 Open Access Article
Open Access ArticleAerobic oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) C bond cleavage of aromatic alkenes by a high valency iron-containing perovskite catalyst†
C bond cleavage of aromatic alkenes by a high valency iron-containing perovskite catalyst†
Satomi
Shibata
,
Keigo
Kamata
 * and
Michikazu
Hara
* and
Michikazu
Hara
 *
*
Laboratory for Materials and Structures, Institute of Innovative Research, Tokyo Institute of Technology, Nagatsuta-cho 4259, Midori-ku, Yokohama-city, Kanagawa 226-8503, Japan. E-mail: kamata.k.ac@m.titech.ac.jp
First published on 1st March 2021
Abstract
High valency iron-containing perovskite catalyst BaFeO3−δ could efficiently promote the additive-free oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage of various aromatic alkenes to the corresponding aldehydes or ketones using O2 as the sole oxidant. This system was applicable to the gram-scale oxidation of 1,1-diphenylethylene, in which 2.71 g (75% yield) of the analytically pure ketone could be isolated.
C bond cleavage of various aromatic alkenes to the corresponding aldehydes or ketones using O2 as the sole oxidant. This system was applicable to the gram-scale oxidation of 1,1-diphenylethylene, in which 2.71 g (75% yield) of the analytically pure ketone could be isolated.
The oxidative C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage of alkenes into the corresponding carbonyl compounds is an important reaction in both laboratories and chemical industry because aldehydes and ketones are useful synthetic intermediates for the production of perfumes, dyes, and pharmaceuticals.1–3 Stoichiometric oxidants such as O3, m-chloroperbenzoic acid, KMnO4, CrO2Cl2, RuO2, and OsO4 are typically utilized to accomplish efficient oxidative C
C bond cleavage of alkenes into the corresponding carbonyl compounds is an important reaction in both laboratories and chemical industry because aldehydes and ketones are useful synthetic intermediates for the production of perfumes, dyes, and pharmaceuticals.1–3 Stoichiometric oxidants such as O3, m-chloroperbenzoic acid, KMnO4, CrO2Cl2, RuO2, and OsO4 are typically utilized to accomplish efficient oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage (Scheme 1(a)),2,4 although these methods have disadvantages such as a requirement for specific equipment due to the instability of O3 and the use of excess toxic and expensive reagents and/or solvents. To address these issues, research has been conducted on catalytic oxidative C
C bond cleavage (Scheme 1(a)),2,4 although these methods have disadvantages such as a requirement for specific equipment due to the instability of O3 and the use of excess toxic and expensive reagents and/or solvents. To address these issues, research has been conducted on catalytic oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage reactions based on second- or third-row transition metal salts and complexes (Ru, W, Os, In, Pd, Mo, Re, etc.) with NaIO4, NaClO, KHSO5, tert-butyl hydroperoxide (TBHP), and H2O2 as oxidants and/or radical initiators (Scheme 1(b)); (ref. 2, 5, 6) however, most of these reactions are homogeneous, and have some problems in the separation and recyclability of expensive catalysts from reaction mixtures that include co-products of the oxidants. In contrast, the development of effective heterogeneous catalysts based on naturally abundant and easily available first-row transition metals with molecular oxygen (O2) is a strongly desired and challenging research subject. Although heterogeneous catalyst systems based on first-row transition metals such as Cu, Ti, Mn, Co, Fe, and V have been reported for aerobic C
C bond cleavage reactions based on second- or third-row transition metal salts and complexes (Ru, W, Os, In, Pd, Mo, Re, etc.) with NaIO4, NaClO, KHSO5, tert-butyl hydroperoxide (TBHP), and H2O2 as oxidants and/or radical initiators (Scheme 1(b)); (ref. 2, 5, 6) however, most of these reactions are homogeneous, and have some problems in the separation and recyclability of expensive catalysts from reaction mixtures that include co-products of the oxidants. In contrast, the development of effective heterogeneous catalysts based on naturally abundant and easily available first-row transition metals with molecular oxygen (O2) is a strongly desired and challenging research subject. Although heterogeneous catalyst systems based on first-row transition metals such as Cu, Ti, Mn, Co, Fe, and V have been reported for aerobic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage, there is plenty of room for improvement with respect to the activity, selectivity, substrate scope, and need for additives (Table S1, ESI†).
