Open Access Article
Open Access ArticleBreaking the symmetry: C1-salans with (N–H) backbones†
Jack Devonport ,
John Spencer
,
John Spencer * and
George E. Kostakis
* and
George E. Kostakis *
*
Department of Chemistry, School of Life Sciences, University of Sussex, Brighton, BN1 9QJ, UK. E-mail: G.Kostakis@sussex.ac.uk; j.spencer@sussex.ac.uk
First published on 30th July 2021
Abstract
We disclose a synthetic route that providess an unprecedented library of C1 salan ligands endowed with (N–H) backbones, previously limited to N-methylated backbones. Efforts to identify a generic complexation protocol to yield the corresponding Cu(II)–salan complexes demonstrate the scope and limitations of this approach.
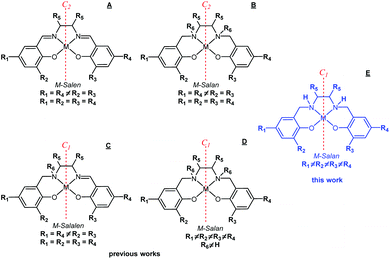
Salens, salans and salalens (Scheme 1) are metal chelators derived from 2-hydroxybenzaldhydes (salicylaldehydes) and 1,2-diamines.1 They offer a tetradentate [ONNO] coordination environment and bind to a wide range of transition metal ions.2–9 The corresponding complexes have been extensively researched for a wide range of applications ranging from catalysis, sensors, biomimetics and medicinal bioinorganic molecules.6–8,10–12 Salans have increased flexibility, thus yielding complexes with less restricted coordination geometries. Salens and their complexes tend to be susceptible to hydrolysis,13 which limits their use in applications that require aqueous, acidic or basic media.14 Symmetric, C2-salen and salan ligands account for the majority of the structures studied thus far due to their ease of synthesis (Scheme 1, A and B).1,6–8,10–12 Breaking of the C2-symmetry was deemed to be a means to expand the repertoire of complexes for studies, inter alia, in biomimetics, magnetism, sensing, catalysis. This can be achieved by varying the salicylaldehyde derivative, amine linker or via amine N-functionalisation (Scheme 1, C and D).15 Although non-symmetric C1 ligands permit finer control over electronic and steric properties, solubility studies remain limited compared to similar symmetric structures. For salens, C1-ligands have been developed using modular synthetic strategies,16 but this approach is limited to scaffolds containing 1,2-diaminocyclohexane or 1,2-phenylenediamine backbones.16–18 Access to ethylene-based C1-salens can be achieved via metal templated synthesis involving first, the formation of a ‘half-unit’ Cu(II) complex, followed by condensation of a second salicylaldehyde derivative.19 However, metal templating limits significantly the scope of the ligands and complexes that can be studied. C1-salan and salalens based on the N,N′-dimethylethylenediamine backbone have been established and their complexes investigated in catalysis.7,20–22 Synthesis of non-symmetric type ligands requires two steps; mono-condensation of salicylaldehyde with a secondary diamine followed by N-alkylation with a bromomethyl derivative (Scheme 1, C and D).23,24 Future investigations of C1-salan ligands and complexes are greatly limited by the necessity for a secondary amine backbone and both salicylaldehyde and bromomethyl starting materials.
We recently reported that Cu(II) C2-salan and C2-salen based complexes promote A3 coupling reactions in open air.25 In order to expand this study, our first effort was to break the symmetry and modify the ligand scaffold in the C2-salan complexes. The simplest route towards Non-Symmetric C1-Salan Ligands (NSSL) containing secondary diamine (N–H) backbones is the reduction of the corresponding C1-salen (Scheme 1, E). Surprisingly, no complexes derived from ethylene-based NSSLs have been deposited in the CCDC,26 and, to the best of our knowledge, no ligands of this type have previously been reported. In this work, we present a two-step synthetic method that yields C1-NSSLs bearing two distinct salicylaldehyde derivatives. The presented simple synthetic strategy uses commercially available reagents, avoids, for the majority of cases, column chromatographic purification and allows for broad structural diversification.
