Acid-promoted hydride transfer from an NADH analogue to a Cr(iii)–superoxo complex via a proton-coupled hydrogen atom transfer†
Abstract
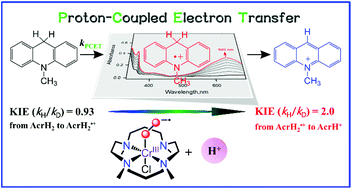
The sequential transfer of an electron, a proton and an electron in a hydride transfer from dihydronicotinamide adenine dinucleotide (NADH) and its analogues has never been separated well. In addition, the effect of acids on hydride transfer from an NADH analogue to a metal–superoxo species has yet to be reported. We report herein the first example of an acid-promoted hydride transfer from an NADH analogue, 10-methyl-9,10-dihydroacridine (AcrH2), to a Cr(III)–superoxo complex, [(TMC)CrIII(O2)]2+, in the presence of HOTf in MeCN at 233 K. The acid-promoted hydride transfer from AcrH2 to [(TMC)CrIII(O2)]2+ occurs via a proton-coupled hydrogen atom transfer from AcrH2 to [(TMC)CrIII(O2)]2+ to produce a radical cation (AcrH2˙+) with an inverse deuterium isotope effect (KIE) of 0.93(5). AcrH2˙+ decayed via a proton transfer from AcrH2˙+ to AcrH2 with a KIE of 2.0(1), followed by the reaction of 10-methylacridinyl radical (AcrH˙) with [(TMC)CrIII(H2O2)]3+ to produce a 10-methylacridinium ion (AcrH+) and [(TMC)CrIII]3+. This work provides valuable insights into the mechanism of hydride transfer of NADH analogues by metal–superoxo intermediates, such as the switchover of the reaction mechanism from a one-step to a separated multi-step pathway in the presence of an acid.


 Please wait while we load your content...
Please wait while we load your content...