Aqueous-phase effects on ethanol decomposition over Ru-based catalysts†
Abstract
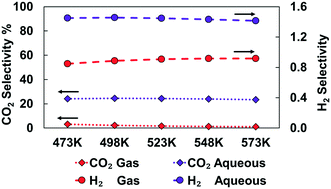
The effects of an aqueous phase on ethanol decomposition for hydrogen production over a Ru(0001) catalyst surface model have been investigated from first principles. Solvent effects on the reaction mechanism and kinetic parameters have been quantified with the help of a microkinetic reactor model, density functional theory, and an implicit solvation scheme (iSMS). Our calculations indicate that in both vapor- and aqueous-phase reaction environments, the ethanol decomposition starts with acetaldehyde formation on the surface, some of which further dehydrogenates to ketenyl species (CHCO), where the C–C bond cleaves to form methylidyne (CH) and CO. In the vapor phase, adsorbed CH gets hydrogenated to methane, and CO desorbs or undergoes methanation reducing the amount of hydrogen produced. In contrast, under aqueous phase reaction conditions, the methanation is inhibited, and the water–gas shift (WGS) reaction is accelerated, leading to complete conversion of CO to CO2 and H2. Calculations indicate that the observed reaction behavior under aqueous phase reforming conditions originates primarily from the higher water chemical potential, and implicit solvent models predict only a small solvation effect.


 Please wait while we load your content...
Please wait while we load your content...