Firstly, a salan ‘half-unit’ (Table 1) is prepared via the reductive amination of N-Boc-ethylenediamine and salicylaldehyde followed by Boc-deprotection (Table 1). The salan ‘half-unit’ can be synthesised on a multigram scale in very good yield (8.97 g, 78% over 2-steps). A second, different salicylaldehyde unit can then be introduced (Table 1) to yield NSSLs. In almost all cases, highly pure NSSLs precipitate as solids after quenching the reduction mixture with water and allowing the aqueous solution to stand overnight. Work-up and chromatography were only required for NSSL-2, which was isolated as an oil. NSSLs were isolated in moderate to good yields and characterised by 1H-NMR, 13C-NMR spectroscopy, HRMS and FTIR (see ESI†). The provided table enlists only ten examples in which R1 = R2 = H; however, the variations in the structure are almost endless. R3 and R4 were chosen for study as these sites are expected to impact complexation by altering pKa, inductive effect, or coordination sphere (R3) and inducing electronic effects (R4).
To further demonstrate the scope of the synthetic strategy, Cyclohexane based Non-Symmetric Salans Ligands (CyNSSL) were also synthesised starting from trans-N-Boc-1,2-cyclohexanediamine. Related symmetric ligands have been investigated as potential anti-cancer cytotoxic agents. Therefore, our novel non-symmetric ligands greatly increases the scope of possible structures accessible for anti-cancer research.27,28 A range of CyNSS ligands were synthesised (Table 2) and characterised with 1H-NMR, 13C-NMR, HRMS and FTIR (see ESI Fig. S1–S53†).
After successfully expanding the family of NSSLs, we attempted to develop a generic complexation protocol to yield the corresponding M-NSS complexes. It is well known that salan ligands can undergo metal-catalysed oxidative dehydrogenation,29,30 and the rate of this process is metal dependant; in organic solutions (Co(II) ≫ Ni(II) ≫ Cu(II)).29,31 We chose NSSL-1, NSSL-2 and NSSL-3, as they contain bulky substituents at the R3 position to sterically crowd the metal centre, as well as NSSL-4 and NSSL-5 that contain substituents at R4 that can induce further steric/electronic effects.
The complexation reactions of the corresponding cyclohexane (CyNSSL) analogues were also investigated. Bearing all these in mind, we chose to study complexations with Cu(II) ions to minimise the formation of oxidised derivatives. The general complexation protocol can be summarised in three steps; (1) heat-assisted complexation with CuCl2 in methanol under aerobic conditions, (2) purification of the resultant complexes with basified column chromatography to remove any free ligand, (3) crystallisation of the compounds under aerobic conditions, in various solvents followed by characterisation via single crystal X-ray crystallography, where possible (see ESI†).
During complexation, all the solutions turned a deep green colour upon the addition of CuCl2 other than for NSSL-3 (Table 3, entry 3), which turned to a dark brown colour and no complex could be isolated. The purified compounds were then characterised using CHN analysis, HRMS, FTIR, UV-Vis, TGA (see ESI Fig. S54–S74†). The complexes showed similar absorption patterns in their UV-Vis spectra with an intense metal to ligand charge transfer (MLCT) band at ∼380 nm and a broader d-d transition band at ∼550 nm (Fig. S72†). Where possible, thermogravimetric analysis was carried out to gain insight into the thermal stability of the formed complexes. Complexes derived from sterically bis-substituted bulky ligands (Table 3, entries 1 & 6) began to degrade at ∼100 °C. In contrast, all the other studied complexes were stable up to 175–250 °C (Fig. S73 and S74†). The general notion is that the complexes formed from CyNSSLs are more thermally stable. The formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, via oxidative dehydrogenation was observed when complexing ligands with tert-butyl substituents close to the coordination cage (Table 3; entries 1, 2, 6 & 7). The formation of the C
N bonds, via oxidative dehydrogenation was observed when complexing ligands with tert-butyl substituents close to the coordination cage (Table 3; entries 1, 2, 6 & 7). The formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds was evidenced in their HRMS (Fig. S63, S64, S67 and S68†) and by a characteristic strong absorption at ∼1650 cm−1 in their FTIR spectra (Fig. S54, S55, S58 and S59†). This observation may suggest that more sterically crowded ligands undergo oxidative dehydrogenation more readily then their less sterically encumbered counterparts.
