Open Access Article
Open Access ArticleNon-noble MNP@MOF materials: synthesis and applications in heterogeneous catalysis
Nejat Redwan
Habib
 a,
Esther
Asedegbega-Nieto
a,
Esther
Asedegbega-Nieto
 b,
Abi M.
Taddesse
a and
Isabel
Diaz
b,
Abi M.
Taddesse
a and
Isabel
Diaz
 *c
*c
aDepartment of Chemistry, Haramaya University, Haramaya, Ethiopia
bDepartamento de Química Inorgánica y Técnica, Facultad de Ciencias, UNED, c/Senda del Rey no. 9, 28040, Madrid, Spain
cInstituto de Catálisis y Petroleoquímica, CSIC, c/Marie Curie 2, 28049 Madrid, Spain. E-mail: idiaz@icp.csic.es; Tel: +34 91 585 4785
First published on 15th June 2021
Abstract
Transition metals have a long history in heterogeneous catalysis. Noble or precious transition metals have been widely used in this field. The advantage of noble and precious metals is obvious in ‘heterogeneous catalysis’. However, the choice of Earth abundant metals is a sustainable alternative due to their abundance and low cost. Preparing these metals in the nanoscale dimension increases their surface area which also increases the catalytic reactions of these materials. Nevertheless, metals are unstable in the nanoparticle form and tend to form aggregates which restrict their applications. Loading metal nanoparticles (MNPs) into highly porous materials is among the many alternatives for combating the unstable nature of the active species. Among porous materials, highly crystalline metal–organic frameworks (MOFs), which are an assembly of metal ions/clusters with organic ligands, are the best candidate. MOFs, on their own, possess catalytic activity derived from the linkers and metal ions or clusters. The catalytic properties of both non-noble metal nanoparticles (MNPs) and MOFs can be improved by loading non-noble MNPs in MOFs yielding MNP@MOF composites with a variety of potential applications, given the synergy and based on the nature of the MNP and MOF. Here, we discussed the synthesis of MNP@MOF materials and the applications of non-noble MNP@MOF materials in heterogeneous catalysis.
1. Introduction
Heterogeneous catalysis based on transition metal nanoparticles is becoming an important field in many industrial applications.1–4 The nanosize effect makes these materials exhibit different properties compared to the corresponding bulk materials.5–8 The exposure of active sites increases due to the high surface area of these materials.9 Noble and precious metal nanoparticles (MNPs) such as platinum group metals for heterogeneous catalysis have been explored in many research studies.10–17 It is stated that these materials have unique electronic, chemical, and optical properties depending on the type of noble metal and synthesis conditions.18–20 On another note, non-noble MNPs have attracted attention in heterogeneous catalysis from the perspective of cost as well as their environmentally friendly nature.21 Also, by exploring different synthetic conditions and selecting the appropriate non-noble metal, researchers are able to develop non-noble MNP based catalysts which are as efficient as noble MNP based catalysts for heterogeneous catalysis applications.22Besides their high catalytic activities, working with non-noble MNPs is not an easy task due to their thermodynamically unstable nature. Owing to their instability, they tend to form larger aggregates, leading to a decrease in the number of active sites and hence a decrease in catalytic activity due to the lowering of high surface area to volume ratios.23,24 A multitude of options have been proposed to inhibit such aggregations. The focus has been centered on porous materials used as a support for MNPs to decrease aggregation. Mahugo et al. stated that using a support is also important for reducing sintering of MNPs during synthesis or catalytic activity, which increases the reuse of the catalysts as well as protects active sites.23 Porous silica and carbon, zeolites and metal–organic frameworks (MOFs) have so far been reported as a support for MNPs.25
Within the known porous materials, research on MOFs has been growing exponentially.26,27 MOFs are an assembly of organic ligands with metal ions/clusters.28 The possibility of using different organic ligands and metal clusters or ions is partly responsible for the vast variety of MOFs designed since their first synthesis in 1999.28,29 MOFs have intrinsic properties for gas adsorption and separation30,31 and heterogeneous catalysis32,33 and are therefore applied in reactions such as photocatalysis34,35 and biocatalysis36–39 and even in the field of biomedicine.40
Experiments show that combining MNPs with MOFs creates different synergies, which overcome the drawbacks of the individual components and increase the catalytic activities of the composites.26,41
This paper highlights the synthesis of MNP@MOF composites with emphasis on the possible synergistic effects derived from combining non-noble MNP@MOFs reported in different works which have already been published so far.
2. MNP@MOF composite synthesis
To date, different synthetic methods have been already explored for MNP@MOF composite synthesis. The synthesis conditions are contingent upon the type of metal and MOF involved as well as the intended applications of the formed composites. Generally, three approaches have been discussed for the synthesis of MNP@MOF composites, as depicted in Fig. 1, wherein different synthetic procedures have been schematized. The first approach called “ship in bottle” consists of placing the preformed MOF into a metal precursor, usually a metal salt solution, for MNP synthesis followed by reducing the metal precursor to its metallic state or the solid state reaction that involves the mixing of the metal salt precursor with the MOF followed by the necessary steps in order to reduce the metal precursor to its metallic form. The second approach known as “bottle around ship” involves the addition of the preformed MNPs during the synthesis of the MOF.42 The last approach, the “one step synthesis”, as its name implies is based on a one step synthetic procedure whereby both MNP and MOF precursors are mixed resulting in the corresponding composite MNP@MOF.43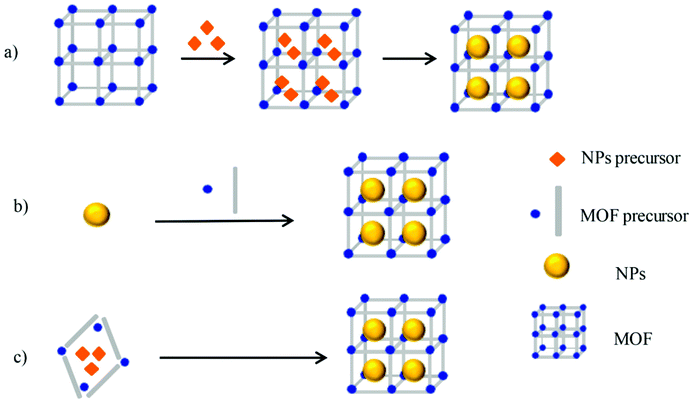 | ||
| Fig. 1 (a) Ship-in-bottle, (b) bottle-around-ship and (c) one-step synthesis. Reproduced from ref. 43 with permission from MDPI, copyright 2017. | ||
The ship-in-bottle approach includes procedures such as solid grinding,44,45 liquid impregnation,46 double solvent impregnation,47,48 chemical vapour deposition49,50 and thermal decomposition.51,52 These methods generally afford MNPs supported on the MOF surface, though the successful incorporation of MNPs in MOF pores has also been reported.53 Controlling the guest morphology, location and structure proves to be challenging when using these synthesis routes.43 This challenge emerges from the microstructure of the MOF, as determined by inner surface characterization, interactions of the MOF with MNPs or the MNP precursor, the pore surface environment of the MOF and the progression of MOF pore wetting and filling.42 In these methods, to control the location and size of MNPs in the MNP@MOF composite, the following criteria should be considered among many others. These include prior information about the cavity of the MOF with which the metal precursor will be mixed and knowledge of the percent weight loading of MNPs, and the interaction between the MOF cavity and metal precursor should also be studied.54
Bottle-around-ship approaches encompass a self-sacrificing template method48 and emulsion-based interfacial synthesis.55 Compared to the ship-in-bottle approaches, location and size control of MNPs can be achieved. The main reason for this is that, unlike the ship-in-bottle approaches, these synthesis methods encapsulate the preformed nanoparticles in the MOF precursor materials. However, the demerit is that there is a possibility of affecting the MOF structure when using these methods and not all types of MOFs are suitable for such procedures; so far, UiO-66 and ZIF-8 are mostly reported.42,43
Unlike the above stepwise procedures, one-step synthesis is known for its facile and direct approach.56,57 Kinetic control is necessary since the nucleation and growth of the MOF and MNP are obviously different.58 In contrast, it would be difficult to obtain MNPs encapsulated in the MOF framework. Surfactants are often applied to obtain small MNPs which also hinder the generation of MOFs.59
In any of the three methods, the final question would be whether the MNPs have been successfully and homogenously incorporated into the porous network of the MOF without occupying all available porosity, or avoiding physical mixtures of surface metal phase formation. The challenge in studying MNP@MOF composites is the difficulty of revealing the growth of MNPs in the pores of the MOFs via convectional characterization techniques, thus, advanced characterization techniques, including transmission electron microscopy and related spectroscopic techniques may be required to circumvent this challenge.23,60,61
3. Synergetic effects of the MNP@MOF composite
Formation of the MNP@MOF composite triggers the corresponding synergy depending on the type of MOF, MNP and the formed composite.42The first synergy which will be explained is surface plasmon, in which photons transform into heat when surface plasmon active materials are simulated by light. This type of synergy is observed on the MNP@MOF composite when MNP is successfully encapsulated in the MOF framework. Thus, surface plasmon active MNPs, once irradiated by light, heat up the surrounding MOF to discharge the material adsorbed on the framework.55,62,63 This synergy is mostly reported for adsorption–desorption and drug release applications.42,54 The surface plasmonic effect of noble MNPs, such as Pt, Pd, Au and so on, is common as reported in different research publications so far.64–66 For non-noble MNPs, Cu is an example which exhibits a surface plasmonic effect.67,68
The second synergy is called size selectivity, which is detected when MNPs are inside the MOF pores. In this synergy the MOF selectively permits the entrance of substrates of smaller size than the MOF's openings but blocks larger substrates, and MNPs inside MOF pores catalyze the reaction.69–80 This type of synergy is extremely useful especially on the industrial scale for selective catalysis involving organic compounds.81 A non-noble MNP@MOF with such type of synergy was reported by Nakatsuka et al.82
The third type of synergy, which is common and appears in most MNP@MOF composites, is MNPs as the active center and the MOF as the stabilizer. This synergy appears irrespective of whether the formed MNPs are outside or inside the framework. This most fundamental synergy has the advantage of preventing aggregation and sintering of MNPs which improves their catalytic activity.83–89
The last type of synergistic effect reported is called electron or energy transfer synergy. This synergy is most significant when using a semi-conducting type of MOF, in which light intensity adjustment can generate electrons and lead to energy transfer from the MOF to MNPs or vice versa. The ligands and metal ions or clusters of MOFs can affect the catalytic activity of the encapsulated or supported MNPs.42,90
In summary, there is sufficient evidence in the literature on the synergy between MNP@MOFs to improve the properties of the composite formed, yet studies to explain this effect in depth are scarce probably due to the difficulty in characterizing such complex systems.
