Myoglobins engineered with artificial cofactors serve as artificial metalloenzymes and models of natural enzymes
Abstract
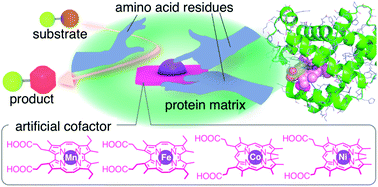
Metalloenzymes naturally achieve various reactivities by assembling limited types of cofactors with endogenous amino acid residues. Enzymes containing metal porphyrinoid cofactors such as heme, cobalamin and F430 exert precise control over the reactivities of the cofactors with protein matrices. This perspective article focuses on our recent efforts to assemble metal complexes of non-natural porphyrinoids within the protein matrix of myoglobin, an oxygen storage hemoprotein. Engineered myoglobins with suitable metal complexes as artificial cofactors demonstrate unique reactivities toward C–H bond hydroxylation, olefin cyclopropanation, methyl group transfer and methane generation. In these cases, the protein matrix enhances the catalytic activities of the cofactors and allows us to monitor the active intermediates. The present findings indicate that placing artificial cofactors in protein matrices provides a useful strategy for creating artificial metalloenzymes that catalyse otherwise unfavourable reactions and providing enzyme models for elucidating the complicated reaction mechanisms of natural enzymes.
- This article is part of the themed collection: 2021 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...