N bonds was evidenced in their HRMS (Fig. S63, S64, S67 and S68†) and by a characteristic strong absorption at ∼1650 cm−1 in their FTIR spectra (Fig. S54, S55, S58 and S59†). This observation may suggest that more sterically crowded ligands undergo oxidative dehydrogenation more readily then their less sterically encumbered counterparts.
| Entry | Ligand | Yielda (%) | –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond(s)b N bond(s)b |
Crystallised species | Solid-state structuree | Coordination environment |
|---|---|---|---|---|---|---|
N.R = No Result.a Yield of product isolated after column chromatography.b Presence of characteristic imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) bond in FTIR and HRMS.c Crystallised from purified post-column product.d Crystallised from pre-column crude product.e L = salan ligand, L′ = salalen ligand, L′′ = salen ligand. N) bond in FTIR and HRMS.c Crystallised from purified post-column product.d Crystallised from pre-column crude product.e L = salan ligand, L′ = salalen ligand, L′′ = salen ligand. |
||||||
| 1 | NSSL-1 | 83 | Y | Salalenc | {Cu2L′2Cl2} | N2OCl2 |
| 2 | NSSL-2 | 41 | Y | N.R | N.R | |
| 3 | NSSL-3 | N.R | N.R | Saland | [CuL(H2O)] | N2O3 |
| 4 | NSSL-4 | 94 | N | N.R | N.R | |
| 5 | NSSL-5 | 79 | N | N.R | N.R | |
| 6 | CyNSSL-1 | 83 | Y | Salenc | [CuL′′] | N2O2 |
| 7 | CyNSSL-1EtOAc | — | — | Salalen | [CuL′] | N2O2 |
| 8 | CyNSSL-2 | 12 | Y | Saland | [CuL] | N2O2 |
| 9 | CyNSSL-3 | 56 | N | Salanc | {[CuL(H2O)0.5][CuL]} 3(H2O) (CH3OH) | N2O3 & N2O2 |
| 10 | CyNSSL-4 | 83 | N | Salanc | [Cu2L2] | N2O3 |
| 11 | CyNSSL-5 | 38 | N | Salanc | {[CuL][Cu2L2]} 2(MeOH) | N2O2 & N2O3 |
To further expand the scope and limitations of this generic complexation protocol, we chose only two ligands (NSS-1 and CyNSS-1) to investigate their complexation behaviour under anaerobic conditions with CuCl2. In both cases, we observed the Cu–salan product as the major product. This observation could be evidenced by IR (Fig. S75†) and the absence of the characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N band at 1600 cm−1 and HRMS. The latter studies showed a molecular ion peak with an isotopic distribution corresponding to the salan product (+2 compared to the original (see Fig. S76 & 77†). Notably, the HRMS of Cu-NSS-1 shows additional small peaks corresponding to the Cu–salen complex indicating that sample Cu-NSS-1 is subject to partial dehydrogenation upon exposure to O2 or during the column conditions. This process could be further corroborated by the presence of a small peak in the IR at 1640 cm−1 corresponding to the C
N band at 1600 cm−1 and HRMS. The latter studies showed a molecular ion peak with an isotopic distribution corresponding to the salan product (+2 compared to the original (see Fig. S76 & 77†). Notably, the HRMS of Cu-NSS-1 shows additional small peaks corresponding to the Cu–salen complex indicating that sample Cu-NSS-1 is subject to partial dehydrogenation upon exposure to O2 or during the column conditions. This process could be further corroborated by the presence of a small peak in the IR at 1640 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (Fig. S75†). The dehydrogenated product is not observed in the HRMS or FTIR of the Cu-CyNSS-1 sample, but the yield of the complexation is significantly lower.