4. Non-noble MNP@MOF composites for heterogeneous catalysis
MOF composite materials are still in the development stage for heterogeneous catalysis. Nonetheless, it has been confirmed so far that MOF composites employed as heterogeneous catalysts have remarkable catalytic actions, reusability and stability.43 Noble MNP@MOF composites for heterogeneous catalysis have been published in many research publications. Non-noble MNP@MOF composites have recently attracted attention in heterogeneous catalysis. Non-noble MNP@MOF composite catalysts are studied in catalytic pollutant removal applications, such as CO and CO2 conversions into HCOOH, CH4, CH3OH, among others.53,91 Catalytic organic reactions and H2 production mostly from chemical hydrides are also experimented with using non-noble MNP@MOF composites.21,924.1. Catalytic CO2 reduction
The CO2 concentration in the environment is constantly increasing. Its implication in the greenhouse effect and long-term climate change is well known. For this reason, CO2 conversion and treatment have been carried out for decades since its negative environmental impact was discovered. CO2 conversion into other industrial chemicals such as HCOOH, CH3OH, CH4 and cyclic carbonate has been experimented with using many catalytic materials.91CO2 methanation into CH4via hydrogenation reaction accounts for its largest hydrogenation product. It is a highly exothermic reaction and can be performed under atmospheric pressure. Noble metal catalysts such as Ru and Rh supported on porous oxides such as SiO2 and Al2O3 are among the noble metal catalysts studied for this reaction.93,94. As for non-noble MNP, Ni has been tested on the same support materials.93 Ni nanoparticles (NPs) supported on the MOF, offer advantages in the perspective of cost and availability compared with noble metals. At the same time, using a highly porous MOF as the support aids in preventing sintering of the active non-noble MNPs and their aggregation.53
Zhen et al. prepared a xNi@MOF-5 series of active catalysts for CO2 methanation by the impregnation method (x = % weight of Ni loading). The benchmark catalyst Ni/SiO2 was used for catalytic comparison with Ni@MOF-5 for methane synthesis.91 It is stated that the catalytic reaction was carried out in the 180 to 320 °C temperature range to avoid the reverse water gas shift (RWGS) reaction. In the methane production process if the temperature is above 320 °C, the RWGS reaction occurs which results in the formation of CO2 as a byproduct. With the reference catalyst (Ni/SiO2), CO2 conversion is 34% at 280 °C while it was 47.2% under the same condition with Ni@MOF-5. 75.09% CO2 conversion was obtained with 10Ni@MOF-5 at 320 °C, while the selectivity towards CH4 was 100%. The specific surface area of 10Ni@MOF-5 is 2961 m2 g−1 which is very high and it has a pore volume of 1.037 cm3 g−1, compared to 10Ni/SiO2 with a specific surface area of 155.6 m2 g−1 and pore volume of 0.8245. This resulted in high Ni NP dispersion (41.8%) in 10Ni@MOF-5 while it is 33.7% in 10Ni/SiO2. The reason behind the high catalytic activity of xNi@MOF-5 is the uniform and highly dispersed Ni NPs in MOF-5.91
The double solvent impregnation method (DSM) and multiple impregnation (MI) are used to synthesize Ni@MiL-100(Cr) composites for CO2 methanation.53 Here, the study concentrates on the effect of synthesis methods on catalytic methane formation under the same conditions. Ni@MOF synthesized by the DSM, with 20 wt% Ni loading, exhibited higher CO2 methanation than the corresponding MI synthesized Ni@MIL-101 composites at 300 °C. The possible explanation given is that Ni@MIL-101 synthesized by the DSM has the Ni (111) facet exposed for the catalytic reaction while that exposed for MI synthesized is Ni (200) (Fig. 2). The potential energy barrier for the Ni (111) facet, which is 10.0 kcal mol−1, calculated by DFT was lower compared with that of Ni (200) (20.3 kcal mol−1) for the dissociation of CO2 into adsorbed carbon monoxide and adsorbed oxygen. The dispersion of Ni NPs is 42.3% for 20Ni@MIL-101(Cr) (DSM) and (41.6%) for 20Ni@MIL-101(Cr) (MI). Furthermore, 20Ni@MIL-101(Cr) synthesized by the DSM at a reaction temperature of 280 °C showed a CH4 TOF (1.2 × 10−3 s−1) value double that of the benchmark catalyst 20Ni/γ-Al2O3 at the same temperature.53
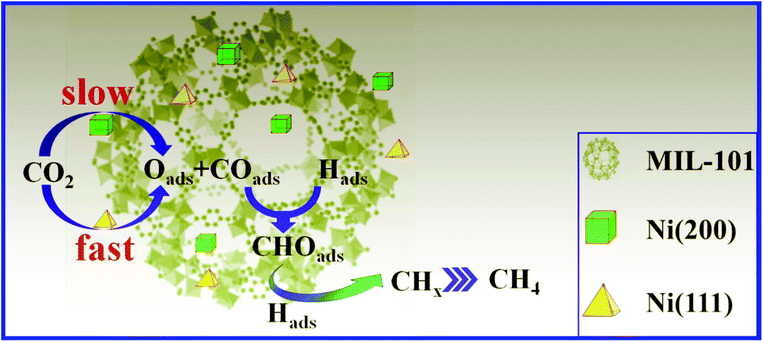 | ||
| Fig. 2 Ni@MiL-101 for CO2 methanation over the Ni (200) facet and Ni (111) facet. Reproduced from ref. 53 with permission from Elsevier, copyright 2017. | ||
Cu nanoparticles encapsulated in UiO-66(Zr), Cu nanocrystals (18 nm), have been utilized for the hydrogenation of CO2 to methanol. CO2 hydrogenation over Cu@UiO-66 at 175 °C and 10 bar using a H2/CO2 molar ratio of 3 showed an initial turnover frequency (TOF) for methanol (MeOH) formation of 3.7 × 10−3 s−1, which is an eight-fold increase in the yield with respect to the reference catalyst Cu/ZnO/Al2O3 (0.45 × 10−3 s−1), with 100% selectivity to methanol. UiO-bpy is another UiO-66(Zr) MOF family, which encapsulated ultrafine Cu/ZnOx to decrease aggregation of Cu NPs and phase separation of ZnOx and Cu. It was tested for CO2 hydrogenation with a H2/CO2 molar ratio of 3 at 40 bar and 250 °C. CuZn@UiO-bpy showed a space–time yield to MeOH (STYMeOH) of 2.59 gMeOH kgCu−1 h−1 at a gas hourly space velocity (GHSV) of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, which was higher than the value (STYMeOH) of 0.83 gMeOH kgCu−1 h−1 obtained with the ternary Cu/ZnO/Al2O3 commercial catalyst in a 6/3/1 ratio under the same experimental conditions. CuZn@UiO-bpy shows 100% selectivity and higher stability (>100 h), when compared with the Cu/ZnO/Al2O3 catalyst which offers 54.8% methanol selectivity.95,96
000 h−1, which was higher than the value (STYMeOH) of 0.83 gMeOH kgCu−1 h−1 obtained with the ternary Cu/ZnO/Al2O3 commercial catalyst in a 6/3/1 ratio under the same experimental conditions. CuZn@UiO-bpy shows 100% selectivity and higher stability (>100 h), when compared with the Cu/ZnO/Al2O3 catalyst which offers 54.8% methanol selectivity.95,96
From the above research works in the catalytic conversion of CO2, it was observed that the use of MOFs as a support for non-noble MNPs results in high dispersions of catalytically active MNPs compared with a traditional oxide support, such as Al2O3 and SiO2. High dispersion of the catalytically active material in supports favors high reaction performance. This is further evidenced by Zhen et al., on the same non-noble MNPs with different supports, where higher catalytic activity is obtained for Ni@MOF-5 compared with Ni/SiO2 under the same reaction conditions.91
Gutterød et al. synthesized Pt NPs encapsulated in UiO-67(Zr) for methanol formation from CO2.97 It is reported that when pressure is increased from 1–8 bar, at 170 °C, a TOF of 0.01 s−1 for methanol formation is obtained and selectivity to methanol also increased from 3 to 19% at a 1/6/3 CO2/H2/He feed molar ratio.97 The TOF of methanol formation is higher than that stated for Cu@UiO-66 at 175 °C and 10 bar pressure, as reported by Rungtaweevoranit et al., but the selectivity to methanol is higher with Cu@UiO-66 at 175 °C and 10 bar. However, it is difficult to compare both results, unless it is carried out using the same optimization techniques96,97.
