuan
,
Gary S.
Nichol
 and
Michael J.
Ingleson
and
Michael J.
Ingleson
 *
*
EaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, UK. E-mail: michael.ingleson@edinburgh.ac.uk
First published on 15th September 2021
Abstract
Amido-zincates containing hydrides are underexplored yet potentially useful complexes. Attempts to access this type of zincate through combining amido-organo zincates and pinacolborane (HBPin) via Zn–C/H–BPin exchange led instead to preferential formation of amide–BPin and/or [amide–BPin(Y)]− (Y = Ph, amide, H), when the amide is hexamethyldisilazide or 2,2,6,6-tetramethylpiperidide and the hydrocarbyl group was phenyl or ethyl. In contrast, the use of a dipyridylamide (dpa) based arylzinc complex led to Zn–C/H–BPin metathesis being the major outcome. Independent synthesis and full characterisation of two LnLi[(dpa)ZnPh2] (L = THF, n = 3; L = PMDETA, n = 1) complexes, 1 and 3, respectively, enabled reactivity studies that demonstrated that these species display zincate type reactivity (by comparison to the lower reactivity of the neutral complex (Me-dpa)ZnPh2, 4, Me-dpa = 2,2′-dipyridyl-N-methylamine). This included 1 performing the rapid deprotonation of 4-ethynyltoluene and also phenyl transfer to α,α,α-trifluoroacetophenone in contrast to neutral complex 4. Complex 1 reacted with one equivalent of HBPin to give predominantly PhBPin (ca. 90%) and a lithium amidophenylzincate containing a hydride unit, complex 7-A, as the major zinc containing product. Complex 7-A transfers hydride to an electrophile preferentially over phenyl, indicating it reacts as a hydridozincate. Attempts to react 1 with >1 equivalent of HBPin or with catecholborane led to more complex outcomes, which included significant borane and dpaZn substituent scrambling, two examples of which were crystallographically characterised. While this work provides proof of principle for Zn–C/H–BPin exchange as a route to form an amido-zincate containing a hydride, amido-organozincates that undergo more selective Zn–C/H–BPin exchange still are required.
Introduction
Zincates, such as monoanionic three coordinate zinc complexes, are receiving increasing attention due to their unique reactivity.1 For example, organozincates ([RnZnX3−n]−n ≥ 1, X = halide, R = hydrocarbyl group) are important nucleophiles in metal-halogen exchange and transmetalation reactions,2 thus can be key species in Negishi cross coupling reactions.3 Another notable class of zincates are the mixed amido-containing zincates that are powerful Brønsted bases able to effect C–H metalations,4 including of challenging substrates such as benzene (Chart 1).5 Detailed studies into (hetero)arene C–H metalation with these zincates demonstrated that all the constituent parts of the zincate (the amide, the group one metal and the hydrocarbyl group) are vital for enabling (hetero)arene metalations. Furthermore, the different roles the amide and alkyl groups fulfil during C–H metalation using amido-alkyl-zincates have been elucidated, specifically the amide is the kinetic and the hydrocarbyl the thermodynamic deprotonation positions, respectively.6 Due to the significant contribution of all components that make up metal-“ate” complexes to controlling reactivity the formation of novel “ate” complexes containing underexplored substituents is important as it has the potential to uncover new reactivity.One rarely explored class of zincates are those containing a hydride substituent.1b The limited reports in this area have focused principally on determining the solution and solid state structures of the hydrido-zincates formed on combining MH or M with R2Zn (M = group one metal).7 Furthermore, there is only one amido-hydrido zincate8 and only one alkoxy-hydrido zincate9 well characterised to date to the best of our knowledge. The use of hydrido-zincates in organic transformations is also limited, and in all cases the hydrido-zincates are formed and used in situ.1b Nevertheless, notable reports from Uchiyama and co-workers using R2Zn/MH combinations revealed highly selective reductions with no competing Brønsted basic reactivity.10 Since this work hydrido-zincates have remained largely overlooked in organic transformations. Changing the latter would be facilitated by the development of alternative routes to access hydrido-zincates, such as methods that use bench stable hydride sources in place of MH or M (M = group 1 metal).
In contrast to anionic zinc hydrides, the chemistry of neutral and cationic molecular zinc hydrides has experienced significant progress, particularly in the past decade.11 This includes the development of multiple synthetic routes to access these zinc hydrides, some of the most common being: complexation of metastable ZnH2 with ligand(s); substituent exchange reactions between LnZn–X and MHn, or between LnZn–OR (or LnZn–NR2) and R4−xSi–Hx or LnZn–NR2 and R2HNBH3.11 More recently, we reported that a metathesis type reaction between neutral and cationic LnZn–R complexes (R = alkyl, aryl, alkenyl or alkynyl) and pinacolborane, HBPin, provides an alternative route to neutral and cationic molecular zinc hydrides (Scheme 1).12 While this approach is related to the formation of zinc-hydrides by reaction of zinc alkyls with LiAlH4 (reported for both neutral organozinc and alkylzincate complexes),13 HBPin is a bench stable hydride source, and the products from Zn–C/H–B exchange, R–BPin, are ubiquitous in synthesis (in contrast to the by-products using LiAlH4, e.g. [R4−XAlHx]−). The formal metathesis of organozincates with HBPin represents an unexplored route to form hydrido-zincate complexes concomitant with organoboranes that could underpin future catalytic developments mediated by zincates. Herein we report our studies into assessing the reactivity of amido-organozincates with HBPin as a potential route to form hydrido-zincates.