N bond (Fig. S75†). The dehydrogenated product is not observed in the HRMS or FTIR of the Cu-CyNSS-1 sample, but the yield of the complexation is significantly lower.
X-ray quality crystals for the copper complexes were grown by suspending either the column purified complexes (Table 3, entries 1, 6, 8, 9 & 10) or crude, pre-column, material (Table 3, entries 2, 3 & 7), in various organic solvents and allowing the solutions to evaporate at ambient temperature under aerobic conditions. Crystallisation experiments were unsuccessful for only two cases (Table 3, entries 4 & 5), while for entry 2, low quality crystallographic data, not provided herein, suggest possible dehydrogenation (see ESI†).
Crystallographic studies reveals that in the solid-state, Cu–salan complexes (Table 3, entries 3, 8, 9, 10 & 11), Cu–salalen complexes (Table 3, entries 1 & 7) and Cu–salen complexes (Table 3, entry 6) are formed as a result of oxidative dehydrogenation (Fig. 1). This oxidative process is solely observed for complexes derived from sterically hindered ligands with tert-butyl substituents. Interestingly, crystallisation of the purified product obtained when complexing CyNSS-1 resulted in both salalen (Table 3, entry 7) and salen (Table 3, entry 6) complexes depending on the crystallisation solvent. Therefore, metal salt, ligand structure and solvent all can affect the oxidative dehydrogenation of salan-metal complexes. Future complexation procedures should be specifically tailored to the ligand used, and the desired complex and all of these parameters should be taken into account.
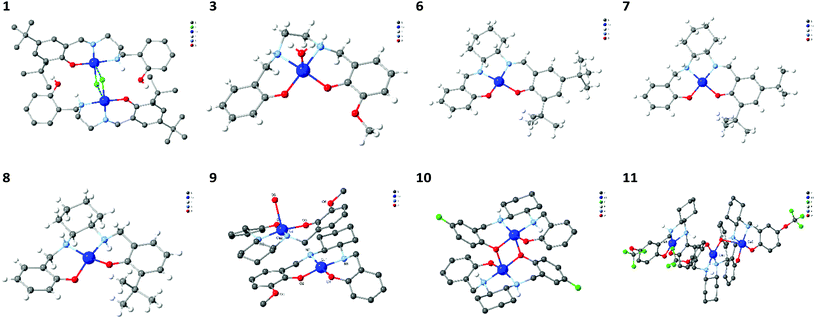 | ||
| Fig. 1 Solid-state structures of Cu(II) complexes summarised in Table 3. Colour coding Cu (blue), C (grey), O (red), N (light blue), Cl/F (green), H (white). In some compounds H-atoms are omitted for clarity. | ||
In the solid state, the Cu(II) complexes were observed to form either monomeric species (Table 3, entries 3, 6, 7, 8 & 9) or dimer species (Table 3, entries 1, 10 & 11). The monomeric species result from a planar arrangement of the nitrogen atoms and oxygen atoms of the ligand complexing to the central Cu(II) ion. The presence of apical solvent molecules results in a 5-cooriniate trigonal pyramidal geometry and can alter the stereochemistry around the amine nitrogen atoms (Table 3, entry 3). Two types of dimeric structure were observed with bridging occurring via the presence of chlorides ions (Table 3, entry 1) or direct coordination of a second Cu(II) via a phenoxide moiety (Table 3, entries 10 & 11). Complexation of NSS-1 resulted in an asymmetric dimer, bridged by two chlorides where one phenol unit remains protonated, forming a 6,5-fused ring system. HRMS data suggest that, in solution, this species is broken down (Fig. S63†). Complexes obtained from the cyclohexene-based ligands that contain electron-withdrawing groups, CyNSS-4 and CyNSS-5, revealed the formation of a dimeric stacked couples (Table 3, entries 9 & 10). In the stacked couples the electron deficient ring is stacked above the electron richer, unsubstituted ring, (R1 = R2 = H). Suitable crystals for the similar ethylene analogues could not be obtained despite our best efforts.