Table 1 summarizes the catalytic activity of Ni NPs loaded on different types of MOFs for methane formation from CO2. Generally, Ni NPs are possible substitutes for noble MNPs in MNP@MOF for methane synthesis from CO2.
4.2. Catalysis for organic compound synthesis
Organic reactions are key procedures that are extensively carried out in industry. Catalytic reactions such as selective oxidation of various alcohols are observed as the most fundamental reactions in organic chemistry.100 Synergistic effects, such as the size selective nature of MOFs and catalytic activity of MNPs make MNP@MOF composite catalysts suitable to participate in highly selective reactions which improve the outcome of these industrial procedures.43 Noble metals such as Pt and Pd dispersed on high surface area supports are used for many organic compound reactions, such as organic compound hydrogenation.101Here, the focus has been placed on non-noble MNP@MOF composites for hydrogenation and oxidation reactions of organic compounds. Zhou et al. stated that supported nano-sized nickel particles were found to be effective in hydrogenation reactions.102 Cu and Ni have been investigated in several research publications as non-noble MNP substitutes in the form of MNP@MOF composites for hydrogenation and oxidation reactions. Table 2 summarizes a list of Cu and Ni NP@MOF composites for hydrogenation and oxidation reactions of organic compounds.
| Catalyst | Type of reaction | Reaction | Reductant/oxidant and solvent | Ref. |
|---|---|---|---|---|
| Cu(II)/Cu(0)@UiO-66-NH2 | Hydrogenation | Styrene to ethylbenzene | N2H4.H2O in ethanol | 104 |
| Ni@MesMOF-1 | Hydrogenation | Styrene to ethylbenzene | H2 gas in methanol | 103 |
| Ni@MesMOF-1 | Hydrogenation | Nitrobenzene to aniline | NaBH4 in methanol | 103 |
| Cu/Cu-BTC | Hydrogenation | Styrene to ethylbenzene | N2H4 in H2O2 | 105 |
| Cu/Cu-BDC | Hydrogenation | Styrene to ethylbenzene | N2H4 in H2O2 | 105 |
| Cu(II)/Cu(0)@UiO-66-NH2 | Oxidation | Cyclohexene to cyclohexen-1-one and 2-cyclohexen-1-ol | tert-Butyl hydroperoxide | 104 |
| Cu/MOF-808(Ce) | Oxidation | Cyclohexane to cyclohexanol/cyclohexanone | tert-Butyl hydroperoxide | 90 |
| Cu/MOF-808(Zr) | Oxidation | Cyclohexane to cyclohexanol/cyclohexanone | tert-Butyl hydroperoxide | 90 |
| Cu/MOF-808(Ce) | Oxidation | CO to CO2 | O2 | 90 |
| Cu/MOF-808(Zr) | Oxidation | CO to CO2 | O2 | 90 |
| Ni/NMOF-Ni | Reduction | 4-Nitrophenol/4-aminophenol | NaBH4 | 106 |
Based on the results summarized in Table 2, various remarks can be made. Supported Ni NPs loaded in the pores of a mesoporous MOF (MesMOF-1) showed high catalytic activity for the hydrogenation of styrene and nitrobenzene. At room temperature, styrene to ethyl benzene conversion was completed in 4 h in methanol medium under H2 reduction. When nitrobenzene was used as the reactant, the reaction was completed within 15 minutes employing NaBH4 as the hydrogen source in methanol solvent. Even after three cycles of reactions, pure aniline formation was confirmed which is an indication of the stability of the framework.103
Cu loaded in UiO-66 MOF, namely Cu(II)@UiO-66-NH2 and Cu(0)@UiO-66-NH2 were used for olefin oxidation and hydrogenation. They served as efficient heterogeneous catalysts under mild experimental conditions. At ambient temperature, styrene hydrogenation is completed after 15 minutes of reaction with a quantitative yield. This makes them potential candidates in providing an alternative to the corresponding conventional noble metal catalysts.104
4.3. Photocatalysis
The basic photocatalytic process includes absorption of light by the semiconducting medium to form electrons (e−) and holes (h+); movement and separation of e− and h+ to the surface of the photocatalyst; followed by oxidation reaction by h+ and reduction reaction by e−. Therefore, increasing light absorption and creating high separation of e− and h+ should be considered when choosing materials as photocatalysts.34,107In MNP@MOF composites, depending on the type of MOF and MNPs, either of the two (MOF or the MNPs) can act as a semiconducting material. The MOF metal ions, clusters or organic linkers can be tailored to obtain a semiconducting MOF. Amino containing ligands are well known for this action. In some types of MNP@MOF composites, photoactive MNPs can be loaded in the pores of the MOF as guest materials. Energy or electron transfer synergy can take place which could be advantageous for the e− and h+ separation during photocatalytic reactions. In Fig. 3 a representation of the possible energy or electron transfer can be seen as indicated in a blue bipodal rectangle from the MOF linker to the encapsulated guest which could be an MNP or the metal nodes of the MOF (pictured in red). There is also the possibility of the MOF acting simply as a support and not actively participating in the photocatalytic reactions.34,108
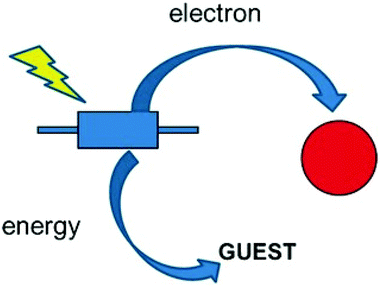 | ||
| Fig. 3 Energy or electron transfer synergy of MNP@MOF composites. Reproduced from ref. 109 with permission from Wiley, copyright 2016. | ||
There are stability concerns when using MOF materials for photocatalysis especially under moderate acidic and basic conditions. MOFs such as UiO-66(Zr) and zeolitic imidazolate frameworks (ZIFs) are known as stable MOFs under these conditions. The high affinity of zirconium towards oxygen ligands in UiO-66(Zr) and the strong bond of anionic nitrogen containing ligands in ZIFs increase the stability of such MOF families.34,110
TiO2@Cu-BTC was synthesized from the titanium(IV) isopropoxide metal precursor by the hydrolysis method under hydrothermal conditions. The photocatalytic reaction of this material was tested for methylene blue (MB) degradation. This material exhibited fast MB degradation compared with the commercial TiO2-P25 photocatalyst. It is stated that the high porosity of Cu-BTC (BTC = benzene-1,3,5-tricarboxylate) contributed to the high contact surface area which increases the photocatalytic activity compared with the benchmark TiO2 nanopowder (Degussa P25) catalyst.111