C bond cleavage, there is plenty of room for improvement with respect to the activity, selectivity, substrate scope, and need for additives (Table S1, ESI†).
Perovskite oxides with the general formula ABO3 are being actively explored for industrial applications, such as piezoelectric, (multi)ferroelectric, magnetic, and superconducting materials.7,8 Moreover, perovskite oxides and related materials have received significant attention as substitutes for noble metal catalysts because of their unique stability, compositional and structural varieties, and controllable physicochemical properties.9,10 However, catalysis over multicomponent perovskites with corner-sharing BO6 octahedra has mainly been investigated for gas-phase reactions (CO/CH4/NO oxidation),10,11 and reports on liquid-phase organic reactions are limited. Therefore, we have focused on the liquid-phase catalysis of hexagonal perovskites with unique face-sharing octahedral units based on high valency metal species. During the course of our investigation on crystalline first-row metal oxide catalysts,12–17 we have successfully synthesized various hexagonal perovskite nanoparticle catalysts for the liquid phase selective oxidation of various organic substrates with O2 as the sole oxidant.15–17 In particular, high valency iron-containing BaFeO3−δ was found to act as an efficient heterogeneous catalyst for the aerobic oxidation of alkanes to the corresponding alcohols and ketones, in sharp contrast to Fe3+/Fe2+ oxides.17 Herein, we apply the superior oxidizing ability of a BaFeO3−δ perovskite catalyst to aerobic oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage. In the presence of BaFeO3−δ various types of aromatic alkenes are converted to the corresponding carbonyl compounds using only O2, without the need for any additives. This study provides the first demonstration of an effective and reusable perovskite oxide catalyst for the oxidative C
C bond cleavage. In the presence of BaFeO3−δ various types of aromatic alkenes are converted to the corresponding carbonyl compounds using only O2, without the need for any additives. This study provides the first demonstration of an effective and reusable perovskite oxide catalyst for the oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage of alkenes.‡
C bond cleavage of alkenes.‡
Perovskite oxides including BaFeO3−δ were synthesized by the sol–gel method using aspartic acid and/or malic acid and characterized by elemental analysis, powder X-ray diffraction (XRD), N2 sorption, scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS) (Fig. S1, ESI†).17 First, the oxidative cleavage of styrene (1a) in benzotrifluoride (PhCF3) using O2 (0.1 MPa) as the sole oxidant in the presence of various types of perovskite oxide and simple oxide catalysts was examined (Fig. 1). Three main products, namely, benzaldehyde (2a), styrene oxide (3a), and benzoic acid (4a), were formed. The reaction did not proceed in the absence of a catalyst under the reaction conditions employed. Among the catalysts tested, BaFeO3−δ exhibited the highest catalytic activity and gave 2a with 68% selectivity in 34% total yield. Another high valency iron-containing SrFeO3 also efficiently catalyzed the oxidation of 1a; however, the intrinsic activity per surface area of SrFeO3 (20 m2 g−1) was lower than that of BaFeO3−δ (11 m2 g−1). In addition, other Fe3+/Fe2+-containing perovskite and simple oxides such as CaFeO2.5, LaFeO3, Fe2O3, and Fe3O4 were much less effective for the present oxidation than BaFeO3−δ. These trends were also observed in the aerobic oxidation of adamantane with iron-containing oxides,17 which indicates the high intrinsic oxidation activity of high valency iron-containing perovskite oxides. Other Mn-, Co-, Ni-, Cu-, and Ru-containing oxides (SrMnO3, BaMnO3, activated MnO2, BaCoO3, LaCoO3, Co3O4, LaNiO3, NiO, CuO, and BaRuO3) were also inactive. In the presence of commercially-available Fe3O4 nanoparticles and montmorillonite K10, which have been reported to be active for the oxidative cleavage of 1a to 2a,18,19 no formation of 2a was observed under the reaction conditions employed.
