 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Elucidating the paramagnetic interactions of an inorganic–organic hybrid radical-functionalized Mn-Anderson cluster†
Chang-Gen
Lin
 ab,
Marie
Hutin
a,
Christoph
Busche
ab,
Marie
Hutin
a,
Christoph
Busche
 a,
Nicola L.
Bell
a,
Nicola L.
Bell
 a,
De-Liang
Long
a,
De-Liang
Long
 *a and
Leroy
Cronin
*a and
Leroy
Cronin
 *a
*a
aWestCHEM, School of Chemistry, The University of Glasgow, Glasgow G12 8QQ, UK. E-mail: deliang.long@glasgow.ac.uk; lee.cronin@glasgow.ac.uk
bState Key Laboratory of Chemical Resource Engineering, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China
First published on 26th January 2021
Abstract
A family of six polyoxometalate-based magnetic compounds were synthesized by anchoring N-oxide type TEMPO radicals onto an Anderson type polyoxometalate cluster. The complexes were structurally characterised by single crystal X-ray diffraction and the intramolecular paramagnetic interactions between TEMPO radicals and Mn ions of the resulting hybrids were investigated in detail by electron paramagnetic resonance and the Evans NMR method.
Polyoxometalates (POMs) represent a vast class of negatively charged metal–oxo clusters1 applicable to catalysis,2 electronics,3,4 and magnetism.5 In particular, it has been reported that a highly redox stable POM, [PMo12O40(VO)2]n−, shows promise for molecular spin-qubit quantum computing, with its molecular magnetism tuneable through electrical manipulation.6 Besides the fascinating redox properties of POMs, their enormous diversity in both size and structure makes them ideal building blocks to accommodate magnetic ions at specific sites, thus leading to magnetic clusters with definite topology and a tuneable coordination environment.7,8
Although big advances have been made in POM-based single molecular magnets, there are currently few examples of such systems based on covalently modified POM clusters. Hence in this context, we envision that it might be interesting to construct POM based magnetic molecules by anchoring stable organic radicals onto a metal–oxo cluster, and investigate the intramolecular magnetic interactions. Here we chose the Mn-Anderson cluster,9 [MnMo6O24{(CH2)3CNH2}]3−, as the platform and covalently attached 2,2,6,6-tetramethyl-piperidine-1-oxyl (TEMPO) radicals on both sides of it. The magnetic properties of the resulting organic–inorganic hybrids were preliminarily monitored by electron paramagnetic resonance (EPR), and the interactions between the TEMPO radicals and the center MnIII ion were investigated systematically using the Evans NMR method, which has rarely been applied in POM chemistry.
The Evans method, first developed in 1959,10 has become a standard method to measure the magnetic susceptibility and unpaired electrons of inorganic complexes by using a NMR spectrometer.11–13 Evans NMR is performed in a diamagnetic solvent, and relies on the effect of the dissolved paramagnetic sample on the chemical shift of a reference compound, usually the solvent.14,15 The data are readily acquired by collecting the 1H NMR spectrum of the paramagnetic sample that contains a capillary insert of pure solvent as a reference.
The general synthetic procedure for the TEMPO-functionalized Mn-Anderson cluster is depicted in Scheme 1. It contains three steps: first, the carboxylic acid modification of the Mn-Anderson cluster using glutaric anhydride; second, N-hydroxysuccinimide (NHS) activation; and third, amidation reaction using 4-amino-TEMPO. The biradical POM hybrid, [(n-C4H9)4N]3[MnMo6O24{(CH2)3CNHCO(CH2)3CONHC9H17NO}2], is designated as 1-Mn. Similarly, an analogous compound 2-Mn, [(n-C4H9)4N]3[MnMo6O24{(CH2)3CNHCO(CH2)2CONHC9H17NO}2], with a shorter alkyl chain, and an AlIII centered Anderson hybrid isostructure 3-Al, [(n-C4H9)4N]3[AlMo6O24{(CH2)3CCH2O-CO(CH2)2CONHC9H17NO}2], were prepared for comparison (see the ESI† for details).