The photocatalytic degradation of MB over core–shell Fe3O4@MIL-100(Fe), as a Fenton-like catalyst, under solar irradiation is shown in Fig. 4. The study compared the photocatalytic performance of the pure MOF and the composite Fe3O4@MIL-100(Fe) counterpart. Here, the MIL-100(Fe) is acting as the photoactive material which means the source of photoinduced e− and h+. As described, the Fe3O4 is spherical in shape with a particle size of 200 nm obtained by TEM for all samples. It is stated that, irrespective of the MOF thickness (10, 20 and 40 cycles which correspond to 10, 50, and 200 nm respectively), the degradation efficiency of Fe3O4@MIL-100(Fe) is higher than that of pure MIL-100(Fe). This is because the presence of a Fe3O4 core relayed the photoinduced h+ of the MOF shell and hence increased the reaction of the e− with H2O2 which results in the formation of hydroxyl radicals. In general, it can be discussed that in this system the production of hydroxyl radicals as a powerful oxidant is enhanced by decreasing e− and h+ recombination which increases the photocatalytic degradation of the MB dye. It is confirmed that without the presence of H2O2 the MB degradation efficiency is very low which indicates that this catalyst works better as the Fenton-like catalyst. An independent study of the effect of the MOF thickness in the core–shell structure of Fe3O4@MIL-100(Fe) described that the optimal thickness for higher activity was found at 20 cycles. The possible reason given was, the MOF thickness obtained from 20 cycles is not too thick to allow the h+ formed from the MOF shell to enter the Fe3O4 core but thick enough to relay the h+ so the e− could react effectively with H2O2. Finally, the magnetic nature of the Fe3O4@MIL-100(Fe) facilitates the easy separation of the photocatalyst from the solution.112
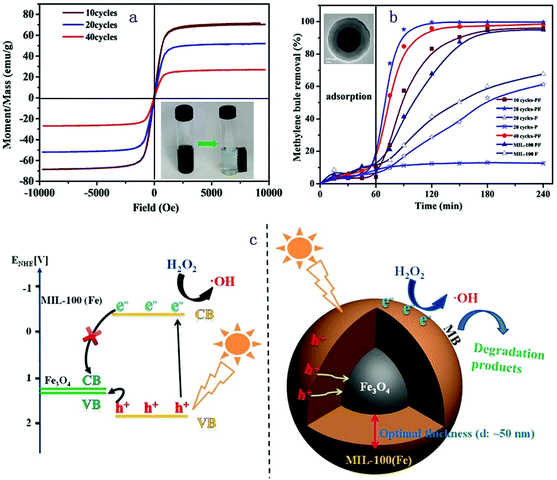 | ||
| Fig. 4 Proposed mechanism for the photo-Fenton like catalyst. Reproduced from ref. 112 with permission from Wiley, copyright 2015. | ||
Obtaining H2 from water splitting has been researched in depth. It is a sustainable way of obtaining hydrogen compared with other possible methods. In the photocatalytic hydrogen evolution reaction (HER), obtaining H2 greatly depends on the co-catalyst used in the system which can reduce the overpotential of the HER.113,114 Pt group metals possess low overpotentials for the HER and have been used as co-catalysts for H2 production from H2O.115 However, it is problematic to scale-up the applications of Pt group catalysts because of their low availability and high cost. It is therefore necessary to find Earth abundant HER co-catalysts that are highly active, stable, low cost and which can show low overpotential for the HER.109,114,116
Ni has been studied as a non-noble metal co-catalyst for the HER. Unlike noble MNPs, Ni NPs easily agglomerate giving rise to aggregates, thus leading to low dispersion and hence, low activity. Therefore, to retain the high activity of the Ni NP catalyst, it should be dispersed in highly porous materials with the aim of obtaining well dispersed active sites. Loading Ni in MOF-5 results in highly dispersed Ni nanoparticles, plus the 3D channel MOF-5 structure provides better collection of charge and movement. Therefore, if highly dispersed and small sized MNPs in a MOF is accomplished, then it would be possible to prepare a non-noble metal catalyst (such as Ni NPs) with low overpotential for the HER.114
Zhen et al.114 developed Eosin Y (EY) sensitized Ni@MOF-5 and Pt@MOF-5 and compared the H2 evolution reaction with these catalysts. Fig. 5 shows a mechanism for photocatalytic H2 evolution over EY sensitized Ni@MOF-5 with triethanolamine (TEOA) as a sacrificial donor, under visible light irradiation. Ni@MOF-5 was prepared by the impregnation method followed by in situ chemical reduction methods. A specific area of 2961 m2 g−1 was measured for Ni@MOF-5 which indicates the Ni NPs do not block the framework of the MOF-5 (2973 m2 g−1), in fact it has a high specific surface area. This is because it was possible to achieve small Ni NPs of only 9 nm. The H2 generation of EY-MOF-5, EY-Ni-nanoparticles and EY-Ni@MOF-5 was reported as 9.67, 49.9 and 302.2 μmol under 2 h irradiation at λ ≥ 420 nm, respectively. It is explained that there is a possible synergy in EY-Ni@MOF-5 as MOF-5 might drive the photogenerated e− from excited EY to the hydrogen evolution active site, in this case Ni NPs, which in turn enhances the photocatalytic hydrogen evolution efficiency. It is stated that hydrogen evolution rates of EY-Ni@MOF-5 are comparable to those of EY-Pt-@MOF-5. EY-Ni@MOF-5 showed a little higher H2 evolution amount in the first 1 h; however, the total H2 evolution amount is just a little lower than that of EY-Pt-@MOF-5. The maximum H2 evolution was 302.2 and 353.7 μmol for EY-Ni@MOF-5 and EY-Pt-@MOF-5, respectively, under 2 h irradiation (λ ≥ 420 nm). It is reported that the as-synthesized Ni@MOF has a longer fluorescence lifetime, larger transient photocurrent and shows a low overpotential of −0.37 V. The apparent quantum efficiency (AQE) of EY-Ni@MOF-5 was also reported as 16.7% under 430 nm illumination.114
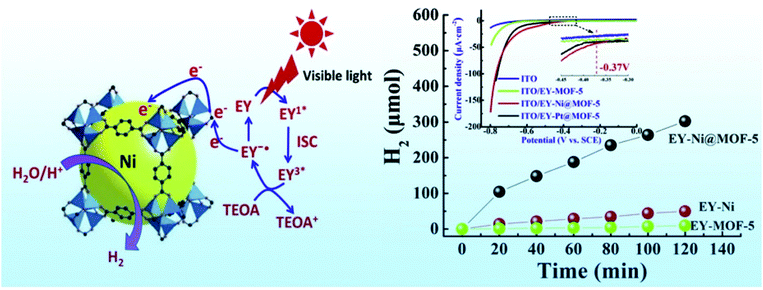 | ||
| Fig. 5 Photocatalytic H2 synthesis over EY sensitized Ni@MOF-5 with TEOA. Reproduced from ref. 114 with permission from Elsevier, copyright 2016. | ||
Catalytic hydrogen generation from liquid chemical hydrides, such as aqueous formic acid (HCOOH),117 ammonia borane (NH3BH3)21,92,118 and hydrazine (N2H4)119 have been reported by MNPs encapsulated in MOF pores. It is stated that, dispersed MNPs inside the MOF could result in enhanced catalytic activity in which the MOF with a high specific surface area and tunable pore size could control the size of MNPs in the confined cavities.43 Wen et al. stated that with the assistance of visible light irradiation an increased hydrogen evolution from ammonia borane is obtained with Ni@MIL-101.21Table 3 summarizes the catalytic activities of noble-metal free MNP loaded MOFs for hydrogen generation from NH3BH3.
4.4. Electrocatalysis
Electrolytic processes such as the oxygen reduction reaction (ORR), oxygen evolution reaction (OER), hydrogen evolution reaction (HER) and carbon dioxide reduction play tremendous roles in energy converting devices such as fuel cells.120MOFs have various characteristics which make them suitable for electrocatalytic reactions. The highly porous nature of MOFs yields high internal surface area, thereby increasing the diffusion of substrates which in turn increases catalytic activity.121 It is possible to create active sites using MOFs deriving their catalytic activity from metal node sites or from the organic linker side.122 However, MOFs have poor conductivity when used directly for the electrocatalytic process.121 So MNPs in the MNP@MOF composite would be a likely solution for the poorly conductive nature of MOFs as well for increasing the stability of the structure.123 In Fig. 6 some examples of the applications of these materials in electrocatalytic processes are illustrated.