For the BaFeO3−δ-catalyzed oxidation of 1a, the O2 pressure had a strong effect on the selectivity to 2a and 3a, although the total yield remained unchanged (Fig. 2(a)). The selectivity to 2a increased from 68% to 87% with an increase in the O2 pressure from 0.1 MPa to 1.0 MPa (Fig. S2, ESI†), which indicates the concentration of O2 is critical to the selective C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage of 1a to 2a. The BaFeO3−δ-catalyzed oxidation systems could be applied to the solvent-free oxidative cleavage of 1a to give 2a in 29% yield (Fig. 2(a)). In this case, the reaction rate per surface area was 1.2 × 10−3 μmol h−1 m−2 and much higher than those (2.0 × 10−4–4.8 × 10−6 μmol h−1 m−2) of previously reported catalysts (Table S1†). The total yield could also be increased to 71% by using tert-amyl alcohol (t-AmOH) as a solvent (Fig. 2(a)).
C bond cleavage of 1a to 2a. The BaFeO3−δ-catalyzed oxidation systems could be applied to the solvent-free oxidative cleavage of 1a to give 2a in 29% yield (Fig. 2(a)). In this case, the reaction rate per surface area was 1.2 × 10−3 μmol h−1 m−2 and much higher than those (2.0 × 10−4–4.8 × 10−6 μmol h−1 m−2) of previously reported catalysts (Table S1†). The total yield could also be increased to 71% by using tert-amyl alcohol (t-AmOH) as a solvent (Fig. 2(a)).
 | ||
Fig. 2 (a) Effect of O2 pressure and solvent on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage reaction of 1a with O2 catalyzed by BaFeO3−δ. aCatalyst (25 mg), 1a (1 mmol), t-AmOH (1 mL), pO2 (1.0 MPa), 363 K, 12 h. bCatalyst (50 mg), 1a (8.8 mmol), pO2 (1.0 MPa), 363 K, 4 h. cCatalyst (50 mg), 1a (1 mmol), PhCF3 (1 mL), pO2 (0.1–1.0 MPa), 363 K, 24 h. (b) Time course for the C C bond cleavage reaction of 1a with O2 catalyzed by BaFeO3−δ. aCatalyst (25 mg), 1a (1 mmol), t-AmOH (1 mL), pO2 (1.0 MPa), 363 K, 12 h. bCatalyst (50 mg), 1a (8.8 mmol), pO2 (1.0 MPa), 363 K, 4 h. cCatalyst (50 mg), 1a (1 mmol), PhCF3 (1 mL), pO2 (0.1–1.0 MPa), 363 K, 24 h. (b) Time course for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage reaction of 1a with O2 catalyzed by BaFeO3−δ. The reaction conditions are the same as those of Fig. 1. C bond cleavage reaction of 1a with O2 catalyzed by BaFeO3−δ. The reaction conditions are the same as those of Fig. 1. | ||
After the oxidation of 1a was completed under the conditions shown in Fig. 1, the used BaFeO3−δ catalyst could be easily recovered from the reaction mixture by simple filtration. No significant leaching of Fe and Ba species into the filtrate was confirmed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis (Fe 0.04% and Ba 0.2% with respect to the fresh BaFeO3−δ). In addition, catalyst precursors (Fe(OAc)2, Ba(OAc)2, and a mixture of Fe(OAc)2 and Ba(OAc)2) were almost inactive for the oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage of 1a to 2a (Fig. 1), which suggests that there was no contribution to the observed catalysis from iron or barium species leached into the reaction solution. There was no significant difference in the XRD patterns and XPS spectra between the fresh and recovered catalysts, although the XRD peaks were slightly shifted, possibly due to the formation of oxygen-deficient BaFeO3−δ (Fig. S3, ESI†). The recovered BaFeO3−δ catalyst could then be reused without a significant change in the total yield or selectivity to each product: selectivity (1a/2a/3a = 68%/24%/8%) at 34% total yield (fresh), selectivity (1a/2a/3a = 65%/25%/10%) at 37% total yield (reused).
C bond cleavage of 1a to 2a (Fig. 1), which suggests that there was no contribution to the observed catalysis from iron or barium species leached into the reaction solution. There was no significant difference in the XRD patterns and XPS spectra between the fresh and recovered catalysts, although the XRD peaks were slightly shifted, possibly due to the formation of oxygen-deficient BaFeO3−δ (Fig. S3, ESI†). The recovered BaFeO3−δ catalyst could then be reused without a significant change in the total yield or selectivity to each product: selectivity (1a/2a/3a = 68%/24%/8%) at 34% total yield (fresh), selectivity (1a/2a/3a = 65%/25%/10%) at 37% total yield (reused).
