Cu boosting the collaborative effect of Ni and H+ in alloyed NiCu/saponite catalysts for hydrogenolysis of glycidol
Abstract
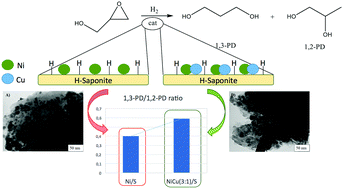
The effect of copper on various acid saponite supported Ni–Cu bimetallic catalysts, prepared with different Ni : Cu ratios, was studied for the liquid phase hydrogenolysis of glycidol on a batch reactor at 393 and 453 K. Characterization of the catalysts showed that Ni and Cu are in close contact as the XRD measurements evidenced the formation of an alloy. H2 chemisorption results revealed that the measured metallic area progressively decreased with an increase in the wt% of copper. In the presence of high metal activity (higher Ni wt%), the formation of 1,2-propanediol (1,2-PD) outweighed, while acid activity led to the formation of dimerization and oligomerization products. The addition of Cu and the increase of the reaction temperature decreased the diol formation but boosted the 1,3-PD/1,2-PD ratio. This could be explained by an improvement of the collaborative effect between the metal Ni and the H+ of the saponite. Therefore, the presence of an appropriate amount of Cu allowed the control of the hydrogenation capacity of Ni and enhanced the collaborative effect of Ni and H+ favouring the formation of 1,3-propanediol with respect to 1,2-propanediol.


 Please wait while we load your content...
Please wait while we load your content...