 Open Access Article
Open Access ArticleSynthesis, structure, and photoluminesence of the chloridoaluminates [BMIm][Sn(AlCl4)3], [BMPyr][Sn(AlCl4)3], and [BMIm][Pb(AlCl4)3]†
Silke
Wolf
,
Mareike
Liebertseder
and
Claus
Feldmann
 *
*
Institut für Anorganische Chemie, Karlsruhe Institute of Technology (KIT), Engesserstrasse 15, D-76131 Karlsruhe, Germany. E-mail: claus.feldmann@kit.edu; Fax: +49 721 608 47021; Tel: +49 721608 42855 Tel: +49 721 608 42856 Web: http://www.kit.edu
First published on 27th May 2021
Abstract
[BMIm][Sn(AlCl4)3] (1) ([BMIm]: 1-butyl-3-methylimidazolium), [BMPyr][Sn(AlCl4)3] (2) ([BMPyr]: 1-butyl-1-methyl-pyrrolidinium), and [BMIm][Pb(AlCl4)3] (3) are obtained by reaction of SnCl2/PbCl2 in [BMIm]Cl/[BMPyr]Cl/AlCl3-based ionic liquids. The colourless crystals of the title compounds contain infinite 1∞[M(AlCl4)3]n− chains (M: Sn, Pb) that are separated by the voluminous [BMIm]+/[BMPyr]+ cations. The central Sn2+/Pb2+ is coordinated by chlorine in the form of distorted squared anti-prismatic polyhedra. Each Cl atom, in turn, is part of an [AlCl4]− tetrahedron that interlinks Sn2+/Pb2+ to the chain-like building unit. In addition to the novel structural arrangement, all title compounds surprisingly show intense white-light emission. Although Sn2+ and Pb2+ are well-known as dopants in conventional phosphors, efficient luminescence via s–p-transitions of compounds containing Sn2+/Pb2+ in molar quantities and as regular lattice constituents is rare. The emission of [BMIm][Sn(AlCl4)3] and [BMPyr][Sn(AlCl4)3] is very efficient with quantum yields of 51 and 76%, which belong to the highest values known for s–p-based luminescence of Sn2+.
Introduction
The synthesis of complex metal halides was most often performed in solution or via solid-state reactions.1 Usually the dissolution of a metal halide requires the coordination of the metal cation by the respective solvent and often results in coordination compounds with solvent molecules serving as ligands being part of the composition of the complex metal halide.1 Solid-state reactions require high temperatures (typically >300 °C) to guarantee sufficiently fast diffusion in the solid state.1 On the other hand, high temperatures are counterproductive when aiming at kinetically stabilized and thermodynamically metastable compounds. Since activation barriers can be passed, only the thermodynamically most stable compound might be obtained at high temperatures.2 Another difficulty relates to the sensitivity of many metal halides to hydrolysis, which is all the higher the more Lewis-acidic and the higher the charge density of the respective metal cations is. Besides long-known molten salts (e.g. LiCl/KCl mixtures),1,3 ionic liquids turned out as versatile media that combine both the absence of (strongly) coordinating ligands and a good solubility of many metal halides.4 Especially, Lewis-acidic ionic liquids – most often AlCl3-based systems – have already shown great potential to realize new compounds that can be hardly prepared in other solvents.5 Some recent examples include, for instance, the intermetalloid [CuBi8]3+ cluster cation in [CuBi8][AlCl4]2[Al2Cl7],6 the Ge12Fe8 cluster core in Ge12(μ-I)4{Fe(CO)3}8,7 the [Sb10Se10]2+ cation in [Sb10Se10][AlCl4]2,8 the clathrate phases KxGe136 or NaxSi136,9 the direct-gap semiconductor [Bi2Te2Br][AlCl4],10 or the hexanuclear Zr6 cluster in Zr6Br14Fe or Li2Zr6Cl15Mn.11By reaction of SnCl2/PbCl2 in [BMIm]Cl/[BMPyr]Cl/AlCl3-based ionic liquids, we could now prepare the chloridoaluminates [BMIm][Sn(AlCl4)3] (1), [BMPyr][Sn(AlCl4)3] (2), and [BMIm][Pb(AlCl4)3] (3) ([BMIm]: 1-butyl-3-methylimidazolium), ([BMPyr]: 1-butyl-1-methyl-pyrrolidinium). Besides new structural features, the compounds surprisingly show intense s–p-based photoluminescence although luminescence quenching is usually expected for high concentrations of the luminescent centers in the host lattice.