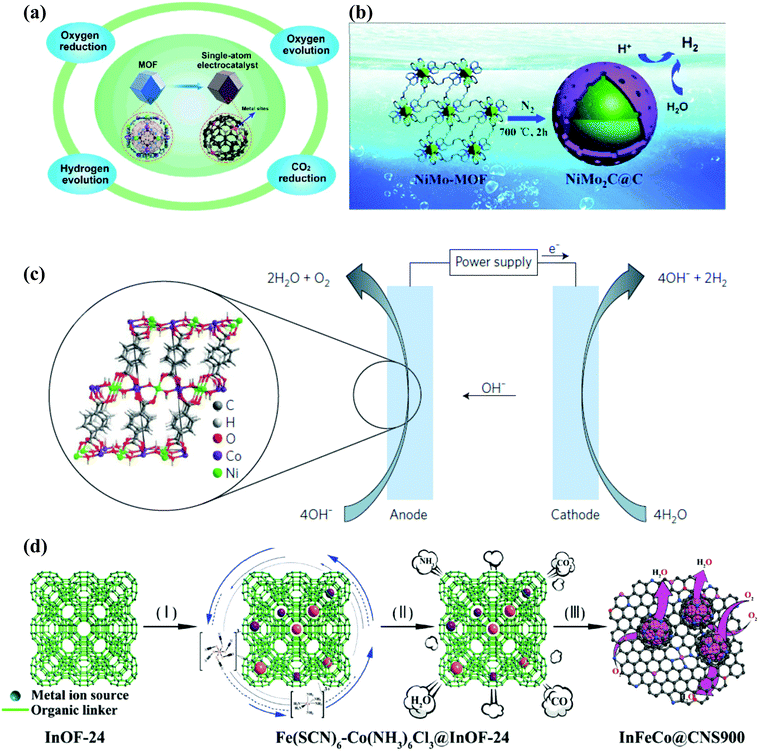 | ||
| Fig. 6 Successful electrocatalytic applications aided by MOF materials: (a) a MOF based single atom metal material employed in electrocatalysis. Reproduced from ref. 124 with permission from Springer, copyright 2019. (b) Graphene coated MOFs used as OER catalysts. Reproduced from ref. 125 with permission from Royal Society of Chemistry, copyright 2017. (c) Thin sheets of Ni–Co MOFs used as OER catalysts. Reproduced from ref. 126 with permission from Nature, copyright 2016. (d) The ORR performed on hierarchically porous 2-dimensional Fe4C and FeCo nanoparticles incorporated in N/S-doped carbon support derived from a MOF template. Reproduced from ref. 68 with permission from Royal Society of Chemistry, copyright 2020. | ||
Among many reasons, the sluggish kinetics (high overpotential of the OER and ORR) impede energy converting devices from being practical. The best and most widely used catalysts for the ORR are Pt-based materials.22,127,128 Nevertheless, as has been discussed throughout this manuscript, there is a need to develop cost-effective catalysts that can readily be efficient for these purposes.22
As an alternative to noble metal catalysts, MnO2@MOF is tested for the ORR. This catalyst shows particularly good catalytic activity for this reaction. The effective catalytic activity of the catalyst is described from the perspective of structure faults and unique electron transfer type during the reaction. The catalytic activity of the active site (MnO2) depends on its structural faults (i.e., De Wolff faults) and structural defects (i.e., micro-twinning) among the polymorphs, which are known to be promising for the ORR. Also, as for the MOF(Fe) itself, the framework allows easy oxygen diffusion which aids in the catalytic activity. A unique structure is formed for MnO2@MOF(Fe), in which ε-MnO2 shows a nanorod morphology with one side strongly holding onto the MOF(Fe) matrix, and the other end protruding which eases the contact with oxygen. All these criteria endow this composite with catalytic activity which is comparable with that of 20% Pt/C. Another quality of this composite is that the electron transfer occurred via an apparent-4-electron system (in a two-step 2-electron route). Here is the possible reaction pathway:22
| O2 + H2O + 2e− → HO2− + OH− | (1) |
| HO2− + H2O + 2e− → 3OH− | (2) |
| 2HO2− → 2OH− + O2 | (3) |
Similarly, in another study, Fe2O3 led to enhanced catalytic performance in water oxidation (very low loading of 0.6 wt%) when it was supported on the surface of a non-precious monometallic MOF, Ni-MOF-74. The results were improved with respect to when iron was absent (Ni-MOF-74) or with a catalyst of the same composition but different loading, and the performance was even higher than that of a commercial IrO2 reference. As a matter of fact, to deliver 10 Am cm−2, an overpotential of 264 mV was needed in contrast to 323 mV in the absence of Fe2O3 or 300 mV for the commercial noble metal oxide.129
MOFs play an outstanding role not only in encapsulating nanoparticles but can also be used as starting blocks to construct porous carbon materials or for the formation of shell structures. The use of these self-sacrificing templates can favor the presence of single atoms with unique and superior catalytic performance with respect to catalysts with a higher number of atomic aggregates. Among the single atom catalysts reported, those involving non-noble metals such as Fe, Co, Ni, Cu, and Mn, as well as Fe–Co sites have been applied in the electrocatalytic ORR, HER, OER, and CO2RR. The success of these reactions can be attributed to the use of MOF template materials, such as ZIF-8, ZIF-67, MIL-101-NH2, and UiO-66-NH2, for the synthesis of the catalysts.124Fig. 6a presents the scheme of the MOF derived single atom electrocatalysts employed in different electrocatalytic reactions as summarized in the above cited review. Other researchers have based their studies on carburization processes of the metal–MOF composite as this can lead to the formation of a graphene shell coating the nanoparticles. In this sense, Xiao et al. carried out the carburization of a bimetallic NiMo–MOF to produce electrocatalysts with excellent performance and prolonged duration of 10 h both under acidic and basic conditions125 (the reaction scheme is depicted in Fig. 6b). The over-potential to reach a current density of 10 mA cm−2 was 169 mV for the former and 181 mV for the latter. The synergistic effect of the components (Mo2C and Ni), graphene coating and porous structure favouring the charge transfer, were key to the remarkable results of these catalysts. More recent studies have shown that for the HER under alkaline media, only 141 mV is needed to attain the current density of 10 mA cm−2 when a yolk–shell based on a nitrogen-doped carbon material derived from a MOF is designed.130 In this referenced work, the yolk was composed of CoP/NC while the shell was made of FeCoP/NC. The obtained structure inhibits the agglomeration of CoP particles which would lead to an increase in the number of accessible active sites. On the other hand, Miner et al. produced thin sheets of a Ni–Co-MOF as OER electrocatalysts used under alkaline conditions. As can be viewed in Fig. 6(c), OH− is oxidized at the anode due to dioxygen while dihydrogen is produced at the cathode upon the reduction of water molecules. Controlling the thickness of the bimetallic MOF was essential for the performance of the generated catalyst.126 Furthermore, given the importance of synthesizing these ultrathin sheets, various preparation methods are being explored. In a recent study, non-noble metal hierarchically porous 2-dimensional nanosheets were prepared with the aid of a MOF template. This material is based on Fe4C and FeCo introduced in doped (N/S) carbon materials. The resulting composite (InFeCo@CNS900) is obtained once the lab prepared MOF (InOF-24) has been conveniently calcined (a synthesis scheme can be viewed in Fig. 6(d)). This synthesized composite was employed as an electrocatalyst offering better ORR activity, high diffusion limited current (5.15 mA cm−2) and stability (91.4% after 24 h) compared to a commercial Pt/C catalyst (diffusion limited current of 5.17 mA cm−2 and stability equal to 83.4% after 24 h).68
After all, owing to the advantageous use of these materials, work on exploration of challenges and prospects regarding the development of MOF-derived catalysts is currently in progress.