We have previously shown that C2-Cu(II)–salen and Cu(II)–salan complexes enable the A3 coupling transformation at room temperature in open air without the need for any additives.25 Spurred by the success of these efforts, the catalytic activity of the non-symmetric complexes in the synthesis of a simple propargylamine was investigated. Under the given experimental conditions (see ESI†), the Cu(II) complexes performed moderately with 1H-NMR conversions ranging from 27–55% (Table S15†). However, variation in the catalytic results exemplifies how future catalytic activity can be tuned by variation in the ligand structure. Despite poor catalytic results, the increased tunability now possible for CyNSSLs offers an opportunity to better understand the structure–activity relationship (SAR) relating to their use as anti-cancer agents.27,28 Potentially leading to the identification of new therapeutic candidates.
To conclude, we have characterised 15 examples of C1-salans with N–H backbones and investigated the use of ten of them to yield the corresponding Cu(II) complexes via a generic synthetic protocol. From the data above, it is evident that in the solid-state, the structure of the crystallised material depends on many factors. The complexation of NSSLs with metal ions cannot be generalised and instead seems to be reliant on the ligand being used and the desired complex. Further development of NSS-complexes may require complexation and crystallisation to take place under anaerobic conditions to avoid oxidative dehydrogenation. It can also be envisaged that adaptation of the complexation procedure could exploit the oxidative dehydrogenation process for the synthesis of novel C1-salen and salalen metal complexes. The possible variations in the ligand and complex framework with this approach are almost endless. A popular chemical vendor offers 100 different salicylaldehyde derivatives; thus, the adaptation of our protocol could allow for the synthesis of 4950 NSSLs from a single diamine backbone. Further variation in the metal centre or diamine backbone used would enable access to a huge array of NSS-complexes. Salan ligands and corresponding metal complexes are already used in a wide range of applications; therefore, our synthetic strategy will have manifold potential applications ranging from coordination chemistry, magnetism, sensing, catalysis to biology and biomedical investigations.
Author contributions
JD devised the project and idea with critical input from J.S and G.E.K. JD synthesised and characterised all ligands and complexes. G.E.K carried out X-ray analysis with help from JD. All authors contributed in the preparation of this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
G.E.K. and J.S. received funding from the School of Life Sciences, the University of Sussex (J.D. PhD fellowship). The National Crystallographic Centre is greatly acknowledged for the data for Cu(II)-NSS-1 and Cu(II)-NSS-3.32Notes and references
- P. G. Cozzi, Chem. Soc. Rev., 2004, 33, 410–421 RSC.
- M. Palucki, P. Hanson and E. N. Jacobsen, Tetrahedron Lett., 1992, 33, 7111–7114 CrossRef CAS.
- T. Katsuki, Coord. Chem. Rev., 1995, 140, 189–214 CrossRef CAS.
- L. Canali and D. C. Sherrington, Chem. Soc. Rev., 1999, 28, 85–93 RSC.
- N. S. Venkataramanan, G. Kuppuraj and S. Rajagopal, Coord. Chem. Rev., 2005, 249, 1249–1268 CrossRef CAS.
- C. Baleizão and H. Garcia, Chem. Rev., 2006, 106, 3987–4043 CrossRef PubMed.
- K. Matsumoto, B. Saito and T. Katsuki, Chem. Commun., 2007, 3619–3627 RSC.
- J. C. Pessoa and I. Correia, Coord. Chem. Rev., 2019, 388, 227–247 CrossRef CAS.
- K. Voronova, L. Homolya, A. Udvardy, A. C. Bényei and F. Joó, ChemSusChem, 2014, 7, 2230–2239 CrossRef CAS.