Results and discussion
Ionic-liquid-based synthesis
[BMIm][Sn(AlCl4)3] (1), [BMPyr][Sn(AlCl4)3] (2), and [BMIm][Pb(AlCl4)3] (3) were prepared by reaction of the respective divalent chlorides SnCl2 and PbCl2 in an ionic liquid established by a mixture of [BMIm]Cl or [BMPyr]Cl and AlCl3 with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 1). All reactions were performed in sealed, Argon-filled glass ampoules by heating to 70–100 °C for 12 hours. The specific temperature is required to guarantee that the reaction mixture is completely liquid at the respective temperature as well as to promote the diffusion of the reactants and the formation of single crystals with suitable crystal quality. Whereas lower temperatures result in a lower yield and a poor crystal quality, higher temperatures lead to a decomposition of the ionic liquid. The formation of the title compounds can be rationalized based on the following reactions:
3 (Fig. 1). All reactions were performed in sealed, Argon-filled glass ampoules by heating to 70–100 °C for 12 hours. The specific temperature is required to guarantee that the reaction mixture is completely liquid at the respective temperature as well as to promote the diffusion of the reactants and the formation of single crystals with suitable crystal quality. Whereas lower temperatures result in a lower yield and a poor crystal quality, higher temperatures lead to a decomposition of the ionic liquid. The formation of the title compounds can be rationalized based on the following reactions:
1:
| SnCl2 + [BMIm]Cl + 3AlCl3 → [BMIm][Sn(AlCl4)3] |
2:
| SnCl2 + [BMPyr]Cl + 3AlCl3 → [BMPyr][Sn(AlCl4)3] |
3:
| PbCl2 + [BMIm]Cl + 3AlCl3 → [BMIm][Pb(AlCl4)3] |
The synthesis of the title compounds requires ionic liquids as the liquid phase. Specific advantages comprise the presence of voluminous cations, the Lewis acidity, the low melting point, the absence of any strong-coordinating solvent molecules, as well as the strict absence of moisture. In comparison to [BMIm]Cl/AlCl3, the system [BMPyr]Cl/AlCl3 results in significantly smaller crystallites. Such behaviour with the cation of the ionic liquid influencing the crystallization and crystal quality is often observed and can be ascribed to the volume of the one or other cation, which fits best with certain crystal structure.4,12
Structure and composition
All title compounds crystallize as colourless, slightly elongated plates with monoclinic lattice symmetry in the space group C2/c (Table 1). They consist of infinite 1∞[M(AlCl4)3]n− chains (M: Sn, Pb), which are separated by the voluminous [BMIm]+/[BMPyr]+ cations (Fig. 2; ESI: Fig. S1 and S2†). The latter naturally stem from the ionic liquid. The most interesting building unit in the title compounds relates to the 1∞[M(AlCl4)3]n− chains, which – to the best of our knowledge – have not been observed until now. In the anionic 1∞[M(AlCl4)3]n− chains, each Sn2+/Pb2+ is surrounded by five [AlCl4]− tetrahedra, resulting in an eightfold coordination of Sn2+/Pb2+ by chlorine (Fig. 2). All-in-all, this coordination leads to a distorted squared anti-prismatic polyhedron. Those two [AlCl4]− tetrahedra that interlink neighboured Sn/Pb atoms in the 1∞[M(AlCl4)3]n− chain coordinate with two Cl atoms to one Sn/Pb atom and with one Cl atom to the neighboured Sn/Pb atom (Fig. 2). Hence, the [AlCl4]− tetrahedra link Sn2+/Pb2+via one corner and one edge, whereas the fourth Cl atom remains non-coordinated. A similar linkage of Sn2+ with [AlCl4]− was yet only reported for the metalorganic coordination compound [(η6-C6(CH3)6)Sn(AlCl4)2]2·3C6H6, which contains a dimeric, molecular [Sn(AlCl4)2]2 unit.13 An additional [AlCl4]− tetrahedron coordinated to Sn/Pb remains isolated and coordinates via two Cl atoms only to one Sn atom (Fig. 2). In the case of Pb2+, examples with a comparable composition and coordination are rare as well. We have previously observed a chain-like connectivity via [AlCl4]− in the carbonyl cluster compound [Pb{Mn(CO)5}3][AlCl4].14 Furthermore, metalorganic compounds such as [Pb(1,2-C6H4Me2)2(AlCl4)2] were reported.15 Most recently, Ruck et al. observed a comparable coordination in Pb[AlCl4]2, however, with a bicapped trigonal prism as polyhedron around Pb2+.16| Data | 1 | 2 | 3 |