5. Summary and future perspectives
Literature reports published so far evidenced the possible synergistic effect arising from the integration of MNPs and MOFs. The synergy between MNPs and MOFs is assumed to arise from the plasmonic effect, steric and size selective effects, chemical environment control and electron or energy and substrate transfer. Although advancements have been made in this dynamic field recently, the metals utilized in the preparation of MNP@MOF composites are mostly Pt group metals, which are expensive and less abundant compared to non-noble MNPs. Non-noble MNP loaded MOF systems are becoming valuable alternatives from sustainability and environmental perspectives.MOFs play a key role in designable structures with clear chemistry. The clear chemistry will be somehow disrupted when additional MNPs are introduced. The possible reasons are the issues related to complex MNPs such as defects and the interactions of MNPs and MOFs. This is the difficulty faced for the future development of these materials. To prepare a size, structure and location controlled MNP@MOF, the properties of MNPs and MOFs independently and their interactions must be studied. The effect of different synthetic conditions such as the additive, solvent and kinetic and thermodynamic parameters have to be experimented with. Detailed investigations for the most part are restricted to the experimental findings of MOFs for different reactions. With respect to the challenge in the characterization of catalysts, increasingly theoretical studies are required apart from the state-of-the art characterization methods currently at hand.
Author contributions
All authors have read and agree to the published version of the manuscript. Conceptualization, N.R.H. and I.D.; investigation, N.R.H.; resources, E.A.N. and I.D.; writing – original draft preparation, N.R.H.; writing – review and editing, E.A.N, A.M.T, and I.D.; supervision, A.M.T. and I.D.; funding acquisition, A.M.T. and I.D.Conflicts of interest
There are no conflicts to declare.Acknowledgements
NRH acknowledges the UNED and Fundación Mujeres por África for the scholarship Lear Africa. This work has been funded by the CSIC Project iCOOP-2019 COOPA20376, and 2019AEP076 as well as the Spanish Agency for International Development Cooperation AECID INNOVACION (2020/ACDE/000373). The support from Haramaya University via a research project HURG_2020_03_02_75 is duly acknowledged.References
- N. Norouzi, M. K. Das, A. J. Richard, A. A. Ibrahim, H. M. El-Kaderi and M. S. El-Shall, Nanoscale, 2020, 12, 19191–19202 RSC.
- B. Lakshminarayana, G. Satyanarayana and C. Subrahmanyam, ACS Omega, 2018, 3, 13065–13072 CrossRef CAS PubMed.
- B. Wei, X. Liu, K. Hua, Y. Deng, H. Wang and Y. Sun, ACS Appl. Mater. Interfaces, 2021, 13, 15113–15121 CrossRef CAS PubMed.
- L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS PubMed.
- F. Yang, D. Deng, X. Pan, Q. Fu and X. Bao, Natl. Sci. Rev., 2015, 2, 183–201 CrossRef CAS.
- X. Zhou, W. Xu, G. Liu, D. Panda and P. Chen, J. Am. Chem. Soc., 2010, 132, 138–146 CrossRef CAS.
- O. M. Wilson, M. R. Knecht, J. C. Garcia-Martinez and R. M. Crooks, J. Am. Chem. Soc., 2006, 128, 4510–4511 CrossRef CAS PubMed.
- P. Suchomel, L. Kvitek, R. Prucek, A. Panacek, A. Halder, S. Vajda and R. Zboril, Sci. Rep., 2018, 8, 1–11 CAS.
- H. R. Moon, D.-W. Lim and M. P. Suh, Chem. Soc. Rev., 2013, 42, 1807–1824 RSC.
- M. Liu, L. Zhou, X. Luo, C. Wan and L. Xu, Catalysts, 2020, 10, 1–34 Search PubMed.
- J. A. Kurzman, L. M. Misch and R. Seshadri, Dalton Trans., 2013, 42, 14653–14667 RSC.
- P. B. Kettler, Org. Proc. Res. Dev., 2003, 7, 342–354 CrossRef CAS.
- A. I. Boronin, E. M. Slavinskaya, A. Figueroba, A. I. Stadnichenko, T. Y. Kardash, O. A. Stonkus, E. A. Fedorova, V. v. Muravev, V. A. Svetlichnyi, A. Bruix and K. M. Neyman, Appl. Catal., B, 2021, 286, 119931 CrossRef CAS.
- B. Han, T. Li, J. Zhang, C. Zeng, H. Matsumoto, Y. Su, B. Qiao and T. Zhang, Chem. Commun., 2020, 56, 4870–4873 RSC.
- J. Gustafson, O. Balmes, C. Zhang, M. Shipilin, A. Schaefer, B. Hagman, L. R. Merte, N. M. Martin, P. A. Carlsson, M. Jankowski, E. J. Crumlin and E. Lundgren, ACS Catal., 2018, 8, 4438–4445 CrossRef CAS.
- M. Qureshi, A. T. Garcia-Esparza, G. Jeantelot, S. Ould-Chikh, A. Aguilar-Tapia, J. L. Hazemann, J. M. Basset, D. Loffreda, T. le Bahers and K. Takanabe, J. Catal., 2019, 376, 180–190 CrossRef CAS.
- J. Shan, C. Ye, S. Chen, T. Sun, Y. Jiao, L. Liu, C. Zhu, L. Song, Y. Han, M. Jaroniec, Y. Zhu, Y. Zheng and S.-Z. Qiao, J. Am. Chem. Soc., 2021, 143, 5201–5211 CrossRef CAS PubMed.
- T. S. Rodrigues, A. G. M. da Silva and P. H. C. Camargo, J. Mater. Chem. A, 2019, 7, 5857–5874 RSC.
- Y. Xu, L. Chen, X. Wang, W. Yao and Q. Zhang, Nanoscale, 2015, 7, 10559–10583 RSC.
- S. Chen, Z. Song, J. Lyu, Y. Guo, B. E. G. Lucier, W. Luo, M. S. Workentin, X. Sun and Y. Huang, J. Am. Chem. Soc., 2020, 142, 4419–4428 CrossRef CAS PubMed.
- M. Wen, Y. Cui, Y. Kuwahara, K. Mori and H. Yamashita, ACS Appl. Mater. Interfaces, 2016, 8, 21278–21284 CrossRef CAS PubMed.
- H. Wang, F. Yin, B. Chen and G. Li, J. Mater. Chem. A, 2015, 3, 16168–16176 RSC.
- R. Mahugo, A. Mayoral, M. Sánchez-Sánchez and I. Diaz, Front. Chem., 2019, 7, 686 CrossRef CAS PubMed.
- Q.-L. Zhu and Q. Xu, Chem, 2016, 1, 220–245 CAS.
- D. Xu, H. Lv and B. Liu, Front. Chem., 2018, 6, 550 CrossRef CAS PubMed.
- R. S. Salama, M. A. Mannaa, H. M. Altass, A. A. Ibrahim and A. E. R. S. Khder, RSC Adv., 2021, 11, 4318–4326 RSC.
- F. H. Wei, D. Chen, Z. Liang, S. Q. Zhao and Y. Luo, RSC Adv., 2017, 7, 46520–46528 RSC.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gandara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018–1023 CrossRef CAS PubMed.
- H. R. Mahdipoor, R. Halladj, E. G. Babakhani, S. Amjad-Iranagh and J. S. Ahari, RSC Adv., 2021, 11, 5192–5203 RSC.
- B. Li, J. P. Dong, Z. Zhou, R. Wang, L. Y. Wang and S. Q. Zang, J. Mater. Chem. C, 2021, 9, 3429–3439 RSC.
- D. Yang and B. C. Gates, ACS Catal., 2019, 9, 1779–1798 CrossRef CAS.
- E. Niknam, F. Panahi, F. Daneshgar, F. Bahrami and A. Khalafi-Nezhad, ACS Omega, 2018, 3, 17135–17144 CrossRef CAS PubMed.
- S.-N. Zhao, G. Wang, D. Poelman and P. van der Voort, Molecules, 2018, 23, 2947 CrossRef PubMed.
- K. Guesh, C. A. D. Caiuby, Á. Mayoral, M. Díaz-García, I. Díaz and M. Sanchez-Sanchez, Cryst. Growth Des., 2017, 17, 1806–1813 CrossRef CAS.
- V. Gascón, M. B. Jiménez, R. M. Blanco and M. Sanchez-Sanchez, Catal. Today, 2018, 304, 119–126 CrossRef.
- V. Gascón, E. Castro-Miguel, M. Díaz-García, R. M. Blanco and M. Sanchez-Sanchez, J. Chem. Technol. Biotechnol., 2017, 92, 2583–2593 CrossRef.
- V. Gascón-Pérez, M. B. Jiménez, A. Molina, R. M. Blanco and M. Sánchez-Sánchez, Catalysts, 2020, 10, 1–16 CrossRef.
- V. Gascón, C. Carucci, M. B. Jiménez, R. M. Blanco, M. Sánchez-Sánchez and E. Magner, ChemCatChem, 2017, 9, 1182–1186 CrossRef.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- X. Gu, Z. Lu, H. Jiang, T. Akita and Q. Xu, J. Am. Chem. Soc., 2011, 133, 11822–11825 CrossRef CAS PubMed.