Results and discussion
Towards selective Zn–C/H–BPin exchange
We targeted a heteroleptic alkali–metal amido-organo zincate that would undergo selective Zn–C/H–BPin exchange to form mixed amido-hydrido-zincates in preference to Zn–N/H–BPin exchange. Selective exchange would help avoid formation of insoluble [ZnH3]− salts which would form in the presence of excess HBPin if both amido and hydrocarbyl ligands rapidly underwent exchange with HBPin. To expedite the search for such systems, a series of amido-organozincates were made in situ following the well-established co-complexation methodology of mixing appropriate monometallic precursors: ZnR′2 and a MNR2 salt.1 Initial studies focused on two sterically demanding, secondary amides, namely hexamethyldisilazide (HMDS) and 2,2,6,6-tetramethylpiperidide (TMP),14 which have progressed the chemistry of alkali–metal zincates significantly. The hypothesis was that sufficient amide steric bulk would retard Zn–N/H–BPin exchange. Combining equimolar amounts of ZnEt2 (1 M in hexanes) and LiHMDS in benzene followed by the addition of one equivalent of HBPin (Table 1, entry 1) led to effectively complete formation of (HMDS)BPin (by 11B NMR spectroscopy, δ11B ≈ 26),15,16 with effectively no (<2%) EtBPin formation (δ11B ≈ 34).16 Changing the organozinc reagent from ZnEt2 to ZnPh2 (entry 2) still led to formation of (HMDS)BPin as the major product. However, PhBPin (δ11B ≈ 31)17 was present in a greater amount relative to EtBPin in the previous reaction (compare Fig. S2 and S4†) alongside broad resonances between +6 to +8 ppm in the 11B NMR spectrum, consistent with [Y2BPin]− (Y = combinations of H, Ph or NR2).18 The observation of more of the desired organoboron product PhBPin (relative to EtBPin) led us to focus further efforts on the reactivity of zincates derived from ZnPh2. To explore the effect of varying the group one metal component of the zincate, LiHMDS was replaced with its potassium counterpart (entry 3), but this did not effect a significant change in site of reactivity as the major products remained (HMDS)BPin along with a broad resonance centered at δ11B = 6 ppm (a chemical shift closely comparable with previously isolated and well characterised [Y(NR2)BPin]− salts).18 It should be noted that in none of these reactions with HMDS (or TMP, vide infra) are any Zn–H resonances observed by 1H NMR spectroscopy indicating that no molecular zinc hydrides are formed.| Entry | MNR2 | ZnR2 | Major producta |
|---|---|---|---|
| a Identity of major product(s) determined by 11B NMR spectroscopy. b 1 eq. of DME added. c THF was added dropwise until a solution was obtained. d 2 eq. HBPin used. For more details see ESI.† | |||
| 1 | LiHMDS | ZnEt2 | R2NBPin |
| 2b | LiHMDS | ZnPh2 | R2NBPin |
| 3c | KHMDS | ZnPh2 | R2NBPin |
| 4c | LiTMP | ZnPh2 | R2NBPin/PhBpin/[R2NBPin(Y)]− |
| 5b,d | LiTMP | ZnPh2 | R2NBPin/PhBpin |
| 6c | KTMP | ZnPh2 | [R2NBPin(Y)]−/PhBPin |
| 7 | (dpa)Li | ZnPh2 | PhBPin |
The more sterically hindered amide TMP was next explored, with equimolar combinations of LiTMP and ZnPh2 addition of one equivalent of HBPin afforded mixtures containing approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios of TMPBPin, PhBPin (δ11B ≈ 25 and 31, respectively) and a broad resonance centered at 6 ppm (presumably [(NR2)YBPin]− anion(s) (Y = H, Ph or NR2)) in contrast to outcomes with HMDS/Ph-zincates (where PhBPin is only observed at ca. 10%). These findings indicate that increasing the amide steric bulk is beneficial for improving selectivity towards Zn–C/H-BPin exchange, but even with TMP this still does not lead to high selectivity. Addition of further HBPin (now two equivalents in total relative to LiTMP/ZnPh2) led to complete consumption of HBPin and formation of an approximately 1
1 ratios of TMPBPin, PhBPin (δ11B ≈ 25 and 31, respectively) and a broad resonance centered at 6 ppm (presumably [(NR2)YBPin]− anion(s) (Y = H, Ph or NR2)) in contrast to outcomes with HMDS/Ph-zincates (where PhBPin is only observed at ca. 10%). These findings indicate that increasing the amide steric bulk is beneficial for improving selectivity towards Zn–C/H-BPin exchange, but even with TMP this still does not lead to high selectivity. Addition of further HBPin (now two equivalents in total relative to LiTMP/ZnPh2) led to complete consumption of HBPin and formation of an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mix of PhBPin and TMPBPin as the major products (by 11B NMR spectroscopy) with minor quantities of borate salts (e.g. Li[PhxBHy], x + y = 4) also observed. This indicates that both Zn–C and Zn–NR2 units undergo complete exchange with HBPin, indicating that the undesired formation of insoluble homoleptic hydridozincates will occur with these systems. Similar results were also observed replacing LiTMP with KTMP.
1 mix of PhBPin and TMPBPin as the major products (by 11B NMR spectroscopy) with minor quantities of borate salts (e.g. Li[PhxBHy], x + y = 4) also observed. This indicates that both Zn–C and Zn–NR2 units undergo complete exchange with HBPin, indicating that the undesired formation of insoluble homoleptic hydridozincates will occur with these systems. Similar results were also observed replacing LiTMP with KTMP.