|---|---|---|---|
| a Restraints were used to address the disorder of the [BMIm]+/[BMPyr]+ cation and include fixed C–C and N–C distances (see deposited data with CSD 2041327–2041329†). | |||
| Sum formula | C8H15N2Cl12Al3Sn | C9H20NCl12Al3Sn | C8H15N2PbAl3Cl12 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c | C2/c |
| Lattice parameters | a = 1506.3(3) pm | a = 1511.0(1) pm | a = 1506(1) pm |
| b = 1556.9(2) pm | b = 1556.3(1) pm | b = 1553(1) pm | |
| c = 1198.6(2) pm | c = 1197.2(1) pm | c = 1188(1) pm | |
| β = 100.2(1)° | β = 96.5(1)° | β = 100.0(1)° | |
| Cell volume | V = 2766.5 × 106 pm3 | V = 2797.1 × 106 pm3 | V = 2735 × 106 pm3 |
| Formula units per cell | Z = 4 | Z = 4 | Z = 4 |
| Calculated density | ρ = 1.835 g cm−3 | ρ = 1.822 g cm−3 | ρ = 2.137 g cm−3 |
| Measurement limits | −20 ≤ h ≤ 20, −21 ≤ k ≤ 21, −14 ≤ l ≤ 16 | −19 ≤ h ≤ 10, −18 ≤ k ≤ 20, −15 ≤ l ≤ 15 | −17 ≤ h < 17; −18 ≤ k < 18, −14 ≤ l < 13 |
| Theta range for data collection | 3.8 to 58.8° | 7.1 to 125.0° | 3.8 to 52.0° |
| Wavelength | Mo-Kα (λ = 0.71073 Å) | Ga-Kα (λ = 1.34013 Å) | Mo-Kα (λ = 0.71073 Å) |
| Linear absorption coefficient | μ = 2.179 mm−1 | μ = 2.404 mm−1 | μ = 7.443 mm−1 |
| Number of reflections | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 461 (3758 independent) 461 (3758 independent) |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 119 (15 119 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727 independent) 727 independent) |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507 (independent 1752) 507 (independent 1752) |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Merging | R int = 0.091 | R int = 0.012 | R int = 0.111 |
| Number of parameters | 167 | 167 | 157 |
| Number of restrains | 60a | 14a | 126a |
| Residual electron density | −0.88 to 0.63 e− × 10−6 pm−3 | −0.68 to 0.87 e− × 10−6 pm−3 | −2.41 to 0.78 e− × 10−6 pm−3 |
| Figures of merit | R 1 (I ≥ 4σI) = 0.035 | R 1 (I ≥ 4σI) = 0.028 | R 1 (I ≥ 4σI) = 0.043 |
| R 1 (all data) = 0.049 | R 1 (all data) = 0.030 | R 1 (all data) = 0.066 | |
| wR2 (all data) = 0.074 | wR2 (all data) = 0.077 | wR2 (all data) = 0.097 | |
| GooF = 1.021 | GooF = 1.079 | GooF = 0.937 | |
The Sn–Cl distances in 1 and 2 range from 287.7(1) to 309.2(1) pm (Table 2). Thereof, the shortest distances occur to non-bridging [AlCl4]− tetrahedra (287.7(1), 289.8(1) pm), longer Sn–Cl distances are observed to corner-coordinating [AlCl4]− tetrahedra (302.6(1), 304.0(1) pm) (Table 2 and Fig. 3). The longest Sn–Cl distances occur for one edge of the edge-coordinating [AlCl4]− tetrahedra (294.8(1) and 306.7(1) pm in 1; 296.3(1) and 309.2(1) pm in 2). These distances are well in agreement with the literature. Thus, [(η6-C6(CH3)6)Sn(AlCl4)2]2·3C6H6 as sole example containing [AlCl4]−-bridged Sn2+ exhibits Sn–Cl distances of 292.0–309.7 pm.13 In [(η6-C6(CH3)6)Sn(AlCl4)2]2·3C6H6, however, one Sn–Cl distance to [AlCl4]− is significantly longer (332.2 pm) and consequently not considered as bridging edge by the authors.13 In comparison to SnCl2 (Sn–Cl: 260.4–330.2 pm), the distances in 1 and 2 are much closer together. For 3, the Pb–Cl distances are 294.7(3)–304.5(3) pm (Fig. 3). These values compare to those in Pb(AlCl4)2 (286.1–345.3 pm)16 and PbCl2 (279.5–308.9 pm) (Table 2).17 The narrow distribution range of the Sn–Cl and Pb–Cl distances in comparison to the reference compounds also indicates the lone-pair of Sn2+/Pb2+ to be stereochemically non-active.