Furthermore, the BaFeO3−δ-catalyzed system was applicable to oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage reactions of various types of alkenes with O2 (1.0 MPa) as the sole oxidant (Table 1). Styrenes with electron-donating p-substituents (1b–1d) were converted into the corresponding aldehydes (2b–2d) as main products, and the formation of their carboxylic acids (4b and 4d) was observed in alkyl substituent-containing styrenes (entries 2–4). Oxidative cleavage of para-halogenated 4-fluorostyrene (1e) and 4-chlorostyrene (1f) also proceeded to afford the corresponding aldehydes (2e and 2f) and carboxylic acids (4e and 4f) (entries 5 and 6). On the other hand, p-nitrostyrene (1g) with a strong electron-withdrawing group was also oxidized to the corresponding aldehyde, although a longer reaction time was required (entry 7). It has been reported for Pt@Fe2O3 and Pd(OAc)2 systems that substrates with electron-withdrawing substituents are less active for the oxidative cleavage of styrene derivatives than those with electron-donating substituents.20,21 Not only monosubstituted styrenes, but also disubstituted styrenes were also oxidized to the corresponding aldehydes and ketones. In the case of 1,2-disubstituted β-methylstyrenes, the trans-isomer (1h) was more reactive than the cis-isomer (1i), and the yields of 2a were 30% and 4% from 1h and 1i, respectively (entries 8 and 9). Similar stereospecificity for more electron-rich but sterically-hindered trans-stilbene (1j) and cis-stilbene (1k) was observed; however, the yields of 2a were low in comparison with 1h and 1i (entries 10 and 11). It has also been reported that trans-isomers are more active than cis-isomers in radical-mediated reactions.22 1,1-Disubstited α-methylstyrene (1l) and 1,1-diphenylethylene (1m) were efficiently converted to acetophenone (2l and benzophenone (2m) in 75% and 70% yields, respectively (entries 12 and 13). In addition, the present system was applicable to the gram-scale reaction of 1m and 2.71 g of analytically pure 2m could be isolated (eqn (1)). The present system was not effective for the oxidative cleavage of aliphatic alkenes (1-octene, 2-octene, and allylbenzene), and such a limitation of scope is similar to previously reported systems based on first-row transition metals (Table S1†).2
C bond cleavage reactions of various types of alkenes with O2 (1.0 MPa) as the sole oxidant (Table 1). Styrenes with electron-donating p-substituents (1b–1d) were converted into the corresponding aldehydes (2b–2d) as main products, and the formation of their carboxylic acids (4b and 4d) was observed in alkyl substituent-containing styrenes (entries 2–4). Oxidative cleavage of para-halogenated 4-fluorostyrene (1e) and 4-chlorostyrene (1f) also proceeded to afford the corresponding aldehydes (2e and 2f) and carboxylic acids (4e and 4f) (entries 5 and 6). On the other hand, p-nitrostyrene (1g) with a strong electron-withdrawing group was also oxidized to the corresponding aldehyde, although a longer reaction time was required (entry 7). It has been reported for Pt@Fe2O3 and Pd(OAc)2 systems that substrates with electron-withdrawing substituents are less active for the oxidative cleavage of styrene derivatives than those with electron-donating substituents.20,21 Not only monosubstituted styrenes, but also disubstituted styrenes were also oxidized to the corresponding aldehydes and ketones. In the case of 1,2-disubstituted β-methylstyrenes, the trans-isomer (1h) was more reactive than the cis-isomer (1i), and the yields of 2a were 30% and 4% from 1h and 1i, respectively (entries 8 and 9). Similar stereospecificity for more electron-rich but sterically-hindered trans-stilbene (1j) and cis-stilbene (1k) was observed; however, the yields of 2a were low in comparison