We next turned our attention to replacing monofunctional amides (TMP/HMDS) with multifunctional amides: it was hypothesised that by preparing zincates containing less nucleophilic amides (e.g. aniline derived) incorporated into a chelating group could preclude Zn–N/H–BPin exchange. 2,2′-Dipyridylamide (dpa) was selected as it has three Lewis basic N-atoms incorporated within a flexible scaffold, favouring chelation to zinc,19 and a significantly lower nucleophilicity amide (potentially disfavouring Zn–N/H–B exchange). Mixing Lidpa with ZnPh2 and reacting this with one equivalent of HBPin afforded PhBPin as the major product (entry 7 and Fig. S14†) with no PinB-NR2 observed (minor borate resonances at δ11B 6–8 ppm were observed consistent with formation of [R2N(Y)BPin]− anions, but these were only 20% the intensity of the PhBPin resonance in the 11B NMR spectra). Importantly, a new sharp resonance in the region 4.5–5.7 ppm (depending on the solvent) consistent with a Zn–H was observed in the 1H NMR spectra suggesting successful formation of a soluble and stable Zn–H species derived from Zn–C/H–BPin exchange. Encouraged by this observation, we set out to identify the constitution of the relevant dpa-based zincates formed prior to addition of HBPin to enable more controlled studies and more insight into the putative hydrido-dpa-zincate.
Zinc-dpa complexes
Deprotonation of 2,2′-dipyridylamine with nBuLi in hexane followed by addition of a THF-solution of ZnPh2 afforded [(THF)3Li(μ-dpa)ZnPh2] (1) in 70% isolated yield (Scheme 2).Deprotonation of dpaH with ZnPh2 in toluene afforded on crystallisation trimeric [(dpaZnPh)3] (2) in 77% isolated yield, which could be converted into mono-zinc complex 1 by addition of one equivalent of PhLi in THF. Addition of the chelating ligand PMDETA (N,N,N′,N′′,N′′-pentamethyldiethylenetriamine) during zincate synthesis afforded the PMDETA analogue of 1, [(PMDETA)Li(μ-dpa)ZnPh2] (3) in 65% isolated yield. In addition, a neutral monomeric Zn-complex incorporating the dpa scaffold was prepared for reactivity comparison studies (vide infra). Adding an equivalent of 2,2′-dipyridyl-N-methylamine (Me-dpa) to a solution of ZnPh2 afforded adduct [(Me-dpa)·ZnPh2] (4). Complexes 1–4 were all fully characterised in solution (comparing well with the data previously reported for other dpa-zincates (e.g. [(TMEDA)2Na2(μ-dpa)2Zn(tBu)2], A, and [Na(THF)6{Zn(tBu)2(dpa)Zn(tBu)2}], B)19 and L2ZnPh2 systems, e.g. TMEDA·ZnPh2)20 and in the solid state by single crystal X-ray diffraction studies. It should be noted that 1H DOSY NMR studies are consistent with 2 persisting as an oligomer in solution by comparison to parameters derived from 1H DOSY studies on monomeric 4 (see ESI, Fig. S38 and S39†).
In the solid state (Fig. 1) 2 is a trimer in which each dpa assumes an anti/anti conformation to act as a bridge between neighbouring Zn atoms. Each Zn atom is in a distorted tetrahedral environment, bonded to the Namido of one dpa unit and chelated within the dipyridyl pocket of another dpa, with its coordination completed with a terminal Ph ligand. Within the trimer, the three Zn-atoms define a plane, to which all three dpa ligands are effectively perpendicular, while the Ph rings are 7.20°, 22.40° and 34.87° tilted to the Zn3 plane. The Zn–C bond distances are in close agreement with that reported for the dimer [(dpaZntBu)2], while the Zn–N bonds are only slightly shortened in 2 (cf. for [(dpaZntBu)2] Zn–N = 2.116(2) Å; 2.079(2) Å; 2.070(2) Å).19
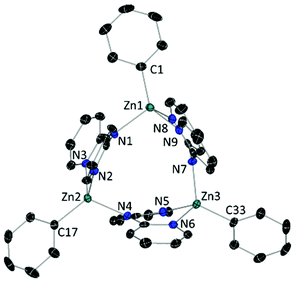 | ||
| Fig. 1 ORTEP-representation of [(dpaZnPh)3] (2) with ellipsoids at 50% probability level. The disorder component, hydrogen atoms and crystallisation solvent have been omitted for clarity. | ||
In both 1 and 3 (Fig. 2a and b), the anionic dpa ligand is also in an anti/anti conformation, but now bridges between a donor-capped Li+ cation coordinated to the Namido with a ZnPh2 unit chelated within the dipyridyl pocket, similar to that observed in zincates A and B.19 Close comparison of 1 and 3 (Table 2) reveal that the tetrahedral environment of Li in 3 is more distorted (τ4 = 0.7) from ideal than in 1 (τ4 = 0.9) but the average angles and Li–Namido bond lengths are identical within error. In both complexes Zn is in a distorted N2C2-tetrahedral environment, displaying essentially identical bond distances, average angles and the extent of distortion from an ideal tetrahedral environment (τ4). However, the C–Zn–C angle is significantly different at 137.17(8)° for 1 and 128.07(4)° for 3, the former angle is even greater than the ZntBu2 species, A (130.31(7)°) and B (131.9(1)°),19 where a larger steric effect from the tBu groups would be expected. Previous studies have shown that the narrowing of the C–Zn–C bond angle in diorganylzinc complexes with C2ZnN2 coordination environments increases the Lewis acidity of Zn-centre.21 However, based on reactivity studies and DFT calculations (vide infra) this C–Zn–C angle disparity does not indicate a significantly different electronic structure for 1 and 3. Finally, the tetrahedrally coordinated Zn centre in 4 (Fig. 2c) exhibits very similar parameters to those found in 3, with a C–Zn–C angle of 126.20(7)°.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Zn–C (Å) | 2.004(2) | 1.987(2)–1.992(2) | 2.0088(11) | 1.9988(18) | 1.973(4) |
| 2.010(2) | 2.0018(10) | 1.9964(19) | 1.971(5) | ||
| Zn–Npy (Å) | 2.0952(16) | 2.035(2)–2.056(2) | 2.0966(9) | 2.1032(15) | 2.059(4) |
| 2.0985(17) | 2.1071(9) | 2.1309(15) | 2.064(4) | ||
| Zn–Namide (Å) | — | 2.047(2)–2.063(2) | — | — | — |
| C–Zn–C (°) | 137.17(8) | — | 128.07(4) | 126.20(7) | 130.60(19) |
| Li–Namide (Å) | 2.047(4) | — | 2.028(2) | — | 2.028(9) |
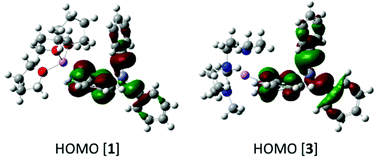
The solid state geometries of complexes 1 and 3 were used for single point calculations at the B3PW91/6-311G(d,p) (H, Li, C, N, O)/lanl2dz (Zn)//PCM(THF) level (PCM = polarisable continuum model). Notably, these calculations show that despite the difference in the C–Zn–C bond angle between 1 and 3 the two structures possess effectively identical NBO charge distributions (e.g. axial ipsoPh![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) charge = −0.499e and −0.506e for 1 and 3, respectively) and frontier orbital energies and character. For example, the HOMO of 1 and 3 (Fig. 3) both contain Zn–C σ bonding character and are effectively identical in energy (−5.02 eV and −5.06 eV), while the charges at Zn are within 0.006e in 1 and 3 (+1.169 and +1.163e, respectively). The most notable difference is in the NBO charge at lithium which is +0.875e for 1 and +0.846e for 3; this small difference is presumably due to a more electrostatic dominated interaction between (THF)O3–Li (in 1) than between (PMDETA)N3–Li in 3.