 | ||
| Fig. 3 Coordination of Sn2+ in [BMIm][Sn(AlCl4)3] (a) and [BMPyr][Sn(AlCl4)3] (b) as well as coordination of Pb2+ in [BMIm][Pb(AlCl4)3] (c). | ||
| Compound | Coordination number | Bond length/pm |
|---|---|---|
| [BMIm][Sn(AlCl4)3] (1) | 8 | 289.8(1), 294.8(1), 302.6(1), 306.7(1) (all 2×) |
| [BMPyr][Sn(AlCl4)3] (2) | 8 | 287.7(1), 296.3(1), 304.0(1), 309.2(1) (all 2×) |
SnCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
5 | 266.4, 278.2, 305.8, 321.9, 330.2 |
[(C6(CH3)6)Sn(AlCl4)2]2·3C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
5 + η6(C6H6) | 282.2, 304.3, 304.4, 309.7, 332.2 |
| [BMIm][Pb(AlCl4)3] (3) | 8 | 294.7(3), 298.2(3), 301.8(3), 304.5(3) (all 2×) |
PbCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
9 | 279.5(1), 290.8(6) (2x), 303.5(2), 304.5(2) (2×), 308.9(6), 370.2(1) (2×) |
| Na[AlCl4]19 | 4 | 212.9(1), 213.4(1), 214.0(1), 214.2(1) |
As discussed before, Sn2+ and Pb2+ are coordinated in a squared anti-prismatic manner. The Cl⋯Cl⋯Cl angles in the squared plane are 87.1(1) and 93.8(1)° (1), 86.7(1) and 92.6(1)° (2), as well as 87.0 and 93.8° (3). The Cl⋯Cl distances range from 333.2(1) to 392.2(1) pm (1), 335.1(1) to 383.3(1) pm (2), and 334.5(4) to 398.3(4) pm (3). The [AlCl4]− tetrahedra are slightly distorted for all title compounds and exhibit Cl–Al–Cl angles of 101.1(1)–113.6(1)° as well as Al–Cl distances of 207.9(1)–217.4(1) pm. Terminal Cl atoms show shorter distances than bridging Cl atoms. All these distances are in the expected range (e.g., Na[AlCl4]: 212.9(1)–214.2(1) pm).19 Beside the infinite 1∞[M(AlCl4)3]n− chains, the structure and connectivity of both cations [BMIm]+ and [PMPyr]+ are as expected. For the [BMIm]+ cations in 1 and 3, two more-or-less rotated (180°) sites are observed (ESI: Fig. S3†). This situation was tackled by split positions for all atoms of the [BMIm]+ cation with a probability of finding of 50% for each position.
In addition to the crystal structure analysis, the purity and composition of all title compounds were examined by Fourier-transform infrared spectroscopy (FT-IR) and thermal analysis, including differential thermal analysis (DTA) and thermogravimetry (TG). FT-IR spectra predominately show vibrations related to the [BMIm]+/[BMPyr]+ cations (Fig. 4), including ν(C–H) at 3300–2800 cm−1, ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) at 1650–1600 cm−1, as well as the fingerprint area (1500–500 cm−1).20 Furthermore, vibrations of the [AlCl4]− tetrahedra (ν(Al–Cl)) are visible below 600 cm−1 (Fig. 4). Finally, it should be noticed that no ν(O–H) vibrations are observed (3600–3000 cm−1), which indicates the absence of any moisture. Taking the similar composition and structure into account, the thermal behaviour of the title compounds is surprisingly different (Fig. 5). Endothermic peaks in the DTA analysis, first of all, indicate the melting points of the tin-containing compounds at 91 (1) and 108 °C (2). For 3, a melting point is not observed (except for the endothermal peak at 500 °C indicating the melting point of PbCl2). TG exhibits a two-step decomposition for 1 and 3, whereas one-step decomposition is observed for 2. Moreover, 2 is highly stable and starts decomposing only above 400 °C, which is even more remarkable since the compound is already in the liquid state. For all compounds, low quantities of a deep black residue were observed after TG analysis, which was finely distributed all over the crucible surface. In sum, the thermal decomposition can be rationalized based on a total decomposition of the [BMIm]+/[BMPyr]+ cation, evaporation of all Al2Cl6, and elemental Sn and Pb as solid residue.
N) at 1650–1600 cm−1, as well as the fingerprint area (1500–500 cm−1).20 Furthermore, vibrations of the [AlCl4]− tetrahedra (ν(Al–Cl)) are visible below 600 cm−1 (Fig. 4). Finally, it should be noticed that no ν(O–H) vibrations are observed (3600–3000 cm−1), which indicates the absence of any moisture. Taking the similar composition and structure into account, the thermal behaviour of the title compounds is surprisingly different (Fig. 5). Endothermic peaks in the DTA analysis, first of all, indicate the melting points of the tin-containing compounds at 91 (1) and 108 °C (2). For 3, a melting point is not observed (except for the endothermal peak at 500 °C indicating the melting point of PbCl2). TG exhibits a two-step decomposition for 1 and 3, whereas one-step decomposition is observed for 2. Moreover, 2 is highly stable and starts decomposing only above 400 °C, which is even more remarkable since the compound is already in the liquid state. For all compounds, low quantities of a deep black residue were observed after TG analysis, which was finely distributed all over the crucible surface. In sum, the thermal decomposition can be rationalized based on a total decomposition of the [BMIm]+/[BMPyr]+ cation, evaporation of all Al2Cl6, and elemental Sn and Pb as solid residue.