The biradical hybrids were characterized by FT-IR, ESI-MS, elemental analysis and single-crystal X-ray diffraction. Taking 1-Mn as an example, the FT-IR spectrum shows typical bands for an Anderson cluster at 940–890 cm−1 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 650 cm−1 (Mo–O–Mo), while the vibration of the amide bonds can be found at 1645 cm−1. The molecular ion peak of 1-Mn in the ESI-MS spectrum is attributed to m/z = 2174.40 ({[1-Mn]-TBA}1−). The X-ray structure of 1-Mn is shown in Fig. 1. It crystallizes in a triclinic system with a P
O) and 650 cm−1 (Mo–O–Mo), while the vibration of the amide bonds can be found at 1645 cm−1. The molecular ion peak of 1-Mn in the ESI-MS spectrum is attributed to m/z = 2174.40 ({[1-Mn]-TBA}1−). The X-ray structure of 1-Mn is shown in Fig. 1. It crystallizes in a triclinic system with a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Two unique half molecules (α and β) were found in the asymmetrical unit. The Mn atoms in each half molecule locate on an inversion centre to generate the whole molecule. α and β differ in the conformation of TEMPO with the N–O lengths and related C–N–C angles being very close to each other (Fig. 1A). Though slightly different, these geometrical parameters are all within the range of six-membered ring nitroxide.16 In crystal packing mode, each α molecule is surrounded by four β molecules at the ac plane, giving rise to a radical layer which further stacks along the b axis (Fig. 1B). No intermolecular contacts of TEMPO radicals are observed within the packing layer, or between layers. Any two N–O groups in the crystal packing are separated by more than 5 Å.
space group. Two unique half molecules (α and β) were found in the asymmetrical unit. The Mn atoms in each half molecule locate on an inversion centre to generate the whole molecule. α and β differ in the conformation of TEMPO with the N–O lengths and related C–N–C angles being very close to each other (Fig. 1A). Though slightly different, these geometrical parameters are all within the range of six-membered ring nitroxide.16 In crystal packing mode, each α molecule is surrounded by four β molecules at the ac plane, giving rise to a radical layer which further stacks along the b axis (Fig. 1B). No intermolecular contacts of TEMPO radicals are observed within the packing layer, or between layers. Any two N–O groups in the crystal packing are separated by more than 5 Å.
The magnetic properties of these organic–inorganic biradicals were first investigated by EPR spectroscopy. As shown in Fig. 2, all the compounds exhibit the typical three-line signal of TEMPO due to the hyperfine splitting of the radical electrons with the nitrogen nucleus (I = 1),17 which indicates the absence of spin coupling between the two TEMPO groups within the biradical hybrid. This behaviour is expected since the N⋯N distance of the two TEMPO radicals within a molecule is too far (ca. 25 Å) to allow for strong J-coupling between the unpaired electrons.18,19 High-spin manganese(III) (d4, S = 2) is archetypical of non-Kramers ions resulting in MnIII centres, which are “EPR-silent” due to a zero-field-splitting with an energy splitting higher than the microwave frequency used (X-Band).20,21 The possible signals resulting from the excited state could not be detected, due to the strong signal of the TEMPO radical. However, comparison of the EPR spectra of 1-Mn and 2-Mn with that of 3-Al allowed us to conclude that the paramagnetic MnIII has no effect on TEMPO radicals. To further confirm this, the Evans NMR method was adopted to explore the magnetic properties of these biradical hybrids.
 | ||
| Fig. 2 The EPR spectra of 1-Mn, 2-Mn and 3-Al obtained in acetonitrile solutions (1 × 10−4 M) at room temperature. | ||
Even though Evans NMR has been proved to be an effective method to study the solution state magnetic susceptibility of coordination complexes, attempts to extend it in metal–oxo clusters, including the organically modified ones, have to our knowledge not been explored. Thus, in our first trial the paramagnetic properties of the Mn-Anderson cluster were examined (see the ESI† for experimental details). The calculation of the magnetic moment is given by eqn (1):
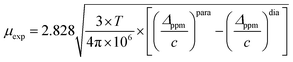 | (1) |
 | (2) |
The implementation of the Evans method in the Mn-Anderson cluster inspired us to go further for biradical hybrids. As shown in Fig. 3, the solvent chemical shifts in both 1-Mn and 3-Al are observed. The corresponding magnetic moments are thus calculated based on eqn (1), and the results are listed in Table 1. The μexp value of 1-Mn is found to be very close to μtheo (low spin), suggesting that MnIII might adopt a low spin state, that is, two unpaired electrons at the outer 3d orbital.