with 1h and 1i (entries 10 and 11). It has also been reported that trans-isomers are more active than cis-isomers in radical-mediated reactions.22 1,1-Disubstited α-methylstyrene (1l) and 1,1-diphenylethylene (1m) were efficiently converted to acetophenone (2l and benzophenone (2m) in 75% and 70% yields, respectively (entries 12 and 13). In addition, the present system was applicable to the gram-scale reaction of 1m and 2.71 g of analytically pure 2m could be isolated (eqn (1)). The present system was not effective for the oxidative cleavage of aliphatic alkenes (1-octene, 2-octene, and allylbenzene), and such a limitation of scope is similar to previously reported systems based on first-row transition metals (Table S1†).2
 | (1) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage reaction of various aromatic alkenes with O2 catalyzed by BaFeO3−δa
C bond cleavage reaction of various aromatic alkenes with O2 catalyzed by BaFeO3−δa
| Entry | Substrate | Time (h) | Product (yield (%)) | |
|---|---|---|---|---|
| a Reaction conditions: catalyst (50 mg), substrate (0.5 mmol), solvent (1 mL), pO2 (1 MPa), 363 K, 24 h. b Epoxide (1% yield). c Epoxide (2% yield). d 373 K. e Catalyst (25 mg). | ||||
| 1 |
 1a
1a
|
24 |
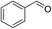 2a (30)
2a (30) |
 4a (4)
4a (4) |
| 2 |
 1b
1b
|
12 |
 2b (26)
2b (26) |
 4b (14)
4b (14) |
| 3b |
 1c
1c
|
6 |
 2c (30)
2c (30) |
 4c (1)
4c (1) |
| 4c |
 1d
1d
|
24 |
 2d (27)
2d (27) |
 4d (10)
4d (10) |
| 5 |
 1e
1e
|
24 |
 2e (34)
2e (34) |
 4e (17)
4e (17) |
| 6 |
 1f
1f
|
24 |
 2f (47)
2f (47) |
 4f (6)
4f (6) |
| 7d |
 1g
1g
|
96 |
 2g (32)
2g (32) |
|
| 8 |
 1h
1h
|
12 |
 2a (30)
2a (30) |
 3h (4)
3h (4) |
| 9 |
 1i
1i
|
24 |
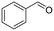 2a (4)
2a (4) |
|
| 10 |
 1j
1j
|
24 |
 2a (12)
2a (12) |
 3j (4)
3j (4) |
| 11 |
 1k
1k
|
24 |
 2a (1)
2a (1) |
|
| 12 |
 1l
1l
|
24 |
 2l (75)
2l (75) |
|
| 13e |
 1m
1m
|
30 |
 2m (70)
2m (70) |
|
H2 temperature-programmed reduction (H2-TPR) analysis was conducted to compare the intrinsic oxidation ability of BaFeO3−δ to those of other iron-based perovskite oxides (Fig. S4, ESI†). The H2 consumption per surface area below 573 K decreased in the order of BaFeO3−δ (3.1 × 10−2 mmol m−2) > SrFeO3 (8.3 × 10−3 mmol m−2) > LaFeO3 (1.3 × 10−3 mmol m−2) > CaFeO2.5 (6.2 × 10−4 mmol m−2), which is reasonable given the high reactivity of BaFeO3–δ. The time course for the oxidative cleavage of 1a to 2a with 0.1 MPa of O2 catalyzed by BaFeO3−δ is shown in Fig. 2(b). The reaction proceeded with an induction period, and only 2a was observed at the initial stage of the reaction. The selectivity to 2a then gradually decreased with an increase in the selectivity to 3a and 4a. This induction period completely disappeared upon the addition of a radical initiator (TBHP; 0.3 equiv. relative to 1a, Fig. S5(a), ESI†), and the addition of a radical scavenger (2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) or 2,6-di-tert-butyl-p-cresol (BHT); 1 equiv. relative to 1a) from the begging and in the middle of the reaction completely suppressed the progress of the reaction (Fig. S6, ESI†). A similar effect of a radical initiator and scavenger have been observed in the KSF montmorillonite systems, for which a radical-type mechanism has been proposed.19 When BaFeO3−δ was removed by hot filtration after 16 h, the reaction did not stop and proceeded in a similar way to that without the filtration step (Fig. S3, ESI†). Such phenomena were also reported for the aerobic oxidation of sulfides with MIL-101 catalysts via a radical-chain mechanism.23 The reaction did not proceed at all under an Ar atmosphere (Fig. S5(b), ESI†), which suggests that BaFeO3−δ does not act as a stochiometric oxidant, but as a catalyst for the present oxidation.