 | ||
| Fig. 4 Infrared spectra of [BMIm][Sn(AlCl4)3] (1), [BMPyr][Sn(AlCl4)3] (2) and [BMIm][Pb(AlCl4)3] (3). | ||
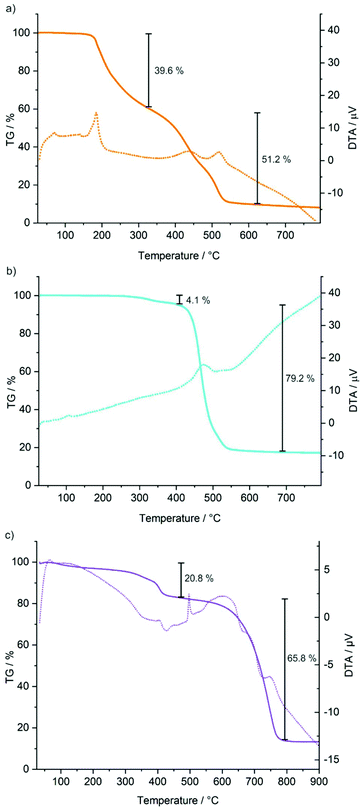 | ||
| Fig. 5 Thermal analysis with thermogravimetry (TG) and differential thermal analysis (DTA) of [BMIm][Sn(AlCl4)3] (a) and [BMPyr][Sn(AlCl4)3] (b) as well as of [BMIm][Pb(AlCl4)3] (c). | ||
Photoluminescence properties
Sn2+ as well as Pb2+ are well-known for their luminescence originating from s–p transitions.21 These transitions are parity-allowed and can be very efficient. Thus, they are also used in commercial phosphors. Selected examples comprise Pb2+-doped UV-emitting phosphors such as CaSiO3:Pb2+ or the Sn2+-doped fluorescent-lamp phosphors Ba2Li2Si2O7:Sn2+, Ca3(PO4)2:Sn2+ and Ca5(PO4)3F:Sn2+ (Table 3).22,23 Herein, Sn2+/Pb2+ as a luminescent center is present as a dopant in a non-luminescent host lattice with a typical concentration range of 5–10 mol%.21,22 This concentration range is normally essential to avoid concentration quenching, which otherwise results in a drastic reduction of the emission efficiency due to continuous energy exchange between directly neighboured luminescent centers.21 For conventional phosphors, moreover, metal oxides are used most often as host lattice, since they only exhibit low-energy lattice vibrations at room temperature, so that vibrational loss processes are low as well. In addition to these conventional phosphors, organic–inorganic hybrid tin/lead halides have only entered the literature since 2019 in the wake of the discovery of the methylammonium lead iodide perovskite as a promising new solar absorber.24 Here, efficient fluorescence has been reported, particularly for hybrid tin/lead bromides and iodides (Table 3). However, due to the small bandgap, excitation and/or emission occur via valence-band-to-conduction-band transitions (especially for the respective iodides), which superimpose the s–p transitions of Sn2+/Pb2+.24,25 Such semiconductor-based luminescence leads to high quantum yields of nanoparticles. For instance, [(PEA)4SnBr6][(PEA)Br]·(CCl2H2)2 exhibits excellent efficiency as a zero-dimensional quantum dot (90%),26 whereas two-dimensional infinite layers of the compound have only a marginal quantum yield (0.1%) (Table 3).| Compound | Maximum of excitation/nm | Maximum of emission/nm | Quantum yield/% |
|---|---|---|---|
| PEA: phenylethylammonium; Bmpip: 1-butyl-1-methyl-piperidinium. | |||
| [BMIm][Sn(AlCl4)3] (1) | 291 | 448 | 51 |
| [BMPyr][Sn(AlCl4)3] (2) | 296 | 453 | 76 |
| [BMIm][Pb(AlCl4)3] (3) | 350 | 416, 571 | 2 |
| Ba2Li2Si2O7:Sn2+ (5 mol%)22 | 254 | 429 | 60–70 |
| Ca3(PO4)2:Sn2+ (5 mol%)23a | 254 | 646 | 80–90 |
| Ca5(PO4)3F:Sn2+ (10 mol%)23b | 254 | 413 | 80–90 |
| [C6N2H16Cl]2[SnCl6]29a | 365 | 450 | 8 |
Cs4SnBr6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29b 29b |
365 | 534 | 21 |
| [C6H13NH3]2[SnBr4]30 | 335 | 618 | 35 |
| [C8H17NH3]2[SnBr4]30 | 344 | 610 | 82 |
| [C12H25NH3]2[SnBr4]30 | 338 | 603 | 60 |
| [C18H35NH3]2[SnBr4]30 | 339 | 623 | 52 |
0D-[(PEA)4SnBr6][(PEA)Br]·(CCl2H2)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
304 | 566 | 90 |
2D-[(PEA)4SnBr6][(PEA)Br]·(CCl2H2)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
304 | 566 | 0.1 |
| [Bmpip]2[SnBr4]25a | 405 | 665 | 75 |
| 2D-[C8H17NH3]2[SnI4]25b | 340 | 670 | 95 |