- T. Katsuki, Chem. Soc. Rev., 2004, 33, 437 RSC.
- T. Katsuki, Synlett, 2003, 281–297 CrossRef CAS.
- A. Erxleben, Inorg. Chim. Acta, 2018, 472, 40–57 CrossRef CAS.
- E. Y. Tshuva and D. Peri, Coord. Chem. Rev., 2009, 253, 2098–2115 CrossRef CAS.
- I. Correia, J. C. Pessoa, M. T. Duarte, M. F. M. Da Piedade, T. Jackush, T. Kiss, M. M. C. A. Castro, C. F. G. C. Geraldes and F. Avecilla, Eur. J. Inorg. Chem., 2005, 2005, 732–744 CrossRef.
- A. W. Kleij, Eur. J. Inorg. Chem., 2009, 2009, 193–205 CrossRef.
- E. J. Campbell and S. T. Nguyen, Tetrahedron Lett., 2001, 42, 1221–1225 CrossRef CAS.
- D. M. Boghaei and S. Mohebi, Tetrahedron, 2002, 58, 5357–5366 CrossRef CAS.
- X. Zheng, C. W. Jones and M. Weck, Chem. – Eur. J., 2006, 12, 576–583 CrossRef PubMed.
- L. Rigamonti, F. Demartin, A. Forni, S. Righetto and A. Pasini, Inorg. Chem., 2006, 45, 10976–10989 CrossRef CAS PubMed.
- A. Pilone, K. Press, I. Goldberg, M. Kol, M. Mazzeo and M. Lamberti, J. Am. Chem. Soc., 2014, 136, 2940–2943 CrossRef CAS PubMed.
- A. Pilone, N. De Maio, K. Press, V. Venditto, D. Pappalardo, M. Mazzeo, C. Pellecchia, M. Kol and M. Lamberti, Dalton Trans., 2015, 44, 2157–2165 RSC.
- M. Cozzolino, V. Leo, C. Tedesco, M. Mazzeo and M. Lamberti, Dalton Trans., 2018, 47, 13229–13238 RSC.
- A. Huber, L. Müller, H. Elias, R. Klement and M. Valko, Eur. J. Inorg. Chem., 2005, 2005, 1459–1467 CrossRef.
- A. Cohen, A. Yeori, J. Kopilov, I. Goldberg and M. Kol, Chem. Commun., 2008, 2149–2151 RSC.
- S. I. Sampani, V. Zdorichenko, M. Danopoulou, M. C. Leech, K. Lam, A. Abdul-Sada, B. Cox, G. J. Tizzard, S. J. Coles, A. Tsipis and G. E. Kostakis, Dalton Trans., 2020, 49, 289–299 RSC.
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef.
- J. Gao, Y. G. Liu, Y. Zhou and R. A. Zingaro, ChemMedChem, 2007, 2, 1723–1729 CrossRef CAS PubMed.
- J. Gao and R. A. Zingaro, MedChemComm, 2012, 3, 326–332 RSC.
- A. Böttcher, H. Elias, E. G. Jäger, H. Langfelderova, M. Mazur, L. Müller, H. Paulus, P. Pelikan, M. Rudolph and M. Valko, Inorg. Chem., 1993, 32, 4131–4138 CrossRef.
- A. Böttcher, H. Elias, L. Müller and H. Paulus, Angew. Chem., Int. Ed. Engl., 1992, 31, 623–625 CrossRef.
- I. Correia, A. Dornyei, T. Jakusch, F. Avecilla, T. Kiss and J. C. Pessoa, Eur. J. Inorg. Chem., 2006, 2006, 2819–2830 CrossRef.
- S. J. Coles and P. A. Gale, Chem. Sci., 2012, 3, 683–689 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR, 13C-NMR, HRMS, FTIR, TG, IR, UV-Vis. CCDC 2086033–2086040. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1dt01950c |
| This journal is © The Royal Society of Chemistry 2021 |