These results indicate the BaFeO3−δ-catalyzed oxidation of 1a to 2a likely involves a radical mechanism where BaFeO3−δ would activate 1a to form an active radical species such as a benzyl radical, which has been often suggested for Fe and Mn catalysts (Fig. 3(a)).2 The selectivity to 3a decreased with an increase in the O2 pressure and the selectivity to 3a and 4a increased with a decrease in the selectivity to 2a; therefore, 3a would be formed by the aerobic epoxidation of 1a with 2a as a co-reductant.24 At high O2 pressure, radical species likely react with O2 to form peroxy intermediates followed by rearrangement to 2a (Fig. 3(a), pathway A). On the other hand, at low O2 pressure radical species would attack hydrogen atom of 2a followed by reaction with O2 to form a peracid, which can promote the epoxidation of 1a to 3a with the co-production of 4a (Fig. 3(a), pathway B). 4a is also formed by the aerobic oxidation of 2a (Fig. 3(a), pathway C). Density functional theory (DFT) calculations were performed to confirm the possible reaction pathways for the formation of 2a, 3a, and 4a from 1a and O2 (Fig. 3(b)). The reaction of 1a with O2 to form an intermediate with a four-membered dioxyethane moiety was calculated to be endothermic by 26 kJ mol−1; therefore, the pathway via this intermediate proposed for Co, Cu, and Cr catalysts would be thermodynamically unfavorable.2,25,26 On the other hand, not only the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage oxidation of 1a with O2 to 2a and HCHO (exothermic by −303 kJ mol−1), but also the epoxidation of 1a to 3a with peroxybenzoic acid from 2a and O2 (exothermic by −271 kJ mol−1) were thermodynamically favourable, which is in good agreement with the proposed reaction pathways.
C bond cleavage oxidation of 1a with O2 to 2a and HCHO (exothermic by −303 kJ mol−1), but also the epoxidation of 1a to 3a with peroxybenzoic acid from 2a and O2 (exothermic by −271 kJ mol−1) were thermodynamically favourable, which is in good agreement with the proposed reaction pathways.
 | ||
| Fig. 3 (a) Proposed reaction pathways and (b) computational free energy diagrams of the aerobic oxidative cleavage reaction of 1a and related reactions. Energies are shown in kJ mol−1. | ||
In conclusion, the high valency iron-based BaFeO3−δ perovskite oxide could act as a heterogeneous catalyst for the aerobic oxidative C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage of various aromatic alkenes to the corresponding carbonyl compounds with O2 as the sole oxidant.
C bond cleavage of various aromatic alkenes to the corresponding carbonyl compounds with O2 as the sole oxidant.
This study was funded in part by JSPS KAKENHI Grant numbers JP20J11604 and JP18H01786, JST A-STEP (JPMJTR20TG), and the “Creation of Life Innovative Materials for Interdisciplinary and International Researcher Development” program of MEXT.
Author contributions
S. S. performed the experimental investigation and the data analysis with the help of K. K. S. S. and K. K. wrote the paper. The draft was reviewed by S. S., K. K., and M. H.Conflicts of interest
There are no conflicts to declare.Notes and references
- F. E. Kühn, R. W. Fischer, W. A. Herrmann and T. Weskamp, in Transition Metals for Organic Synthesis, ed. M. Beller and C. Bolm, Wiley-VCH, Weinheim, 2004, vol. 2, p. 427 Search PubMed.
- P. Spannring, P. C. A. Bruijnincx, B. M. Weckhuysen and R. J. M. Klein Gebbink, Catal. Sci. Technol., 2014, 4, 2182–2209 RSC.
- G. Urgoitia, R. SanMartin, M. T. Herrero and E. Domínguez, ACS Catal., 2017, 7, 3050–3060 CrossRef CAS.
- R. Willand-Charnley, T. J. Fisher, B. M. Johnson and P. H. Dussault, Org. Lett., 2012, 14, 2242–2245 CrossRef CAS PubMed.