charge = −0.499e and −0.506e for 1 and 3, respectively) and frontier orbital energies and character. For example, the HOMO of 1 and 3 (Fig. 3) both contain Zn–C σ bonding character and are effectively identical in energy (−5.02 eV and −5.06 eV), while the charges at Zn are within 0.006e in 1 and 3 (+1.169 and +1.163e, respectively). The most notable difference is in the NBO charge at lithium which is +0.875e for 1 and +0.846e for 3; this small difference is presumably due to a more electrostatic dominated interaction between (THF)O3–Li (in 1) than between (PMDETA)N3–Li in 3.
Reactivity studies of Zn-dpa complexes
To determine their relative reactivity, the various zinc complexes were applied in selected transformations. We started by investigating the deprotonation of two equivalents of the terminal alkyne 4-ethynyltoluene. This was slow using neutral zinc complex 4, only proceeding to 41% after 24 h at room temperature. Contrastingly, 1 completely consumed all two equivalents of the terminal alkyne within two hours at room temperature affording the new bimetallic complex [(THF)3Li(μ-dpa)Zn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4Me)2] (5, Fig. 4) as the major product. The disparity between the reactivity of 1 and 4 is an indicator of zincate character in 1.
CC6H4Me)2] (5, Fig. 4) as the major product. The disparity between the reactivity of 1 and 4 is an indicator of zincate character in 1.
 | ||
Fig. 4 Formation of [(THF)3Li(μ-dpa)Zn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4Me)2] (5). ORTEP representation of 5 with ellipsoids at 30% probability level and hydrogen atoms omitted for clarity. CC6H4Me)2] (5). ORTEP representation of 5 with ellipsoids at 30% probability level and hydrogen atoms omitted for clarity. | ||
Single crystal X-ray diffraction analysis of 5 revealed it to have an essentially isostructural Li-dpa-Zn core to 1 and 3 (Table 2). The most notable metric is the C–Zn–C angle which is different between 1 and 5, with 5 having a compressed C–Zn–C angle (130.60(19)°) relative to that in 1 (137.17(8)°), despite both having an identical lithium environment. Solution studies on 5 were precluded due to the poor solubility of crystals of 5, even in THF. Furthermore, during reactions to form 5 small amounts of two other alkynyl-containing species also were observed which could correspond to different dpaZn structural motif(s) or to products obtained by alkyne deprotonation partially proceeding through Li–Namido fragment. The same minor species are observed even when 5 was prepared by reacting dialkynylzinc with Lidpa. Finally, compound 3 reacts with two equivalents of 4-ethynyltoluene in a comparable manner to 1, with all alkyne consumed within 2 hours using 3, indicating similar reactivity between 1 and 3 in metalation reactions supporting the comparable electronic structures in the Ph2Zn-dpa units of 1 and 3 found by DFT calculations.
We next turned our attention to phenyl transfer to α,α,α-trifluoroacetophenone (to facilitate reaction monitoring by 19F NMR spectroscopy). While neutral complexes 2 and 4 afforded no transfer of a phenyl group (by 19F NMR spectroscopy) even after days, complex 1 displayed significant reactivity and afforded 2,2,2-trifluoro-1,1-diphenylethanol (6) in 71% yield after only 2 h reaction time at room temperature (Scheme 3). This observation is in line with previous reports on the sluggish reactivity of neutral diorganozinc reagents towards aldehydes and ketones that can be enhanced by formation of zincates.22 This further indicates that 1 displays zincate type reactivity. Monitoring the reaction of 1/α,α,α-trifluoroacetophenone in situ (prior to work up) revealed formation of resonances consistent with 2 as the only observed zinc containing by-product from phenyl transfer. In contrast to fast phenyl transfer with 1, compound 3 led to no phenyl transfer to α,α,α-trifluoroacetophenone after 2 h by 19F NMR spectroscopy (though slow phenyl transfer does occur at longer reaction times). Given the similar calculated electronic structures and comparable reactivity towards terminal alkynes observed for 1 and 3 this disparity was surprising. It is attributed to the lithium centre in 1 being more accessible for coordination of a carbonyl and that this Lewis acid activation of the ketone accelerates phenyl transfer (consistent with observations from reported DFT calculations on lithium-zincates reacting with carbonyls).10 In contrast, PMDETA would bind lithium more strongly and disfavour coordination of the ketone to lithium. A similar observation has been previously reported where TMEDA addition retards nucleophile transfer from a lithium zincate to carbonyl containing electrophiles.23 This hypothesis is supported further by the addition of PMDETA to 1 resulting in formation of 3, confirming the stronger binding affinity that PMDETA has towards lithium in these lithium zincates relative to three molecules of THF.