- Q. Yang, Q. Xu and H.-L. Jiang, Chem. Soc. Rev., 2017, 46, 4774–4808 RSC.
- W. Xiang, Y. Zhang, H. Lin and C. Liu, Molecules, 2017, 22, 2103 CrossRef PubMed.
- T. Ishida, M. Nagaoka, T. Akita and M. Haruta, Chem. – Eur. J., 2008, 14, 8456–8460 CrossRef CAS PubMed.
- H.-L. Jiang, B. Liu, T. Akita, M. Haruta, H. Sakurai and Q. Xu, J. Am. Chem. Soc., 2009, 131, 11302–11303 CrossRef CAS PubMed.
- K. Leus, P. Concepcion, M. Vandichel, M. Meledina, A. Grirrane, D. Esquivel, S. Turner, D. Poelman, M. Waroquier, V. van Speybroeck, G. van Tendeloo, H. García and P. van der Voort, RSC Adv., 2015, 5, 22334–22342 RSC.
- C. Hou, Q. Xu, Y. Wang and X. Hu, RSC Adv., 2013, 3, 19820–19823 RSC.
- Z. Li, R. Yu, J. Huang, Y. Shi, D. Zhang, X. Zhong, D. Wang, Y. Wu and Y. Li, Nat. Commun., 2015, 6, 8248 CrossRef CAS PubMed.
- S. Hermes, M.-K. Schröter, R. Schmid, L. Khodeir, M. Muhler, A. Tissler, R. W. Fischer and R. A. Fischer, Angew. Chem., Int. Ed., 2005, 44, 6237–6241 CrossRef CAS PubMed.
- F. Schröder, D. Esken, M. Cokoja, M. W. E. van den Berg, O. I. Lebedev, G. van Tendeloo, B. Walaszek, G. Buntkowsky, H.-H. Limbach, B. Chaudret and R. A. Fischer, J. Am. Chem. Soc., 2008, 130, 6119–6130 CrossRef PubMed.
- G. Li, H. Kobayashi, K. Kusada, J. M. Taylor, Y. Kubota, K. Kato, M. Takata, T. Yamamoto, S. Matsumura and H. Kitagawa, Chem. Commun., 2014, 50, 13750–13753 RSC.
- M. Mukoyoshi, H. Kobayashi, K. Kusada, M. Hayashi, T. Yamada, M. Maesato, J. M. Taylor, Y. Kubota, K. Kato, M. Takata, T. Yamamoto, S. Matsumura and H. Kitagawa, Chem. Commun., 2015, 51, 12463–12466 RSC.
- W. Zhen, F. Gao, B. Tian, P. Ding, Y. Deng, Z. Li, H. Gao and G. Lu, J. Catal., 2017, 348, 200–211 CrossRef CAS.
- B. Rungtaweevoranit, Y. Zhao, K. M. Choi and O. M. Yaghi, Nano Res., 2016, 9, 47–58 CrossRef.
- L. He, Y. Liu, J. Liu, Y. Xiong, J. Zheng, Y. Liu and Z. Tang, Angew. Chem., Int. Ed., 2013, 52, 3741–3745 CrossRef CAS PubMed.
- K. Wang, W. Zhao, Q. Zhang, H. Li and F. Zhang, ACS Omega, 2020, 5, 16183–16188 CrossRef CAS PubMed.
- H. Liu, L. Chang, L. Chen and Y. Li, J. Mater. Chem. A, 2015, 3, 8028–8033 RSC.
- H. Liu, L. Chang, C. Bai, L. Chen, R. Luque and Y. Li, Angew. Chem., Int. Ed., 2016, 55, 5019–5023 CrossRef CAS PubMed.
- L. Chen, R. Luque and Y. Li, Chem. Soc. Rev., 2017, 46, 4614–4630 RSC.
- C. Li, Q. Zhang and A. Mayoral, ChemCatChem, 2020, 12, 1248–1269 CrossRef CAS.
- C. Wiktor, M. Meledina, S. Turner, O. I. Lebedev and R. A. Fischer, J. Mater. Chem. A, 2017, 5, 14969–14989 RSC.
- Y. Zhao, N. Kornienko, Z. Liu, C. Zhu, S. Asahina, T.-R. Kuo, W. Bao, C. Xie, A. Hexemer, O. Terasaki, P. Yang and O. M. Yaghi, J. Am. Chem. Soc., 2015, 137, 2199–2202 CrossRef CAS PubMed.
- L. E. Kreno, N. G. Greeneltch, O. K. Farha, J. T. Hupp and R. P. van Duyne, Analyst, 2014, 139, 4073–4080 RSC.
- P. Verma, K. Yuan, Y. Kuwahara, K. Mori and H. Yamashita, Appl. Catal., B, 2018, 223, 10–15 CrossRef CAS.
- S. Wieghold, L. Nienhaus, F. L. Knoller, F. F. Schweinberger, J. J. Shepherd, J. W. Lyding, U. Heiz, M. Gruebele and F. Esch, Phys. Chem. Chem. Phys., 2017, 19, 30570–30577 RSC.
- S. Kunwar, M. Sui, P. Pandey, Z. Gu, S. Pandit and J. Lee, Sci. Rep., 2019, 9, 1–14 CAS.
- C. L. Huang, G. Kumar, G. D. Sharma and F. C. Chen, Appl. Phys. Lett., 2020, 116, 253302 CrossRef CAS.
- Q. Huang, Y. Guo, X. Wang, L. Chai, J. Ding, L. Zhong, T. T. Li, Y. Hu, J. Qian and S. Huang, Nanoscale, 2020, 12, 10019–10025 RSC.
- Z. Guo, C. Xiao, R. v. Maligal-Ganesh, L. Zhou, T. W. Goh, X. Li, D. Tesfagaber, A. Thiel and W. Huang, ACS Catal., 2014, 4, 1340–1348 CrossRef CAS.
- G. Lu, S. Li, Z. Guo, O. K. Farha, B. G. Hauser, X. Qi, Y. Wang, X. Wang, S. Han, X. Liu, J. S. DuChene, H. Zhang, Q. Zhang, X. Chen, J. Ma, S. C. J. Loo, W. D. Wei, Y. Yang, J. T. Hupp and F. Huo, Nat. Chem., 2012, 4, 310–316 CrossRef CAS PubMed.
- J. Zhou, P. Wang, C. Wang, Y. T. Goh, Z. Fang, P. B. Messersmith and H. Duan, ACS Nano, 2015, 9, 6951–6960 CrossRef CAS PubMed.
- P. Wang, J. Zhao, X. Li, Y. Yang, Q. Yang and C. Li, Chem. Commun., 2013, 49, 3330–3332 RSC.
- C.-H. Kuo, Y. Tang, L.-Y. Chou, B. T. Sneed, C. N. Brodsky, Z. Zhao and C.-K. Tsung, J. Am. Chem. Soc., 2012, 134, 14345–14348 CrossRef CAS PubMed.
- Y. Yang, F. Wang, Q. Yang, Y. Hu, H. Yan, Y.-Z. Chen, H. Liu, G. Zhang, J. Lu, H.-L. Jiang and H. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 18163–18171 CrossRef CAS PubMed.
- B. Xi, Y. C. Tan and H. C. Zeng, Chem. Mater., 2016, 28, 326–336 CrossRef CAS.
- Z. Xu, W. Zhang, J. Weng, W. Huang, D. Tian and F. Huo, Nano Res., 2016, 9, 158–134 CrossRef CAS.
- Q. Yang, Q. Xu, S.-H. Yu and H.-L. Jiang, Angew. Chem., Int. Ed., 2016, 55, 3685–3689 CrossRef CAS PubMed.
- C. J. Stephenson, J. T. Hupp and O. K. Farha, Inorg. Chem., 2016, 55, 1361–1363 CrossRef CAS PubMed.
- C. J. Stephenson, J. T. Hupp and O. K. Farha, Inorg. Chem. Front., 2015, 2, 448–452 RSC.
- S. Aguado, S. El-Jamal, F. Meunier, J. Canivet and D. Farrusseng, Chem. Commun., 2016, 52, 7161–7163 RSC.
- K. Wang, W. Zhao, Q. Zhang, H. Li and F. Zhang, ACS Omega, 2020, 5, 16183–16188 CrossRef CAS PubMed.
- K. Nakatsuka, T. Yoshii, Y. Kuwahara, K. Mori and H. Yamashita, Chem. – Eur. J., 2018, 24, 898–905 CrossRef CAS PubMed.