 | ||
| Fig. 3 The Evans NMR spectra of 1-Mn and 3-Al. Square and round dots indicate the solvent peaks of H2O and DMSO, respectively. The chemical shifts of these solvents are labelled in the graph. | ||
| μ exp | μ theo (high) | μ theo (low) | |
|---|---|---|---|
| The last two columns are the theoretical magnetic moments with the number of unpaired electrons given in brackets. | |||
| Mn-Anderson clusters9 | 4.69 ± 0.04 | 4.90 (4) | — |
| 1-Mn | 5.22 ± 0.13 | 6.93 (6) | 4.90 (4) |
| 2-Mn | 5.16 ± 0.15 | 6.93 (6) | 4.90 (4) |
| 3-Al | 2.23 ± 0.09 | 2.83 (2) | — |
| MnIII in 1-Mn | 4.72 ± 0.14 | 4.90 (4) | 2.83 (2) |
| MnIII in 2-Mn | 4.65 ± 0.16 | 4.90 (4) | 2.83 (2) |
However, this finding is contradictory to that suggested by X-ray diffraction, as all the Mn–O bond lengths of 1-Mn are similar to those of previously reported Mn-Anderson POMs (Fig. S1†), which strongly indicates that the MnIII ion in the biradical hybrid is still at its high spin state.9 Similar phenomena are also observed in the case of 2-Mn. As for 3-Al, μcalc is much smaller than μtheo, which implies a weak antiferromagnetic coupling of the magnetic centres.
As such, in order to obtain the magnetic moment of MnIII in 1-Mn, we hypothesize that the paramagnetic contribution of TEMPO may be eliminated by using 3-Al as a reference compound, since these biradical hybrids are similar in both structure and molecular mass.15 To prove this, a modified equation (eqn (3)) is used to calculate the magnetic moment of the MnIII ion in 1-Mn.
 | (3) |
As listed in Table 1, the μcalc of MnIII in 1-Mn is found to be 4.72, which is very close to that of the Mn-Anderson cluster (4.69). This result is in agreement with the EPR (non-Kramers ion) and X-ray data and indicates a high spin state of MnIII. In the case of 2-Mn, the μexp of MnIII is calculated as 4.65, which further validates that there is no spin coupling between MnIII and the TEMPO radicals.
Conclusions
In conclusion, we have presented here an example of magnetic molecules that are prepared by covalently anchoring TEMPO radicals onto Anderson clusters. The resulting organic–inorganic biradicals have been fully characterized by various techniques such as ESI-MS and single-crystal X-ray diffraction. The solution state magnetic interactions between the TEMPO radicals and MnIII ions have been investigated by EPR spectroscopy and Evans NMR. Though it seems that there might exist some spin coupling, close investigation reveals that such ‘false’ interaction is due to the magnetic susceptibility calculation of TEMPO radicals using the Evans method, which turns out to be unsuitable. Therefore, the paramagnetic contribution of TEMPO in such biradical hybrids should be taken into account to get the actual spin state of MnIII.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by EPSRC (Grant No. EP/J015156/1, EP/L023652/1, EP/I033459/1, and EP/K023004/1) and ERC for an Advanced Grant (670467 SMART-POM). C.-G. L. is thankful for the financial support from China Scholarship Council and the National Natural Science Foundation of China (21901016).Notes and references
- (a) D.-L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736 CrossRef CAS; (b) A. Proust, B. Matt, R. Villanneau, G. Guillemot, P. Gouzerh and G. Izzet, Chem. Soc. Rev., 2012, 41, 7605 RSC; (c) A. V. Anyushin, A. Kondinski and T. N. Parac-Vogt, Chem. Soc. Rev., 2020, 49, 382 RSC; (d) N. I. Gumerova and A. Rompel, Chem. Soc. Rev., 2020, 49, 7568 RSC.