In regard of the aforementioned considerations, the colourless title compounds 1–3 represent wide-band-gap materials and contain Sn2+/Pb2+ in molar quantities. Nevertheless, they show considerable yellowish to white emission, which is clearly visible for the naked eye under UV-excitation (Fig. 6). Excitation and emission spectra indicate strong absorption at 280–320 nm and intense emission at 400–550 nm for [BMIm][Sn(AlCl4)3] and [BMPyr][Sn(AlCl4)3] (Table 3 and Fig. 7). [BMIm][Pb(AlCl4)3] can be excited at 320–550 nm and emits at 400–700 nm (Table 3 and Fig. 7). The observed decay times are in a range of 1 × 10−7 to 1.0 × 10−6 s, which is expected for parity-allowed s–p transitions of Sn2+/Pb2+. The broad peak widths for excitation and especially for emission are also expected for s–p transitions21 and can be assigned to a 1S0 ground state of the s2 electrons. The excited state is represented by 3P0, 3P1, 3P2 and 1P1 states with 3P1 → 1S0 as preferred relaxation to the ground state.27 For the heavy Pb2+, 3P0 → 1S0 transition is also possible due to spin–orbit coupling and explains the double-band structure of excitation and emission as well as the very broad emission in the case of [BMIm][Pb(AlCl4)3].21
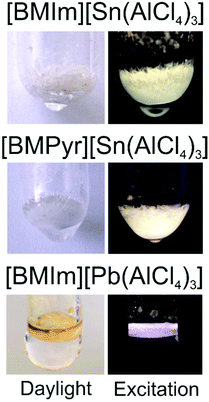 | ||
| Fig. 6 Photos showing [BMIm][Sn(AlCl4)3], [BMPyr][Sn(AlCl4)3], and [BMIm][Pb(AlCl4)3] in daylight and under UV-excitation. | ||
 | ||
| Fig. 7 Photoluminescence spectra of [BMIm][Sn(AlCl4)3] and [BMPyr][Sn(AlCl4)3] (a) as well as of [BMIm][Pb(AlCl4)3] (b) (dashed line: excitation; solid line: emission). | ||
The efficiency of the photoluminescence processes was quantified by determining the absolute quantum yield following the method given by Friend et al.28 Whereas the quantum yield of [BMIm][Pb(AlCl4)3] is low (2%), the quantum yields of [BMIm][Sn(AlCl4)3] and [BMPyr][Sn(AlCl4)3] are surprisingly high with values of 51% and 76%, respectively (Table 3). Comparing the quantum yield of the Sn2+-containing compounds with literature data, it is first noticeable that there are only few examples with quantum yields around 80% even among the long-optimized conventional Sn2+-doped phosphors. Here, Ba2Li2Si2O7:Sn2+, Ca3(PO4)2:Sn2+ or Ca5(PO4)3F:Sn2+ belong to those with the highest quantum yield of Sn2+-doped phosphors (Table 3).22,23 For ionic organic–inorganic hybrid tin chlorides and bromides with s–p-based luminescence of Sn2+ (e.g. [C6N2H16Cl]2[SnCl6], Cs4SnBr6),29 the quantum yields are so far also significantly lower than for the title compounds 1 and 2. In a seminal work, Deng et al. recently correlated the quantum yield of hybrid tin bromides [CnH2n+1NH3]2[SnBr4] (n = 6–18) with the length of the alkyl chain and the distance between the Sn2+ centers and observed a maximum for n = 8 with a quantum yield of 82% (Table 3).30 This points to the necessity of large distances between the luminescent Sn2+ centers to avoid concentration quenching. With increasing length of the alkyl chain, however, the influence of vibrational loss processes related to the organic cation is increased and reduces the quantum yield. [BMIm][Sn(AlCl4)3] and [BMPyr][Sn(AlCl4)3] in this regard are unique as they – to the best of our knowledge – exhibit the highest quantum yield known for s–p-based luminescence of Sn2+. This finding can be ascribed to the rigid coordination of Sn2+ in the 1∞[Sn(AlCl4)3]n− chain and the large distance between the luminescent centers (Sn2+⋯Sn2+: 644.2(1) pm (1) and 647.1(1) pm (2)) with the [AlCl4]− tetrahedra serving as spacers. Especially, [BMPyr][Sn(AlCl4)3] is obviously a good compromise between a large distance and a rigid coordination of the luminescent centers.
