An organometallic catalase mimic with exceptional activity, H2O2 stability, and catalase/peroxidase selectivity†
Abstract
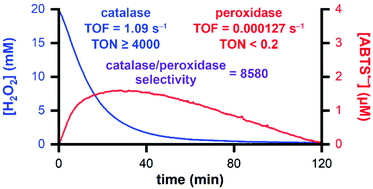
Manganese–porphyrin and –salen redox therapeutics catalyze redox reactions involving O2˙−, H2O2, and other reactive oxygen species, thereby modulating cellular redox states. Many of these complexes perform catalase reactions via high-valent Mn–oxo or –hydroxo intermediates that oxidize H2O2 to O2, but these intermediates can also oxidize other molecules (e.g., thiols), which is peroxidase reactivity. Whether catalase or peroxidase reactivity predominates depends on the metal–ligand set and the local environment, complicating predictions of what therapeutic effects (e.g., promoting vs. suppressing apoptosis) a complex might produce in a given disease. We recently reported an organoruthenium complex (Ru1) that catalyzes ABTS˙− reduction to ABTS2− with H2O2 as the terminal reductant. Given that H2O2 is thermodynamically a stronger oxidant than ABTS˙−, we reasoned that the intermediate that reduced ABTS˙− would also be able to reduce H2O2 to H2O. Herein we demonstrate Ru1-catalyzed H2O2 disproportionation into O2 and H2O, exhibiting an 8,580-fold faster catalase TOF vs. peroxidase TOF, which is 89.2-fold greater than the highest value reported for a Mn–porphyin or –salen complex. Furthermore, Ru1 was 120-fold more stable to H2O2 than the best MnSOD mimic (TON = 4000 vs. 33.4) Mechanistic studies provide evidence that the mechanism for Ru1-catalyzed H2O2 disproportionation is conserved with the mechanism for ABTS˙− reduction. Therapeutic effects of redox catalysts can be predicted with greater accuracy for catalysts that exhibit exclusively catalase activity, thereby facilitating the development of future redox therapeutic strategies for diseases.


 Please wait while we load your content...
Please wait while we load your content...