With confirmation of zincate type reactivity exhibited by 1 in hand we explored next the reactivity of 1 towards HBPin targeting an amido-hydrido-zincate by Zn–C/HBPin exchange.
Reactivity of 1 towards pinacolborane
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) resonances (they do not correspond to C–H moieties based on HSQC NMR experiments and are not observed in reactions using DBPin in place of HBPin). Along with these the 1H NMR spectra contained broad aromatic resonances for dpa and Zn–Ph, sharper aromatic resonances corresponding to PhBPin, and THF resonances that remain shifted relative to free THF consistent with THF coordination to Li+ as observed in 1. The 11B NMR spectra showed PhBPin as the major product and a minor product associated with a very broad resonance centered at 5 ppm. This very broad 11B resonance is in the region expected for [PinB(Y)2]−, Y = Ph and/or NR2.18,25 The observation of PhBPin as the major boron containing product and new Zn–H species formed from equimolar 1/HBPin suggests successful formation of a mixed amido-phenyl-hydrido-zincate derived from 1, tentatively assigned as [(THF)3Li(μ-dpa)ZnPh(H)] (7-A) (Fig. 5 and 6). 7Li NMR spectra for 7-A do not show any Li–H scalar coupling in contrast to the only (to our knowledge) other previously reported lithium amido-hydrido zincate.8 Repeated attempts to isolate or crystallise any Zn–H species from these reaction mixtures were unsuccessful in our hands.
resonances (they do not correspond to C–H moieties based on HSQC NMR experiments and are not observed in reactions using DBPin in place of HBPin). Along with these the 1H NMR spectra contained broad aromatic resonances for dpa and Zn–Ph, sharper aromatic resonances corresponding to PhBPin, and THF resonances that remain shifted relative to free THF consistent with THF coordination to Li+ as observed in 1. The 11B NMR spectra showed PhBPin as the major product and a minor product associated with a very broad resonance centered at 5 ppm. This very broad 11B resonance is in the region expected for [PinB(Y)2]−, Y = Ph and/or NR2.18,25 The observation of PhBPin as the major boron containing product and new Zn–H species formed from equimolar 1/HBPin suggests successful formation of a mixed amido-phenyl-hydrido-zincate derived from 1, tentatively assigned as [(THF)3Li(μ-dpa)ZnPh(H)] (7-A) (Fig. 5 and 6). 7Li NMR spectra for 7-A do not show any Li–H scalar coupling in contrast to the only (to our knowledge) other previously reported lithium amido-hydrido zincate.8 Repeated attempts to isolate or crystallise any Zn–H species from these reaction mixtures were unsuccessful in our hands.
Mass spectrometry on equimolar 1/HBPin reactions only showed fragmentation products such as [dpaZnPh]˙+. It should be noted that combining HBPin/1 in aromatic solvents also formed some insoluble material, however performing these reactions in more polar solvents, e.g. THF, led to more complex mixtures by NMR spectroscopy, (though PhBPin is still the dominant boron containing species when reactions are performed in THF), thus aromatic solvents were the preferred reaction media.
On standing in benzene 1/HBPin mixtures slowly produced more insoluble material over several days, with the only significant species ultimately left in solution being PhBPin. The amount of PhBPin formed was quantified versus an internal standard which revealed >90% of HBPin had converted into PhBPin indicating that Zn–Ph/H–BPin exchange is the dominant outcome on combining equimolar 1 and HBPin. Finally, attempts to access the putative amido-hydrido-zincate 7-A by other routes were unsuccessful (see ESI† for more details).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 combinations of 1 and HBPin.
Complex 1 was combined with two equivalents of HBPin in benzene, with arene solvents again essential as the use of THF leads to more complex outcomes (including rapid formation of [BH4]−, [PhBH3]− and other new B–H species). Combining in d6-benzene a 1
2 combinations of 1 and HBPin.
Complex 1 was combined with two equivalents of HBPin in benzene, with arene solvents again essential as the use of THF leads to more complex outcomes (including rapid formation of [BH4]−, [PhBH3]− and other new B–H species). Combining in d6-benzene a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of 1
2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBPin led to complete consumption of HBPin with the 1H NMR spectrum containing resonances consistent with a single zinc-dpa containing complex, termed 7-B, with key resonances including: a 1H integral (relative to dpa) Zn–
HBPin led to complete consumption of HBPin with the 1H NMR spectrum containing resonances consistent with a single zinc-dpa containing complex, termed 7-B, with key resonances including: a 1H integral (relative to dpa) Zn–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) at 5.10 ppm, and resonances for a single Zn–Ph unit, along with one set of well-defined dpa resonance (Fig. 5). A HMBC NMR experiment contained a cross peak between the ipso Zn–CPh and the Zn–H confirming they are bound to the same zinc centre. It should be noted that dpa also remains bound to the Zn(Ph)H moiety in this complex based on 1H DOSY NMR studies (Fig. 7). An internal standard was added and integration revealed that the dpaZn(Ph)H species 7-B corresponded to 70% of the amount of 1 originally present, confirming it is the major product, but that some dpa containing material had precipitated. The addition of three equivalents of HBPin to 1 led to the same soluble zinc complex 7-B, with one equivalent of HBPin left unreacted (by 11B NMR spectroscopy). This confirms that dpa ligation of zinc disfavours formation of insoluble homoleptic hydridozincates and that a 2
at 5.10 ppm, and resonances for a single Zn–Ph unit, along with one set of well-defined dpa resonance (Fig. 5). A HMBC NMR experiment contained a cross peak between the ipso Zn–CPh and the Zn–H confirming they are bound to the same zinc centre. It should be noted that dpa also remains bound to the Zn(Ph)H moiety in this complex based on 1H DOSY NMR studies (Fig. 7). An internal standard was added and integration revealed that the dpaZn(Ph)H species 7-B corresponded to 70% of the amount of 1 originally present, confirming it is the major product, but that some dpa containing material had precipitated. The addition of three equivalents of HBPin to 1 led to the same soluble zinc complex 7-B, with one equivalent of HBPin left unreacted (by 11B NMR spectroscopy). This confirms that dpa ligation of zinc disfavours formation of insoluble homoleptic hydridozincates and that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction stoichiometry between HBPin and 1 leads to the 7-B species. The 11B NMR spectrum of the 1
1 reaction stoichiometry between HBPin and 1 leads to the 7-B species. The 11B NMR spectrum of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 1 /HBPin reaction showed only PhBPin and a very broad resonance centered at 5 ppm. As 7-B could not be isolated in our hands, it can only be tentatively assigned as the analogue of 7-A where a YBPin molecule is bound to the anilido N in place of [Li(THF)3]+.