- P. Daw, R. Petakamsetty, A. Sarbajna, S. Laha, R. Ramapanicker and J. K. Bera, J. Am. Chem. Soc., 2014, 136, 13987–13990 CrossRef CAS PubMed.
- R. Noyori, M. Aoki and K. Sato, Chem. Commun., 2003, 1977–1986 RSC.
- Y. Zhu, W. Zhou and Z. Shao, Small, 2017, 13, 1603793 CrossRef PubMed.
- A. S. Bhalla, R. Guo and R. Roy, Mater. Res. Innovations, 2000, 4, 3–26 CrossRef CAS.
- S. Royer, D. Duprez, F. Can, X. Courtois, C. Batiot-Dupeyrat, S. Laassiri and H. Alamdari, Chem. Rev., 2014, 114, 10292–10368 CrossRef CAS PubMed.
- K. Kamata, Bull. Chem. Soc. Jpn., 2019, 92, 133–151 CrossRef CAS.
- X. Zhang, C. Pei, X. Chang, S. Chen, R. Liu, Z.-J. Zhao, R. Mu and J. Gong, J. Am. Chem. Soc., 2020, 142, 11540–11549 CrossRef CAS PubMed.
- Y. Yamaguchi, R. Aono, E. Hayashi, K. Kamata and M. Hara, ACS Appl. Mater. Interfaces, 2020, 12, 36004–36013 CrossRef CAS PubMed.
- E. Hayashi, Y. Yamaguchi, Y. Kita, K. Kamata and M. Hara, Chem. Commun., 2020, 56, 2095–2098 RSC.
- E. Hayashi, Y. Yamaguchi, K. Kamata, N. Tsunoda, Y. Kumagai, F. Oba and M. Hara, J. Am. Chem. Soc., 2019, 141, 890–900 CrossRef CAS PubMed.
- K. Sugahara, K. Kamata, S. Muratsugu and M. Hara, ACS Omega, 2017, 2, 1608–1616 CrossRef CAS PubMed.
- K. Kamata, K. Sugahara, Y. Kato, S. Muratsugu, Y. Kumagai, F. Oba and M. Hara, ACS Appl. Mater. Interfaces, 2018, 10, 23792–23801 CrossRef CAS PubMed.
- S. Shibata, K. Sugahara, K. Kamata and M. Hara, Chem. Commun., 2018, 54, 6772–6775 RSC.
- M. J. Rak, M. Lerro and A. Moores, Chem. Commun., 2014, 50, 12482–12485 RSC.
- C. Schäfer, C. J. Ellstrom and B. Török, Top. Catal., 2018, 61, 643–651 CrossRef.
- H. Hong, L. Hu, M. Li, J. Zheng, X. Sun, X. Lu, X. Cao, J. Lu and H. Gu, Chem. – Eur. J., 2011, 17, 8726–8730 CrossRef CAS PubMed.
- C. A. Hone, A. O'Kearney-McMullan, R. Munday and C. O. Kappe, ChemCatChem, 2017, 9, 3298–3302 CrossRef CAS.
- P. E. Correa, G. Hardy and D. P. Riley, J. Org. Chem., 1988, 53, 1695–1702 CrossRef CAS.
- A. Gómez-Paricio, A. Santiago-Portillo, S. Navalón, P. Concepción, M. Alvaro and H. Garcia, Green Chem., 2016, 18, 508–515 RSC.
- K. Kaneda, S. Haruna, T. Imanaka, M. Hamamoto, Y. Nishiyama and Y. Ishii, Tetrahedron Lett., 1992, 33, 6827–6830 CrossRef CAS.
- S. Rahman, C. Santra, R. Kumar, J. Bahadur, A. Sultana, R. Schweins, D. Sen, S. Maity, S. Mazumdar and B. Chowdhury, Appl. Catal., A, 2014, 482, 61–68 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, ACS Catal., 2011, 1, 836–840 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cy00245g |
| ‡ While oxidative cleavage of styrene with O2 has been reported for some heterogeneous iron-based catalytic systems, there are only two examples of all-inorganic heterogeneous catalysts such as KSF19 montmorillonite and hollow Fe3O4 nanoshells.18 |
| This journal is © The Royal Society of Chemistry 2021 |