Conclusion
The chloridoaluminates [BMIm][Sn(AlCl4)3] (1) ([BMIm]: 1-butyl-3-methylimidazolium) and [BMPyr][Sn(AlCl4)3] (2) ([BMPyr]: 1-butyl-1-methyl-pyrrolidinium), and [BMIm][Pb(AlCl4)3] (3) are obtained by reaction of SnCl2/PbCl2 in a Lewis-acidic ionic liquid based on mixtures of [BMIm]Cl or [BMPyr]Cl and AlCl3. The crystal structures of the title compounds are characterized by a novel infinite 1∞[M(AlCl4)3]n− chain (M: Sn, Pb) that is separated by the voluminous [BMIm]+/[BMPyr]+ cations. Herein, the central Sn2+/Pb2+ is coordinated by chlorine in the form of distorted squared anti-prismatic polyhedra. Surprisingly, all compounds show emission of white to yellowish light. Although Sn2+/Pb2+ are well-known as luminescent centers, efficient luminescence of ionic, wide-band-gap compounds containing the luminescent center as regular lattice constituents (instead of doping) is unusual. In this regard, [BMIm][Sn(AlCl4)3] and [BMPyr][Sn(AlCl4)3] with 51 and 76% show surprisingly high quantum yields, which belong to the highest values observed for s–p transitions on Sn2+. This finding can be ascribed to the rigid coordination of Sn2+ in the 1∞[Sn(AlCl4)3]n− chains and the large distance between the [AlCl4]−-bridged luminescent Sn2+ centers.Experimental section
Synthesis of compounds
Analytical equipment
Fourier-transform infrared (FT-IR) spectra were recorded on a Bruker Vertex 70 FT-IR spectrometer (Bruker). The samples were measured as pellets in KBr. Thus, 300 mg of dried KBr and 0.5–1.0 mg of the sample were carefully pestled and pressed to a thin pellet.
Thermogravimetry (TG) was carried out with a Netzsch STA 449 F3 Jupiter device, using α-Al2O3 as crucible material and reference. Buoyancy effects were corrected by baseline subtraction of a blank measurement. The samples were measured under dried nitrogen up to 800 °C with a heating rate of 10 K min−1. The reaction gas was analyzed via IR-coupling by a Bruker Vertex 70 FT-IR spectrometer (Bruker). The Netzsch software PROTEUS Thermal Analysis (Version 5.2.1) was used for graphical illustration.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Deutsche Forschungsgemeinschaft (DFG) for funding in the Priority Program SPP1708 “Material synthesis near room temperature”. Moreover, we acknowledge the Karlsruhe Nano Micro Facility (KNMF) and Prof. Dr D. Fenske and Dr A. Eichhöfer for data collection on a Stoe StadiVari diffractometer with Ga-metal-jet source.References
- Reviews: (a) A. R. West, Solid State Chemistry and its Applications, Wiley, Chichester, 2014 Search PubMed; (b) J. D. Corbett, J. Alloys Compd., 2006, 418, 1–20 CrossRef CAS; (c) G. Meyer, Prog. Solid State Chem., 1982, 14, 141–219 CrossRef CAS.
- C. Feldmann, Angew. Chem., Int. Ed., 2013, 52, 7610–7611 CrossRef CAS PubMed.
- Review: C. R. Boston, Adv. Molten Salt Chem., 1971, 1, 129–163 CAS.
- Review: (a) E. Ahmed and M. Ruck, Dalton Trans., 2011, 40, 9347–9357 RSC; (b) D. Freudenmann, S. Wolf, M. Wolff and C. Feldmann, Angew. Chem., Int. Ed., 2011, 50, 11050–11060 CrossRef CAS PubMed; (c) J. Dupont, Acc. Chem. Res., 2011, 44, 1223–1231 CrossRef CAS PubMed.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS PubMed.
- M. Knies, M. Kaiser, A. Isaeva, U. Mueller, Th. Doert and M. Ruck, Chem. – Eur. J., 2018, 24, 127–132 CrossRef CAS PubMed.
- S. Wolf, W. Klopper and C. Feldmann, Chem. Commun., 2018, 54, 1217–1220 RSC.
- E. Ahmed, A. Isaeva, A. Fiedler, M. Haft and M. Ruck, Chem. – Eur. J., 2011, 17, 6847–6852 CrossRef CAS.
- P. Simon, Z. Tang, W. Carrillo-Cabrera, K. Chiong, B. Bohme, M. Baitinger, H. Lichte, Y. Grin and A. M. Guloy, J. Am. Chem. Soc., 2011, 133, 7596–7601 CrossRef CAS PubMed.
- K. Biswas, Q. Zhang, I. Chung, J. H. Song, J. Androulakis, A. J. Freeman and M. G. Kanatzidis, J. Am. Chem. Soc., 2010, 132, 14760–14762 CrossRef CAS PubMed.
- C. E. Runyan and T. Hughbanks, J. Am. Chem. Soc., 1994, 116, 7909–7910 CrossRef CAS.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2007 Search PubMed.
- H. Schmidbaur, T. Probst, O. Steigelmann and G. Müller, Z. Naturforsch., 1989, 44b, 1175–1178 CrossRef.
- S. Wolf, D. Fenske, W. Klopper and C. Feldmann, Dalton Trans., 2019, 48, 4696–4701 RSC.
- (a) W. Frank and F.-G. Wittmer, Chem. Ber., 1997, 130, 1731–1732 CrossRef CAS; (b) T. Auel and E. L. Amma, J. Am. Chem. Soc., 1968, 90, 5941–5942 CrossRef CAS.
- M. Knies, M. Lê Anh, U. Keßler and M. Ruck, Z. Naturforsch., B: J. Chem. Sci., 2020, 75, 117–123 CrossRef CAS.