2 1 /HBPin reaction showed only PhBPin and a very broad resonance centered at 5 ppm. As 7-B could not be isolated in our hands, it can only be tentatively assigned as the analogue of 7-A where a YBPin molecule is bound to the anilido N in place of [Li(THF)3]+.
 | ||
| Fig. 7 1H DOSY NMR spectrum of reaction mixture 1 with 2 eq. of HBPin in C6D6 at room temperature after 45 min. | ||
On standing the δ1H 5.10 resonance slowly decreases in intensity, concomitant with the very broad δ11B 5 ppm resonance being replaced with several other boron resonances. The most intense new resonances correspond to [BH4]− species (δ11B = −40 ppm quintet) and a sharper δ11B resonance at +5 ppm (consistent with [diamido–BPin]−).18 This decomposition is consistent with previous work on the reactivity of anionic nucleophiles with HBPin, that form metastable [HBPin(Y)]− anions that undergo substituent scrambling, this indicates that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 1/HBpin reaction mixture contains significant [(Y)HBPin]−.24,25 Attempts to crystallise these 1
2 1/HBpin reaction mixture contains significant [(Y)HBPin]−.24,25 Attempts to crystallise these 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reaction mixtures to isolate the Zn–H species 7-B also were unsuccessful, with the only species isolated being a small quantity of the redistribution product, 8, containing both [BH4]− and [(R2N)2BPin]− moieties consistent with the +5 and −40 ppm resonances observed in the 11B NMR spectra. The PMDETA analogue, 3, displayed comparable outcomes to combinations of 1 with HBPin, undergoing Zn–Ph/H–BPin metathesis (albeit more slowly than 1) to give PhBPin and Zn–H 7-B (δ1H 5.1 ppm), with scrambling to form BHx species (x > 1) also observed on standing. Finally, attempts to use catecholborane, HBCat, in place of HBPin led to much more complex outcomes, rapidly forming new boron containing compounds including: [BCat2]−, species containing BH2 and BH3 units and [BH4]−. Single crystals of a substituent scrambled product, [dpa3Zn2][BCat2] (9), were isolated from these mixtures (see ESI, Fig. S132†). These observations indicate that both the borane and dpaZn fragments can undergo exchange processes, and that these occur much more rapidly with BCat derived species.
2 reaction mixtures to isolate the Zn–H species 7-B also were unsuccessful, with the only species isolated being a small quantity of the redistribution product, 8, containing both [BH4]− and [(R2N)2BPin]− moieties consistent with the +5 and −40 ppm resonances observed in the 11B NMR spectra. The PMDETA analogue, 3, displayed comparable outcomes to combinations of 1 with HBPin, undergoing Zn–Ph/H–BPin metathesis (albeit more slowly than 1) to give PhBPin and Zn–H 7-B (δ1H 5.1 ppm), with scrambling to form BHx species (x > 1) also observed on standing. Finally, attempts to use catecholborane, HBCat, in place of HBPin led to much more complex outcomes, rapidly forming new boron containing compounds including: [BCat2]−, species containing BH2 and BH3 units and [BH4]−. Single crystals of a substituent scrambled product, [dpa3Zn2][BCat2] (9), were isolated from these mixtures (see ESI, Fig. S132†). These observations indicate that both the borane and dpaZn fragments can undergo exchange processes, and that these occur much more rapidly with BCat derived species.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1/HBPin on addition of α,α,α-trifluoroacetophenone led to consumption of the δ1H 5.55 ppm Zn–H species and formation of 2 as the major zinc containing species. After work up 2,2,2-trifluoro-1-phenylethanol, 10, derived from hydride transfer from zinc was the major product with minimal product observed derived from phenyl transfer, 6 (Scheme 4). This is consistent with previous studies using hydrido zincates that demonstrated preferential hydride transfer over hydrocarbyl.10 Monitoring the reaction in situ revealed no significant ketone hydroboration (product 14, Scheme 4) had formed by 19F NMR spectroscopy, precluding the presence of any significant amount of [H(Y)BPin]− anion in the 1
1 1/HBPin on addition of α,α,α-trifluoroacetophenone led to consumption of the δ1H 5.55 ppm Zn–H species and formation of 2 as the major zinc containing species. After work up 2,2,2-trifluoro-1-phenylethanol, 10, derived from hydride transfer from zinc was the major product with minimal product observed derived from phenyl transfer, 6 (Scheme 4). This is consistent with previous studies using hydrido zincates that demonstrated preferential hydride transfer over hydrocarbyl.10 Monitoring the reaction in situ revealed no significant ketone hydroboration (product 14, Scheme 4) had formed by 19F NMR spectroscopy, precluding the presence of any significant amount of [H(Y)BPin]− anion in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction mixture. This further supports hydrido-zincate 7-A being the major product derived from a 1
1 reaction mixture. This further supports hydrido-zincate 7-A being the major product derived from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1/HBPin combination, and that 7-A can react selectively as a source of hydride. 7-A was also effective for the reduction of a number of other ketones, including an enolisable ketone, affording alcohols 11–13 in reasonable yield.
