Improving the rate of the copper-catalyzed Henry reaction by surface plasmon excitation of gold nanoparticles†
Abstract
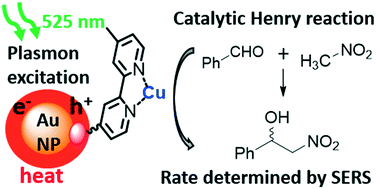
Green LED plasmon excitation (525 nm) of colloidal gold nanoparticles (NPs), onto which a copper(II) complex was grafted, in the presence of nitrobenzaldehyde and nitromethane in DMF lead to the formation of the corresponding nitroaldol with high efficiency. Kinetic studies using 1H NMR and Raman spectroscopies showed that the reaction was faster under irradiation with green light, especially when in close proximity to the NPs (ca. 100% conversion after 100 min). After reaction completion, it was possible to recover the nanocatalyst and run two subsequent reactions. Green LED excitation of plasmonic nanocatalyst is shown to be a straightforward and efficient method for accelerating the rate of the Henry reaction with a lower energy demand and over a shorter reaction time when compared to the reaction performed under classical conditions.


 Please wait while we load your content...
Please wait while we load your content...