- S.-S. Wang and G.-Y. Yang, Chem. Rev., 2015, 115, 4893 CrossRef CAS.
- C. Busche, L. Vila-Nadal, J. Yan, H. N. Miras, D.-L. Long, V. P. Georgiev, A. Asenov, R. H. Pedersen, N. Gadegaard, M. M. Mirza, D. J. Paul, J. M. Poblet and L. Cronin, Nature, 2014, 515, 545 CrossRef CAS.
- C. Kato, R. Machida, R. Maruyama, R. Tsunashima, X.-M. Ren, M. Kurmoo, K. Inoue and S. Nishihara, Angew. Chem., Int. Ed., 2018, 57, 13429 CrossRef CAS.
- J. M. Clemente-Juan, E. Coronado and A. Gaita-Ariño, Chem. Soc. Rev., 2012, 41, 7464 RSC.
- J. Lehmann, A. Gaita-Ariño, E. Coronado and D. Loss, Nat. Nanotechnol., 2007, 2, 312 CrossRef CAS.
- C. Ritchie, A. Ferguson, H. Nojiri, H. N. Miras, Y.-F. Song, D.-L. Long, E. Burkholder, M. Murrie, P. Kögerler, E. K. Brechin and L. Cronin, Angew. Chem., Int. Ed., 2008, 47, 5609 CrossRef CAS.
- S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, A. Camón, M. Evangelisti, F. Luis, M. J. Martínez-Pérez and J. Sesé, J. Am. Chem. Soc., 2012, 134, 14982 CrossRef CAS.
- P. R. Marcoux, B. Hasenknopf, J. Vaissermann and P. Gouzerh, Eur. J. Inorg. Chem., 2003, 2406 CrossRef CAS.
- D. F. Evans, J. Chem. Soc., 1959, 2003 RSC.
- A. A. Pavlov, G. L. Denisov, M. A. Kiskin, Y. V. Nelyubina and V. V. Novikov, Inorg. Chem., 2017, 56, 14759 CrossRef CAS.
- P. Lommens, P. Tack, L. V. Elst, I. V. Driessche, L. Vincze and D. Sinnaeve, Dalton Trans., 2018, 47, 3755 RSC.
- T. Tezgerevska, E. Rousset, R. W. Gable, G. N. L. Jameson, E. C. Sañudo, A. Starikova and C. Boskovic, Dalton Trans., 2019, 48, 11674 RSC.
- D. H. Grant, J. Chem. Educ., 1995, 72, 39 CrossRef CAS.
- C. Piguet, J. Chem. Educ., 1997, 74, 815 CrossRef CAS.
- H. Karoui, F. L. Moigne, O. Ouari and P. Tordo, in Stable Radicals, ed. R. G. Hicks, 2010, Wiley, West Sussex, UK Search PubMed.
- A. L. Barra, L. C. Brunel and J. B. Robert, Chem. Phys. Lett., 1990, 165, 107 CrossRef CAS.
- C. Song, K.-N. Hu, C.-G. Joo, T. M. Swager and R. G. Griffin, J. Am. Chem. Soc., 2006, 128, 11385 CrossRef CAS.
- J. Wang, L. Hou, W. R. Browne and B. L. Feringa, J. Am. Chem. Soc., 2011, 133, 8162 CrossRef CAS.
- D. P. Goldberg, J. Tesler, J. Krzystek, A. G. Montalban, L.-C. Brunel, A. G. M. Barrett and B. M. Hoffman, J. Am. Chem. Soc., 1997, 119, 8722 CrossRef CAS.
- M. M. Roessler and E. Salvadori, Chem. Soc. Rev., 2018, 47, 2534 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedure, ESI-MS spectra, EPR spectra, Evans NMR spectra, and X-ray crystallographic data. CCDC 2001278–2001283. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0dt04149a |
| This journal is © The Royal Society of Chemistry 2021 |