1 1/HBPin combination, and that 7-A can react selectively as a source of hydride. 7-A was also effective for the reduction of a number of other ketones, including an enolisable ketone, affording alcohols 11–13 in reasonable yield.
 | ||
| Scheme 4 Top and bottom, disparate outcomes on reaction of Zn–H species 7-A and 7-B derived from 1/HBPin with α,α,α-trifluoroacetophenone. Middle, alcohols formed by carbonyl reduction using 7-A. | ||
In contrast, the combination of the reaction mixture derived from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 1/HBPin with one equivalent of α,α,α-trifluoroacetophenone led to formation of a significant quantity of the hydroboration product 14 (by 19F NMR spectroscopy), indicating the presence of significant [(Y)HBPin]− species. Furthermore, the Zn–H resonance at 5.10 ppm is not consumed on addition of this ketone, indicating that ketone reduction is achieved by a B–H species not a Zn–H complex. Note, in a control reaction using HBPin under identical conditions no hydroboration of α,α,α-trifluoroacetophenone is observed. The formation of significant hydroboration product 14, combined with the spectroscopic data, suggests that on using two equivalents of HBPin (with respect to 1) the outcome involves a single Zn–C/H–BPin exchange followed by formation of a [H(Y)BPin]− species that is metastable (towards substituent redistribution).
2 1/HBPin with one equivalent of α,α,α-trifluoroacetophenone led to formation of a significant quantity of the hydroboration product 14 (by 19F NMR spectroscopy), indicating the presence of significant [(Y)HBPin]− species. Furthermore, the Zn–H resonance at 5.10 ppm is not consumed on addition of this ketone, indicating that ketone reduction is achieved by a B–H species not a Zn–H complex. Note, in a control reaction using HBPin under identical conditions no hydroboration of α,α,α-trifluoroacetophenone is observed. The formation of significant hydroboration product 14, combined with the spectroscopic data, suggests that on using two equivalents of HBPin (with respect to 1) the outcome involves a single Zn–C/H–BPin exchange followed by formation of a [H(Y)BPin]− species that is metastable (towards substituent redistribution).
Conclusions
In summary, controlling selectivity on the addition of pinacolborane to amido-containing organozincates is challenging, with three outcomes occurring, often in competition: (i) Zn–N/H–BPin exchange; (ii) Zn–C/H–BPin exchange; (iii) transfer of an anionic group from the zincate to HBPin to form [(Y)HBPin]− species. The use of dipyridylamide (dpa) to ligate zinc produces zincates where the two pyridyl nitrogens bind Zn while the anilido nitrogen binds Li. The (donor)Li(dpa)ZnPh2 complexes exhibit zincate type reactivity, and in reaction with pinacolborane lead to improved selectivity for Zn–C/H–BPin exchange and no over-reaction (to from [ZnH3]−) on addition of excess HBPin, presumably due to the lower nucleophilicity of the anilido N and the chelate effect. This enables the in situ synthesis of a mixed amido-hydrido zincate 7-A as the major product, with initial reactivity studies using 7-A demonstrating it functions as a selective hydride transfer agent. However, selectivity during the Zn–C/H–BPin exchange step still needs to be improved for catalytic applications. Furthermore, the addition of excess pinacolborane results in formation of both zinc hydride 7-B and [(Y)HBpin]−, the latter which are unstable with respect to substituent scrambling.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No. 769599). Crystallographic data for 8 measured remotely26 at beam line I-19 of Diamond Light Source (award CY22240). We thank Mr Juraj Bella for help with multi-dimensional NMR experiments. We thank Dr Faye Cruickshank at the Mass Spectrometry facility at the University of Edinburgh for carrying out air-sensitive HRMS analysis.References
- For recent reviews covering this area see: (a) S. D. Robertson, M. Uzelac and R. E. Mulvey, Chem. Rev., 2019, 119, 8332 CrossRef CAS PubMed; (b) Organo-di-Metallic Compounds (or Reagents), in Topics in Organometallic Chemistry, ed. M. Uchiyama, C. Wang and Z. Xi, 2014, Springer, p. 47 Search PubMed.
- (a) For a review see: D. Tilly, F. Chevallier, F. Mongin and P. C. Gros, Chem. Rev., 2014, 114, 1207 CrossRef CAS PubMed; (b) For more work see (and references therein): N. Balkenhohl, D. S. Zeigler, A. Desaintjean, L. J. Bole, A. R. Kennedy, E. Hevia and P. Knochel, Angew. Chem., Int. Ed., 2019, 58, 12898 CrossRef PubMed.
- P. Eckert, S. Sharif and M. G. Organ, Angew. Chem., 2021, 60, 12224 CrossRef CAS PubMed.
- R. E. Mulvey, F. Mongin, M. Uchiyama and Y. Kondo, Angew. Chem., Int. Ed., 2007, 46, 3802 CrossRef CAS PubMed.
- P. C. Andrikopoulos, D. R. Armstrong, H. R. L. Barley, W. Clegg, S. H. Dale, E. Hevia, G. W. Honeyman, A. R. Kennedy and R. E. Mulvey, J. Am. Chem. Soc., 2005, 127, 6184 CrossRef CAS PubMed.
- D. Nobuto and M. Uchiyama, J. Org. Chem., 2008, 73, 1117 CrossRef CAS PubMed.
- For representative examples see: (a) E. C. Ashby, K. C. Nainan and H. S. Prasad, Inorg. Chem., 1977, 16, 349 Search PubMed; (b) G. J. Kubas and D. F. Shriver, J. Am. Chem. Soc., 1970, 92, 1949 CrossRef CAS; (c) A. Lennartson, M. Håkansson and S. Jagner, Angew. Chem., Int. Ed., 2007, 46, 6678 CrossRef CAS PubMed.
- R. Campbell, D. Cannon, P. García-Álvarez, A. R. Kennedy, R. E. Mulvey, S. D. Robertson, J. Sassmannshausen and T. Tuttle, J. Am. Chem. Soc., 2011, 133, 13706 CrossRef CAS PubMed.
- W. Marciniak, K. Merz, M. Moreno and M. Driess, Organometallics, 2006, 25, 4931 CrossRef CAS.
- (a) M. Uchiyama, S. Furumoto, M. Saito, Y. Kondo and T. Sakamoto, J. Am. Chem. Soc., 1997, 119, 11425 CrossRef CAS; (b) M. Uchiyama, S. Nakamura, T. Ohwada, M. Nakamura and E. Nakamura, J. Am. Chem. Soc., 2004, 126, 10897 CrossRef CAS PubMed.
- For reviews see: (a) K. Y. Zhizhin, N. N. Mal'tseva, G. A. Buzanov and N. T. Kuznetsov, Russ. J. Inorg. Chem., 2014, 59, 1665 CrossRef CAS; (b) A.-K. Wiegand, A. Rit and J. Okuda, Coord. Chem. Rev., 2016, 314, 71 CrossRef CAS. For select recent reports using molecular zinc hydrides see: (c) F. Ritter, T. P. Spaniol, I. Douair, L. Maron and J. Okuda, Angew. Chem., 2020, 59, 23335 CrossRef CAS PubMed; (d) R. K. Sahoo, N. Sarkar and S. Nembenna, Angew. Chem., 2021, 60, 11991 CrossRef CAS PubMed.
- (a) R. J. Procter, M. Uzelac, J. Cid, P. J. Rushworth and M. J. Ingleson, ACS Catal., 2019, 9, 5760 CrossRef CAS; (b) M. Uzelac, K. Yuan and M. J. Ingleson, Organometallics, 2020, 39, 1332 CrossRef CAS.
- E. C. Ashby and J. J. Watkins, Inorg. Chem., 1973, 12, 2493 CrossRef CAS.
- R. E. Mulvey and S. D. Robertson, Angew. Chem., Int. Ed., 2013, 52, 11470 CrossRef CAS PubMed.
- (a) Y. Gu, Y. Shen, C. Zarate and R. Martin, J. Am. Chem. Soc., 2019, 141, 127 CrossRef CAS PubMed.
- M. Rauser, C. Ascheberg and M. Niggemann, Angew. Chem., Int. Ed., 2017, 56, 11570 CrossRef CAS PubMed.
- L. P. Press, A. J. Kosanovich, B. J. McCulloch and O. V. Ozerov, J. Am. Chem. Soc., 2016, 138, 9487 CrossRef CAS PubMed.
- (a) A. D. Bage, T. A. Hunt and S. P. Thomas, Org. Lett., 2020, 22, 4107 CrossRef CAS PubMed; (b) L. E. Lemmerz, D. Mukherjee, T. P. Spaniol and J. Okuda, J. Organomet. Chem., 2019, 894, 39 CrossRef CAS; (c) M. P. Crockett, C. C. Tyrol, A. S. Wong, B. Li and J. A. Byers, Org. Lett., 2018, 20, 5233 CrossRef CAS PubMed; (d) L. Zhang and L. Jiao, Chem. Sci., 2018, 9, 2711 RSC.
- (a) D. R. Armstrong, J. A. Garden, A. R. Kennedy, R. E. Mulvey and S. D. Robertson, Angew. Chem., Int. Ed., 2013, 52, 7190 CrossRef CAS PubMed; (b) D. R. Armstrong, E. V. Brouillet, A. R. Kennedy, J. A. Garden, M. Granitzka, R. E. Mulvey and J. J. Trivett, Dalton Trans., 2014, 43, 14409 RSC.
- A. Hernan-Gomez, S. A. Orr, M. Uzelac, A. R. Kennedy, S. Barroso, X. Jusseau, S. Lemaire, V. Farina and E. Hevia, Angew. Chem., Int. Ed., 2018, 57, 10630 CrossRef CAS PubMed.
- B. Mirabi, W. C. Poh, D. Armstrong, A. J. Lough and U. Fekl, Inorg. Chem., 2020, 59, 2621 CrossRef CAS PubMed.
- (a) A. Metzger, S. Bernhardt, G. Manolikakes and P. Knochel, Angew. Chem., Int. Ed., 2010, 49, 4665 CrossRef CAS PubMed; (b) D. R. Armstrong, W. Clegg, P. Garcia-Alvarez, M. D. McCall, L. Nuttall, A. R. Kennedy, L. Rosso and E. Hevia, Chem. – Eur. J., 2011, 17, 4470 CrossRef CAS PubMed.
- A. Vaughan and R. D. Singer, Tetrahedron Lett., 1995, 36, 5683 CAS.
- (a) I. P. Query, P. A. Squier, E. M. Larson, N. A. Isley and T. B. Clark, J. Org. Chem., 2011, 76, 6452 CrossRef CAS PubMed; (b) For a recent review on this topic see: A. D. Bage, K. Nicholson, T. A. Hunt, T. Langer and S. P. Thomas, ACS Catal., 2020, 10, 13479 CrossRef CAS.
- For a discussion on the mechanism of substituent scrambling of dioxaborolanes by Lewis base see: S. A. Westcott, H. P. Blom, T. B. Marder, R. T. Baker and J. C. Calabrese, Inorg. Chem., 1993, 32, 2175 CrossRef CAS.
- N. T. Johnson, P. G. Waddell, W. Clegg and M. R. Probert, Crystals, 2017, 7, 360 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental procedures, crystallographic data and additional figures. CCDC 2101027–2101033. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1dt02580e |
| This journal is © The Royal Society of Chemistry 2021 |