- R. L. Sass, E. B. Brackett and T. E. Brackett, J. Phys. Chem., 1963, 67, 2863–2864 CrossRef CAS.
- J. M. van den Berg, Acta Crystallogr., 1961, 14, 1002–1003 CrossRef.
- (a) G. Mairesse, P. Barbier and J.-P. Wignacourt, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1979, 35, 1573–1580 CrossRef; (b) W. Scheinert and A. Weiss, Z. Naturforsch., A: Phys. Sci., 1976, 31, 1354–1369 CrossRef.
- (a) Y. Jeon, J. Sung, C. Seo, H. Lim, H. Cheong, M. Kang, B. Moon, Y. Ouchi and D. Kim, J. Phys. Chem. B, 2008, 112, 4735–4740 CrossRef CAS PubMed; (b) T. Liu, Y. Danten, J. Grondina and R. Vilarb, J. Raman Spectrosc., 2016, 47, 449–456 CrossRef CAS.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer, Berlin, 1994 Search PubMed.
- W. M. Yen, S. Shionoya and H. Yamamoto, Phosphor Handbook, CRC Press, Boca Raton, 2006 Search PubMed.
- (a) E. R. Kreidler, J. Electrochem. Soc., 1971, 118, 923–929 CrossRef CAS; (b) H. J. Jenkins, A. H. McKeag and P. W. Ranby, J. Electrochem. Soc., 1949, 96, 1–12 CrossRef CAS.
- (a) S. Lee, C. Zhou, J. Neu, D. Beery, A. Arcidiacono, M. Chaaban, H. Lin, A. Gaiser, B. Chen, T. E. Albrecht-Schmitt, T. Siegrist and B. Ma, Chem. Mater., 2020, 32, 374–380 CrossRef CAS; (b) Q. Zhang, Y. Ji, Z. Chen, D. Vella, X. Wang, Q.-H. Xu, Y. Li and G. Eda, J. Phys. Chem. Lett., 2019, 10, 2869–2873 CrossRef CAS PubMed; (c) C. Wu, T. Wu, Y. Yang, J. A. McLeod, Y. Wang, Y. Zou, T. Zhai, J. Li, M. Ban, T. Song, X. Gao, S. Duhm, H. Sirringhaus and B. Sun, ACS Nano, 2019, 13, 1645–1654 CAS; (d) H. Zhu, Y. Fu, F. Meng, X. Wu, Z. Gong, Q. Ding, M. V. Gustafsson, M. T. Trinh, S. Jin and X.-Y. Zhu, Nat. Mater., 2015, 14, 636–643 CrossRef CAS PubMed.
- (a) V. Morad, Y. Shynkarenko, S. Yakunin, A. Brumberg, R. D. Schaller and M. V. Kovalenko, J. Am. Chem. Soc., 2019, 141, 9764–9768 CrossRef CAS PubMed; (b) A. Wang, Y. Guo, Z. Zhou, X. Niu, Y. Wang, F. Muhammad, H. Li, T. Zhang, J. Wang, S. Nie and Z. Deng, Chem. Sci., 2019, 10, 4573–4579 RSC.
- L.-J. Xu, H. Lin, S. Lee, C. Zhou, M. Worku, M. Chaaban, Q. He, A. Plaviak, X. Lin, B. Chen, M.-H. Du and B. Ma, Chem. Mater., 2020, 32, 4692–4698 CrossRef CAS.
- B. Gaveau, E. Mihokova, M. Nikl, K. Polak and L. S. Schulman, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 6938–6943 CrossRef CAS.
- J. C. de Mello, H. F. Wittmann and R. H. Friend, Adv. Mater., 1997, 9, 230–232 CrossRef CAS.
- (a) G. Song, M. Li, Y. Yang, F. Liang, Q. Huang, X. Liu, P. Gong, Z. Xia and Z. Lin, J. Phys. Chem. Lett., 2020, 11, 1808–1813 CrossRef CAS PubMed; (b) L. Tan, W. Wang, Q. Li, Z. Luo, C. Zou, M. Tang, Z. Min;, L. Zhang, J. He and Z. Quan, Chem. Commun., 2020, 56, 387–390 RSC.
- Y. Liu, A. Wang, J. Wu, C. Wang, Z. Li, G. Hu, S. Sui, J.-X. She, W. Meng, W. Li and Z. Deng, Mater. Adv., 2021, 2, 1320–1327 RSC.
- J. Koziskova, F. Hahn, J. Richter and J. Kozisek, Acta Chim. Slov., 2016, 9, 136–140 CrossRef CAS.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- (a) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed; (b) G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- DIAMOND Version 4.2.2 – Crystal and Molecular Structure Visualization, Crystal Impact GbR, Bonn, 2016 Search PubMed.
- (a) A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148–155 CrossRef CAS PubMed; (b) A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2041327–2041329. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0dt03766d |
| This journal is © The Royal Society of Chemistry 2021 |