- S. Gao, N. Zhao, M. Shu and S. Che, Appl. Catal., A, 2010, 388, 196–201 CrossRef CAS.
- H. Li, Z. Zhu, F. Zhang, S. Xie, H. Li, P. Li and X. Zhou, ACS Catal., 2011, 1, 1604–1612 CrossRef CAS.
- M. Yadav and Q. Xu, Chem. Commun., 2013, 49, 3327–3329 RSC.
- Y. Huang, T. Ma, P. Huang, D. Wu, Z. Lin and R. Cao, ChemCatChem, 2013, 5, 1877–1883 CrossRef CAS.
- C. Hou, G. Zhao, Y. Ji, Z. Niu, D. Wang and Y. Li, Nano Res., 2014, 7, 1364–1369 CrossRef CAS.
- V. Pascanu, F. Carson, M. V. Solano, J. Su, X. Zou, M. J. Johansson and B. Martín-Matute, Chem. – Eur. J., 2016, 22, 3729–3737 CrossRef PubMed.
- F. Carson, V. Pascanu, A. Bermejo Gómez, Y. Zhang, A. E. Platero-Prats, X. Zou and B. Martín-Matute, Chem. – Eur. J., 2015, 21, 10896–10902 CrossRef CAS PubMed.
- X. He, B. G. Looker, K. T. Dinh, A. W. Stubbs, T. Chen, R. J. Meyer, P. Serna, Y. Román-Leshkov, K. M. Lancaster and M. Dincă, ACS Catal., 2020, 10, 7820–7825 CrossRef CAS.
- W. Zhen, B. Li, G. Lu and J. Ma, Chem. Commun., 2015, 51, 1728–1731 RSC.
- P. Liu, X. Gu, K. Kang, H. Zhang, J. Cheng and H. Su, ACS Appl. Mater. Interfaces, 2017, 9, 10759–10767 CrossRef CAS PubMed.
- C. Kim, S. Hyeon, J. Lee, W. D. Kim, D. C. Lee, J. Kim and H. Lee, Nat. Commun., 2018, 9, 1–8 CrossRef PubMed.
- A. Karelovic and P. Ruiz, Appl. Catal., B, 2012, 113–114, 237–249 CrossRef CAS.
- B. An, J. Zhang, K. Cheng, P. Ji, C. Wang and W. Lin, J. Am. Chem. Soc., 2017, 139, 3834–3840 CrossRef CAS PubMed.
- B. Rungtaweevoranit, J. Baek, J. R. Araujo, B. S. Archanjo, K. M. Choi, O. M. Yaghi and G. A. Somorjai, Nano Lett., 2016, 16, 7645–7649 CrossRef CAS PubMed.
- E. S. Gutterød, A. Lazzarini, T. Fjermestad, G. Kaur, M. Manzoli, S. Bordiga, S. Svelle, K. P. Lillerud, E. Skúlason, S. Øien-ØDegaard, A. Nova and U. Olsbye, J. Am. Chem. Soc., 2020, 142, 999–1009 CrossRef PubMed.
- H. Cao, W. Wang, T. Cui, H. Wang, G. Zhu and X. Ren, Energies, 2020, 13, 2235 CrossRef CAS.
- Z. W. Zhao, X. Zhou, Y. N. Liu, C. C. Shen, C. Z. Yuan, Y. F. Jiang, S. J. Zhao, L. B. Ma, T. Y. Cheang and A. W. Xu, Catal. Sci. Technol., 2018, 8, 3160–3165 RSC.
- C. Xu, C. Zhang, H. Li, X. Zhao, L. Song and X. Li, Catal. Surv. Asia, 2016, 20, 13–22 CrossRef CAS.
- S. Navalón, M. Álvaro, A. Dhakshinamoorthy and H. García, Molecules, 2019, 24, 3050 CrossRef PubMed.
- L. Zhou, M. Wen, Q. Wu and D. Wu, Dalton Trans., 2014, 43, 7924–7929 RSC.
- Y. K. Park, S. B. Choi, H. J. Nam, D.-Y. Jung, H. C. Ahn, K. Choi, H. Furukawa and J. Kim, Chem. Commun., 2010, 46, 3086 RSC.
- J.-C. Wang, Y.-H. Hu, G.-J. Chen and Y.-B. Dong, Chem. Commun., 2016, 52, 13116–13119 RSC.
- A. K. Kar and R. Srivastava, New J. Chem., 2018, 42, 9557–9567 RSC.
- T. Guo, C. Wang, N. Zhang, Y. Zhang, T. Chen, X. Xing, Z. Lu and L. Wen, Cryst. Growth Des., 2020, 20, 6217–6225 CrossRef CAS.
- L. Shen, R. Liang and L. Wu, Chin. J. Catal., 2015, 36, 2071–2088 CrossRef CAS.
- X. Deng, Z. Li and H. García, Chem. – Eur. J., 2017, 23, 11189–11209 CrossRef CAS PubMed.
- A. Dhakshinamoorthy, A. M. Asiri and H. García, Angew. Chem., Int. Ed., 2016, 55, 5414–5445 CrossRef CAS PubMed.
- Z. Jin and H. Yang, Nanoscale Res. Lett., 2017, 12, 539 CrossRef PubMed.
- N. T. Binh, P. T. Thu, N. T. H. Le, D. M. Tien, H. T. Khuyen, L. T. K. Giang, N. T. Huong and T. D. Lam, Int. J. Green Nanotechnol., 2015, 12, 447–455 CrossRef.
- H. Zhao, L. Qian, H. Lv, Y. Wang and G. Zhao, ChemCatChem, 2015, 7, 4148–4155 CrossRef CAS.
- F. Song, W. Li and Y. Sun, Inorganics, 2017, 5, 40 CrossRef.
- W. Zhen, J. Ma and G. Lu, Appl. Catal., B, 2016, 190, 12–25 CrossRef CAS.
- L. Zhang, K. Doyle-Davis and X. Sun, Energy Environ. Sci., 2019, 12, 492–517 RSC.
- X.-J. Kong, Z. Lin, Z.-M. Zhang, T. Zhang and W. Lin, Angew. Chem., Int. Ed., 2016, 55, 6411–6416 CrossRef CAS PubMed.
- F. Ke, L. Wang and J. Zhu, Nanoscale, 2015, 7, 8321–8325 RSC.
- P.-Z. Li, K. Aranishi and Q. Xu, Chem. Commun., 2012, 48, 3173–3175 RSC.
- Z. Zhang, S. Zhang, Q. Yao, X. Chen and Z. H. Lu, Inorg. Chem., 2017, 56, 11938–11945 CrossRef CAS PubMed.
- Y. Luo and N. Alonso-Vante, in SPR Electrochemistry, ed. C. Banks and S. McIntosh, RSC Chemistry, London, 2017, vol. 14, pp. 194–256 Search PubMed.
- Z. Song, N. Cheng, A. Lushington and X. Sun, Catalysts, 2016, 6, 116 CrossRef.
- C. Caratelli, J. Hajek, F. G. Cirujano, M. Waroquier, F. X. Llabrés i Xamena and V. van Speybroeck, J. Catal., 2017, 352, 401–414 CrossRef CAS.
- A. Kim, N. Muthuchamy, C. Yoon, S. Joo and K. Park, Nanomaterials, 2018, 8, 138 CrossRef PubMed.
- T. Sun, L. Xu, D. Wang and Y. Li, Nano Res., 2019, 12, 2067–2080 CrossRef CAS.
- X. Li, L. Yang, T. Su, X. Wang, C. Sun and Z. Su, J. Mater. Chem. A, 2017, 5, 5000–5006 RSC.
- E. M. Miner and M. Dinca, Nat. Energy, 2016, 1, 1–2 Search PubMed.
- N. Cheng, L. Zhang, K. Doyle-Davis and X. Sun, Electrochem. Energy Rev., 2019, 2, 539–573 CrossRef.
- Z. Song, L. Zhang, K. Doyle-Davis, X. Fu, J. L. Luo and X. Sun, Adv. Energy Mater., 2020, 10, 1–42 Search PubMed.
- Z. Gao, Z. W. Yu, F. Q. Liu, Y. Yu, X. M. Su, L. Wang, Z. Z. Xu, Y. L. Yang, G. R. Wu, X. F. Feng and F. Luo, Inorg. Chem., 2019, 58, 11500–11507 CrossRef CAS PubMed.
- J. Shi, F. Qiu, W. Yuan, M. Guo and Z. H. Lu, Chem. Eng. J., 2021, 403, 126312 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |