 Open Access Article
Open Access ArticleControlling the selectivity of bimetallic platinum–ruthenium nanoparticles supported on N-doped graphene by adjusting their metal composition†
Christian
Cerezo-Navarrete
 ,
Yannick
Mathieu
,
Yannick
Mathieu
 ,
Marta
Puche
,
Cristina
Morales
,
Patricia
Concepción
,
Luis M.
Martínez-Prieto
,
Marta
Puche
,
Cristina
Morales
,
Patricia
Concepción
,
Luis M.
Martínez-Prieto
 * and
Avelino
Corma
* and
Avelino
Corma
 *
*
ITQ, Instituto de Tecnología Química, Universitat Politècnica de València (UPV), Av. de los Naranjos S/N 46022, Valencia, Spain. E-mail: luismiguel.martinez@csic.es; acorma@itq.upv.es
First published on 5th January 2021
Abstract
Mono and bimetallic platinum–ruthenium nanoparticles have been generated on N-doped graphene (NH2-rGO) following an organometallic approach. Surface and structural studies confirmed the formation of bimetallic MNPs with controlled metal compositions. To evaluate the activity/selectivity of the different materials prepared we used the hydrogenation of acetophenone as a model reaction. We found that both the activity and selectivity of the supported-bimetallic NPs are highly dependent on the support and the atomic composition. The higher the Pt/Ru ratio, the higher the selectivity towards 1-phenylethanol. Indeed, a remarkable activity and selectivity in the hydrogenation of acetophenone was observed for Pt5Ru1@NH2-rGO. The reactivity of these catalysts was also investigated in the hydrogenation of other substrates such as functionalized arenes (i.e. nitrobenzene and benzaldehyde) or hydroxymethylfurfural (HMF), demonstrating that it is possible to control the activity and selectivity of bimetallic Pt–Ru MNPs supported on N-doped graphene by adjusting their metal composition.
Introduction
Supported transition metal catalysts are of great importance for the industrial synthesis of numerous chemicals.1,2 These industrial catalysts are usually based on metals well-dispersed on supports, leading to large metal surface area materials with good interaction between the metal and the support that result in active and stable catalysts. Depending on the nature of the metal active sites required, i.e. single metal atoms, small metal nanoclusters or metal nanoparticles (MNPs), an appropriate catalyst synthesis method that maximizes the surface population of the desired metal site is necessary.3 The final activity and selectivity of the metal sites will depend not only on the specific electronic properties of the metal but also on their modifications by the support that can act as a “ligand”,4 specially in the case of single atoms and subnanometric metal clusters. Therefore, the selection of a support with suitable characteristics (high surface area, functionalization with heteroatoms, basic/acid sites, etc.) is a key parameter for metal stabilization and its catalytic behaviour. In this context, functionalized materials with two-dimensional (2D) nanolayered structures such as N-doped graphenes, are attractive supports for MNPs. The presence of functional groups with donor atoms such as nitrogen groups introduces new active sites that alter the local environment of the metallic nanoparticle and facilitates the synergic interaction between MNPs and the support. The doping of graphene improves the catalytic performance of supported MNPs in many catalytic reactions, including hydrogenation,5 oxidation6 and coupling reactions,7 among others. For example, in a recent study we observed that the basic centres of N-doped reduced graphene oxide (NH2-rGO) next to the active metal sites heterolytically activate H2, enhancing the activity and selectivity of ruthenium nanoparticles in the selective hydrogenation of fatty acids to alcohols.8 There is another way to modify the electronic and geometric characteristics of a given metal cluster or nanoparticle that involves the formation of bimetallic entities where the catalytic properties can be modified,9 controlling the final effect on what occurs in metal materials.10 Here we have combined both effects by generating bimetallic PtRu NPs over N-doped reduced graphene oxide (PtRu/NH2-rGO), looking for the formation of catalysts that present catalytic properties beyond those of Pt and Ru working separately, together with MNPs with neighbouring basic sites that are able to activate H2 in a heterolytic way.Another efficient strategy to modulate the reactivity of MNPs is the use of surface molecules (ligands or metal complexes), which can modify the metal surface–substrate interactions during catalysis and thus change the MNP activity/selectivity. The employment of ligands to decorate/stabilize MNPs and control their surface chemistry is well known in colloidal catalysis,11 but much less used in supported-MNP catalysis, since it is normally assumed that surface ligands partially block the metal active sites and reduce the catalytic activity of the catalysts, which, in principle, is a non-desired effect.12 In the same way, molecular complexes, i.e. organometallic tin compounds such as tetrabutyltin hydride, have been employed to modify MNP surfaces, and thus improve the activity and selectivity of the nanocatalysts.13 However, this increase in selectivity is sometimes accompanied by a decrease in activity, since these organotin species somewhat poison the surface nanoparticle obstructing a considerable number of available active sites.13a On the other hand, bimetallic nanoparticles present new catalytic properties (i.e. selectivity), in comparison to the inherent properties of monometallic NPs, without an evident loss of activity. Even in certain cases, an increase in activity has been observed due to a synergistic effect.14 For example, the incorporation of a more electropositive metal such as iron, tin or ruthenium in Pt NPs increases their selectivity towards the hydrogenation of carbonyl groups in unsaturated aldehydes/ketones. The presence of a second metal, which acts as an electron donor “ligand”, increases the electron density on platinum, and led to the electrophilic activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.15
O bond.15
Herein, we present a series of mono and bimetallic platinum–ruthenium nanoparticles on N-doped graphene, which were obtained by decomposition of the corresponding organometallic precursors, [Pt(NBE)3] (NBE: norbornene) and/or [Ru(COD)(COT)] (COD: cyclooctadiene and COT: cyclooctatriene), under H2 in the presence of the graphene material. To obtain extra anchoring points for MNP stabilization, and to introduce basic centres next to the active metal sites for heterolytic H2 cleavage, N-doped reduced graphene oxide (NH2-rGO) was used as a support. The obtained catalytic systems (Pt/NH2-rGO, Pt5Ru1/NH2-rGO, Pt1Ru1/NH2-rGO, Pt1Ru5/NH2-rGO and Ru/NH2-rGO) have been characterized from the electronic and geometric points of view, and it has been possible to modulate the catalytic activity and selectivity for the hydrogenation of several substrates with different functional groups by means of the supported bimetallic nanoparticles.
Results and discussion
Synthesis, characterization and surface studies
A series of mono and bimetallic PtRu NPs were generated on N-doped reduced graphene oxide (NH2-rGO) by decomposition of the corresponding organometallic precursors under 3 bar H2 pressure in THF at room temperature (r.t) (Scheme 1). Monometallic Pt/NH2-rGO and Ru/NH2-rGO were prepared following a previously described synthetic method,8 using Pt(NBE)3 and Ru(COD)(COT), respectively. Bimetallic systems with different metal compositions were obtained after the co-decomposition of Pt(NBE)3 and Ru(COD)(COT) in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratios (Pt5Ru1/NH2-rGO, Pt1Ru1/NH2-rGO and Pt1Ru5/NH2-rGO). These metal precursors were used due to their fast and similar decomposition rates under H2, which lead to the formation of Pt–Ru alloys.16 The metal contents of the supported NPs (3 wt%) were determined by inductively-coupled plasma (ICP) and X-ray fluorescence analyses (XRF). In all cases, the metal contents and molar ratios are close to the theoretical ones (see Tables S1 and S3, ESI†).
5 molar ratios (Pt5Ru1/NH2-rGO, Pt1Ru1/NH2-rGO and Pt1Ru5/NH2-rGO). These metal precursors were used due to their fast and similar decomposition rates under H2, which lead to the formation of Pt–Ru alloys.16 The metal contents of the supported NPs (3 wt%) were determined by inductively-coupled plasma (ICP) and X-ray fluorescence analyses (XRF). In all cases, the metal contents and molar ratios are close to the theoretical ones (see Tables S1 and S3, ESI†).
 | ||
| Scheme 1 Generation of mono and bimetallic Pt–Ru nanoparticles on NH2-rGO following the organometallic approach. | ||
Transmission electron microscopy (TEM) analysis for the mono and bimetallic NPs on NH2-rGO revealed ultrafine, monodispersed and a narrow distribution of NPs within the 1.2–2.5 nm range, with a mean diameter between 1.5 and 2.1 nm (Fig. 1a–e). In particular, Ru NPs supported on NH2-rGO (Ru/NH2-rGO) present the smallest particles with a mean size of 1.5 ± 0.2 nm, whereas the platinum counterpart, Pt/NH2-rGO, exhibit the largest size (2.1 ± 0.4 nm). Bimetallic NPs show intermediate sizes, and the size increases as the percentage of Pt in the system increases (Pt1Ru5/NH2-rGO: 1.6 ± 0.4 nm, Pt1Ru1/NH2-rGO: 1.7 ± 0.3 nm and Pt5Ru1/NH2-rGO: 1.9 ± 0.3 nm) (Fig. 1a–e). According to the results, it is apparently possible to obtain smaller and less agglomerated NPs when the N-doped reduced graphene oxide is used as a support (i.e. Pt1Ru5/NH2-rGO: 1.9 ± 0.3 nm – see Fig. 1d) instead of the non-doped analogous reduced graphene oxide (i.e. Pt1Ru5/rGO: 2.5 ± 0.5 nm – see Fig. 1f). This phenomenon is very likely due to the fact that N atoms facilitate the interaction between the organometallic precursors and the graphene material during the synthesis, and thus form very small and better-distributed MNPs.8 In addition, the N atoms present in the graphene act as anchoring points for MNPs, increasing their stability and avoiding the agglomeration during catalysis (see hereafter).
 | ||
| Fig. 1 TEM micrographs and the corresponding size histograms of (a) Ru/NH2-rGO, (b) Pt1Ru5/NH2-rGO, (c) Pt1Ru1/NH2-rGO, (d) Pt5Ru1/NH2-rGO, (e) Pt/NH2-rGO and (f) Pt5Ru1/rGO. | ||
High-resolution TEM (HRTEM) analyses confirmed the crystallinity of both mono and bimetallic supported-NPs (Fig. S1, ESI†). Ru in the Ru/NH2-rGO catalyst presents the expected hexagonal close packed (hcp) crystal structure of bulk ruthenium, exhibiting reflections due to the (002), (101) and (002) atomic planes after Fourier analysis (Fig. S1a, ESI†). On the other hand, the HRTEM micrograph of Pt/NH2-rGO shows highly crystalline NPs with a face centered cubic (fcc) structure, typical for bulk Pt. Fourier analysis applied to this image displays reflection planes at (220), (111), (111) and (111) (Fig. S1e, ESI†). Likewise, a fcc crystalline structure is observed for the bimetallic systems, Pt1Ru1/NH2-rGO and Pt5Ru1/NH2-rGO, with inter-planar distances of 2.23 Å and 2.28 Å, respectively, corresponding to the (111) plane in both cases (Fig. S1c and d, ESI†). However, Pt1Ru5/NH2-rGO retains the hcp structure typical of bulk Ru with an inter-planar distance of 2.36 Å that corresponds to the (100) plane (Fig. S1b, ESI†). A similar atomic-packing trend has been previously observed for non-supported Ru–Pt bimetallic NPs with different metal compositions.17 High-angle annular dark-field scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (HAADF-SEM EDX) of the bimetallic systems confirmed the presence of alloy NPs with a homogeneous distribution of Pt and Ru. The Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ru % atomic ratios observed by SEM-EDX were Pt21Ru79, Pt48Ru52 and Pt76Ru24 and are close to those expected for Pt1Ru5/NH2-rGO, Pt1Ru1/NH2-rGO and Pt5Ru1/NH2-rGO, respectively (Fig. S2 and Table S3, ESI†). Such results seem to confirm that the similar decomposition rate of the organometallic precursors used during the synthesis led to the formation of Pt–Ru alloy NPs.16
Ru % atomic ratios observed by SEM-EDX were Pt21Ru79, Pt48Ru52 and Pt76Ru24 and are close to those expected for Pt1Ru5/NH2-rGO, Pt1Ru1/NH2-rGO and Pt5Ru1/NH2-rGO, respectively (Fig. S2 and Table S3, ESI†). Such results seem to confirm that the similar decomposition rate of the organometallic precursors used during the synthesis led to the formation of Pt–Ru alloy NPs.16
Raman spectroscopy is a model technique to characterize graphene materials, thus, both the support NH2-rGO and the mono and bimetallic catalysts were analyzed by this technique. The spectrum of NH2-rGO presents two bands of similar intensity at 1363 cm−1 (band D) and 1603 cm−1 (band G), together with a broad band around ca. 3000 cm−1 (band 2D′), which is typically associated with 1–2 graphene layers (Fig. S3a, ESI†).18 The high D/G ratio indicates the high percentage of defect sites of this material, which is very convenient for MNP stabilization. The Raman spectra after the incorporation of the MNPs on NH2-rGO did not show any significant difference (Fig. S3b–f†). A small decrease of the D/G ratio was observed, indicating a higher sp2 domain, due the incorporation of MNPs.19 However, it was not possible to observe the vibrations due to the interactions of the carbon, oxygen or nitrogen atoms with the metallic nanoparticles (Pt and/or Ru).
XPS analysis confirmed the chemical composition of this N-doped graphene. The C 1s signal of NH2-rGO exhibits a band at 284.6 eV that can be deconvoluted into three peaks (Fig. S4a, ESI†). The central peak at 284.8 eV corresponds to graphitic carbons (sp2), and the other two peaks are attributed to carbon atoms bound to N or O (287.0 eV) and carboxylic groups (288.8 eV). Likewise, the N 1s region shows a signal at 399.6 eV that can also be deconvoluted into three components: (i) the main peak at 399.8 eV that belongs to –NH2 and –NH groups, (ii) a peak at 398.6 eV, which corresponds to pyridine-like N atoms, and (iii) the last one at 401.2 eV which is characteristic of graphitic nitrogen atoms (Fig. S4b, ESI†). It is noteworthy that amino and pyrrolic nitrogen groups (–NH2 and –NH) are the most abundant species in this N-doped graphene, which in turn act as basic centres.8,20
In addition, the metal composition and the oxidation state of the as-synthetized and previously reduced (180 °C under a H2 flow for 5 h) supported metal nanoparticles were studied by XPS analysis of the Ru 3p and Pt 4f regions. Fig. S5 (left-hand side, ESI†) shows the Ru 3p3/2 area of the different as-synthetized catalytic systems with Ru (Ru/NH2-rGO, Pt1Ru5/NH2-rGO, Pt1Ru1/NH2-rGO and Pt5Ru1/NH2-rGO), where a peak with a binding energy (BE) at ca. 463 eV can be observed. The deconvolution of this band presents two different contributions, one at ca. 464 eV corresponding to more oxidized species, which can be attributed to Ru(IV) from the RuO2 layer formed after exposure to air, and another one at ca. 462 eV (corresponding to more reduced species) characteristic of Ru(0).21 Indeed, Fig. S5 (right-hand side, ESI†) shows the XPS spectra of Ru 3p3/2 after reduction and it can be clearly observed that the Ru(0) component at ∼462 eV increases at the expense of the RuO2 one. Similarly, Fig. S6 (ESI†) shows the Pt 4f region before (left-hand side) and after reduction (right-hand side) of Pt/NH2-rGO, Pt1Ru5/NH2-rGO, Pt1Ru1/NH2-rGO and Pt5Ru1/NH2-rGO. In this 4f area, we observe the characteristic asymmetric peaks for platinum metal corresponding to the Pt 4f7/2 and Pt 4f5/2 signals, with a BE in the range of 70–80 eV. More specifically, in all XPS spectra the Pt 4f7/2 band is close to ca. 72 eV, which can be deconvoluted into two components. One at ∼71 eV, characteristic of Pt(0), and another one at ca.73 eV, which is attributed to Pt atoms with a partial positive charge, in part due to the presence of platinum oxides (PtOx) on the MNP surface after air exposure.22 In the same way as Ru, but in a lower degree, after reduction a fraction of PtOx is reduced back to Pt(0). The precise Ru(0), RuO2, Pt(0), PtOx percentages for each sample, before and after reduction, are shown in Table 1. Therefore, it can be concluded that under reduction conditions, very similar to the ones used during the catalytic reactions (i.e. 130 °C and 50 bar H2, see hereafter), supported MNPs are mostly reduced to their respective zero-valent states, which are the active species in hydrogenation reactions, though some oxidized Pt and Ru still exist. Moreover, the surface metal composition of the bimetallic systems was also determined by XPS (Tables S2 and S3, ESI†). All the bimetallic catalytic systems showed an atomic surface composition close to the theoretical ones indicating no surface enrichment with any of the metals forming the bimetallic PtRu NPs.
| Before reduction (%) | After reduction (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Catalyst | Ru0 | RuO2 | Pt0 | PtOx | Ru0 | RuO2 | Pt0 | PtOx |
| Ru/NH2-rGO | 41 | 59 | — | — | 73 | 27 | — | — |
| Pt1Ru5/NH2-rGO | 34 | 66 | 50 | 50 | 64 | 36 | 67 | 33 |
| Pt1Ru1/NH2-rGO | 36 | 64 | 47 | 53 | 65 | 35 | 60 | 40 |
| Pt5Ru1/NH2-rGO | 31 | 69 | 48 | 52 | 62 | 38 | 66 | 34 |
| Pt/NH2-rGO | — | — | 47 | 53 | — | — | 63 | 37 |
Catalytic studies
Firstly, the activity and selectivity of the prepared catalysts have been estimated during the hydrogenation of acetophenone (see Table 2 and Fig. 2). Acetophenone has been chosen as a model molecule because it has two potentially hydrogenable functional groups (i.e. the ketone and the phenyl groups) and the activity and selectivity of the prepared catalysts can be easily compared. It is important to comment that acetophenone (1) can be reduced to 1-cyclohexylethanol (4) through two distinct hydrogenation routes: (i) route A where the ketone is first hydrogenated to 1-phenylethanol (2) before the complete hydrogenation to form (4) and (ii) route B where the aromatic ring is hydrogenated before the ketone to form 1-cyclohexylethanone (3) (see Table 2, top part).| Entry | Catalyst | Conversionb,c (%) | Selectivityb (%) | ||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |||
| a Reactions conditions: 0.5 mmol acetophenone, 0.5 mol% cat. (0.0025 mmol metal), 10 mL THF, 50 bar H2, 130 °C, 20 h. b Conversions and selectivities were determined by GC using dodecane as the internal standard, and confirmed by GC-MS. c Metal-free NH2-rGO showed negligible activity in the hydrogenation of acetophenone under reaction conditions. | |||||||
| 1 | Ru/NH2-rGO | >99 | 0.0 | 1.7 | 90.6 | 0.1 | 7.6 |
| 2 | Pt1Ru5/NH2-rGO | >99 | 0.7 | 0.5 | 92.0 | 1.3 | 5.5 |
| 3 | Pt1Ru1/NH2-rGO | 97.2 | 74.6 | 1.3 | 22.5 | 1.1 | 0.5 |
| 4 | Pt5Ru1/NH2-rGO | 92.2 | 96.0 | 0.3 | 3.0 | 0.4 | 0.3 |
| 5 | Pt/NH2-rGO | 62.9 | 95.4 | 0.4 | 3.5 | 0.3 | 0.4 |
| 6 | Pt5Ru1/rGO | 86.9 | 87.1 | 3.2 | 8.3 | 1.3 | 0.5 |
| 7 | Ru–Bu3Sn/NH2-rGO | 38.0 | 97.2 | 1.4 | 1.0 | 0.4 | 0.0 |
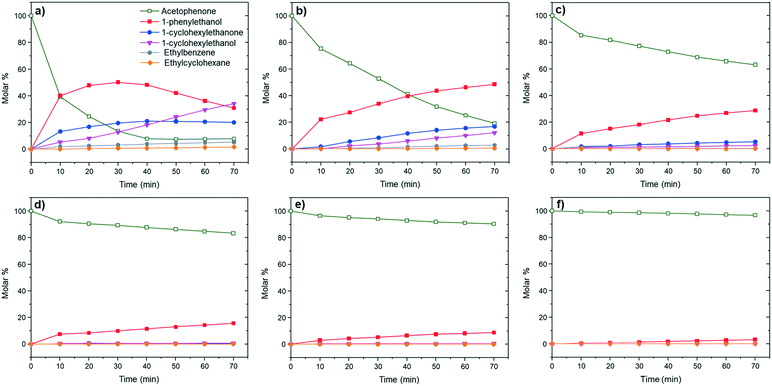 | ||
| Fig. 2 Hydrogenation of acetophenone using (a) Ru/NH2-rGO, (b) Pt1Ru5/NH2-rGO, (c) Pt1Ru1/NH2-rGO, (d) Pt5Ru1/NH2-rGO, (e) Pt/NH2-rGO and (f) Ru–Bu3Sn/NH2-rGO as catalysts (130 °C, 50 bar H2). | ||
Concerning monometallic catalysts and according to the results, it was found that Pt/NH2-rGO is clearly much less active than Ru/NH2-rGO for the hydrogenation of acetophenone. After 1 hour of reaction, Ru/NH2-rGO presents an estimated conversion of around 92%, whereas the conversion with Pt/NH2-rGO is only 8% (see kinetic data in Fig. 2a and e). The tendency observed at the beginning of the reaction is confirmed at higher reaction time (i.e. 20 hours). Almost complete conversion is reached when using the Ru/NH2-rGO catalyst, while a moderate conversion of 63% is obtained with the Pt/NH2-rGO (see Table 2, entries 1 and 5, respectively). With respect to catalyst selectivity, 1-cyclohexylethanol (4) is the main product (i.e. ∼91% of selectivity after 20 hours of reaction – see Table 2, entry 1) when using Ru/NH2-rGO, and hydrodeoxygenation (HDO) products [i.e. ethylbenzene (5) and ethylcyclohexane (6)] are also detected even at short reaction times (see Table 2 entry 1 and Fig. 2a). Meanwhile, the Pt/NH2-rGO catalyst appears to be much more selective to 1-phenylethanol (2) at low (see Fig. 2e) and high conversion rates (i.e. >95% after 20 h reaction – see Table 2, entry 5).
On the other hand, bimetallic catalysts present intermediate reactivities between the monometallic Pt and Ru systems. In general terms, the higher the Pt/Ru ratio, the higher the selectivity towards 1-phenylethanol (2), but the lower the activity. After 20 h, Pt5Ru1/NH2-rGO presents a remarkable activity compared to monometallic Pt/NH2-rGO [conversion of 92% with 96% selectivity towards 1-phenylethanol (2)] (Table 2, entry 4). This bimetallic catalyst of Pt doped with Ru is as selective as Pt/NH2-rGO in the hydrogenation of acetophenone to 1-phenylethanol (2), but much more active. The presence of a small amount of Ru on the catalyst surface boosts the activity of the supported-Pt NPs, but maintaining the selectivity of Pt/NH2-rGO. The incorporation of a less electronegative metal (ruthenium) in Pt NPs increases the electron density of Pt, which led to the electrophilic activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and increases the activity in the hydrogenation of carbonyl groups.15 Increasing the Ru content of the catalytic system (i.e. Pt1Ru1/NH2-rGO catalyst, with a Ru
O bond, and increases the activity in the hydrogenation of carbonyl groups.15 Increasing the Ru content of the catalytic system (i.e. Pt1Ru1/NH2-rGO catalyst, with a Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt molar ratio of 1
Pt molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the initial reaction rate is enhanced, however, it is accompanied by a loss in selectivity (see Fig. 2c). Indeed, after 20 h, the conversion is around ca. 97% with only ca. 75% selectivity to 1-phenylethanol (2) (Table 2, entry 3). This trend was confirmed with Pt1Ru5/NH2-rGO, which presents a higher initial conversion rate but lower selectivity towards 1-phenylethanol (2) (Fig. 2b). After 20 h of reaction time, full conversion is reached for Pt1Ru5/NH2-rGO with a chemoselectivity similar to monometallic Ru/NH2-rGO (Table 2, entries 1 and 2). Altogether, the obtained results clearly indicate that it is possible to control the activity and selectivity of graphene-supported bimetallic Pt–Ru MNPs during the hydrogenation of acetophenone by finely tuning their metal composition.
1), the initial reaction rate is enhanced, however, it is accompanied by a loss in selectivity (see Fig. 2c). Indeed, after 20 h, the conversion is around ca. 97% with only ca. 75% selectivity to 1-phenylethanol (2) (Table 2, entry 3). This trend was confirmed with Pt1Ru5/NH2-rGO, which presents a higher initial conversion rate but lower selectivity towards 1-phenylethanol (2) (Fig. 2b). After 20 h of reaction time, full conversion is reached for Pt1Ru5/NH2-rGO with a chemoselectivity similar to monometallic Ru/NH2-rGO (Table 2, entries 1 and 2). Altogether, the obtained results clearly indicate that it is possible to control the activity and selectivity of graphene-supported bimetallic Pt–Ru MNPs during the hydrogenation of acetophenone by finely tuning their metal composition.
Moreover, it is worth commenting that the formation of benzyl alcohols by the selective hydrogenation of aromatic ketones is normally carried out by homogeneous catalysts.23 When heterogeneous catalysts are used, a mixture of (2), (3) and (4) is generally obtained.24 Nevertheless, MNPs immobilized on basic supports have been recently reported as efficient catalysts for selective hydrogenation reactions.25 Among these catalysts we can find Ru/NH2-rGO, which presents a high selectivity in the hydrogenation of fatty acids,8 due to the presence of basic sites next to the active metal centres that heterolytically split H2 and enhances the hydrogenation of polar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. To confirm this support effect on the new bimetallic catalysts herein presented, we generated Pt5Ru1 MNPs on reduced graphene oxide (rGO). The lack of N-atoms on Pt5Ru1/rGO is reflected in a loss of activity and selectivity compared to Pt5Ru1/NH2-rGO (Table 2, entries 4 and 6). After 20 h, Pt5Ru1/rGO only shows a conversion of 87% with a selectivity to 1-phenylethanol (3) of 87%. Besides, the formation of 3% of 1-cylohexylethanone (4), 8% of 1-cyclohexylethanol (3), and traces of the HDO products (5 and 6) is observed. As the metal composition (see the Experimental part) and MNP size (Fig. 1f) of Pt5Ru1/rGO are in the same range as those of the analogous N-doped catalyst, (i.e. Pt5Ru1/NH2-rGO), we can assume that N-doped graphene enhances the selectivity of the bimetallic catalyst in the hydrogenation of the carbonyl groups in the aromatic ketones. Therefore, the high activity and selectivity observed for Pt5Ru1/NH2-rGO are due to the combination of two factors: (i) the electronic modification of Pt by Ru in the bimetallic nanoparticles that favours their interaction with the carbonyl group and (ii) the cooperative work between the metallic surface and the nitrogen atoms of the support to dissociate H2 heterolytically.
O bonds. To confirm this support effect on the new bimetallic catalysts herein presented, we generated Pt5Ru1 MNPs on reduced graphene oxide (rGO). The lack of N-atoms on Pt5Ru1/rGO is reflected in a loss of activity and selectivity compared to Pt5Ru1/NH2-rGO (Table 2, entries 4 and 6). After 20 h, Pt5Ru1/rGO only shows a conversion of 87% with a selectivity to 1-phenylethanol (3) of 87%. Besides, the formation of 3% of 1-cylohexylethanone (4), 8% of 1-cyclohexylethanol (3), and traces of the HDO products (5 and 6) is observed. As the metal composition (see the Experimental part) and MNP size (Fig. 1f) of Pt5Ru1/rGO are in the same range as those of the analogous N-doped catalyst, (i.e. Pt5Ru1/NH2-rGO), we can assume that N-doped graphene enhances the selectivity of the bimetallic catalyst in the hydrogenation of the carbonyl groups in the aromatic ketones. Therefore, the high activity and selectivity observed for Pt5Ru1/NH2-rGO are due to the combination of two factors: (i) the electronic modification of Pt by Ru in the bimetallic nanoparticles that favours their interaction with the carbonyl group and (ii) the cooperative work between the metallic surface and the nitrogen atoms of the support to dissociate H2 heterolytically.
As mentioned in the introduction, another efficient way to control the selectivity of MNPs is through their surface functionalization with organic molecules or molecular complexes.12,13 However, this surface modification is usually accompanied with a loss of activity. Then, Ru/NH2-rGO was decorated with 0.5 equiv. of tributyltin hydride (Ru–Bu3Sn/NH2-rGO, Fig. S7, ESI†) and tested in the hydrogenation of acetophenone under the standard conditions (20 h at 130 °C and 50 bar H2). The functionalization of Ru/NH2-rGO NPs with this organometallic tin complex increases their selectivity, but considerably decreases their activity (Fig. 2f). Specifically, after 20 h, the conversion is only 38% with a selectivity towards 1-phenylethanol (2) of 97% (Table 2, entry 7). Interestingly, this was not the case for the bimetallic system Pt5Ru1/NH2-rGO, where the activity is much less affected (i.e. 96% of 1-phenylethanol at 92% conversion; Table 2, entry 4). Although the use of surface molecules to control the selectivity is an efficient strategy, the characteristic loss of activity makes a priori the bimetallic systems more attractive catalysts.
The reactivity of Pt5Ru1/NH2-rGO was compared with the monometallic Pt and Ru catalysts for the hydrogenation of other functionalized arenes such as nitrobenzene or benzaldehyde (Table 3). In general, a similar trend was observed for acetophenone, Ru/NH2-rGO being the most active system but the less selective. For example, in the hydrogenation of nitrobenzene (Table 3, entries 8–10), Ru/NH2-rGO not only allows the complete conversion of the substrate, but also shows the higher reactivity for the over reduced product (55% cyclohexylamine). On the other hand, the bimetallic and platinum systems exhibit a similar reactivity, both being highly selective to aniline at full conversion. When comparing the reactivity of these catalytic systems with benzaldehyde (Table 3, entries 11–13), again the monometallic ruthenium catalyst shows the highest activity but the lowest selectivity. After 4 h, Ru/NH2-rGO presents full conversion and 84% selectivity towards benzyl alcohol, whereas the conversion of benzaldehyde with Pt/NH2-rGO is only 67%, being totally selective to the aromatic alcohol. In between, we could find the activity of Pt5Ru1/NH2-rGO, which presents great selectivity of platinum but with a much higher activity (100% selectivity towards the aromatic alcohol at 93% conversion).
| Entry/Catalyst | Substrates | Products | Conditionsa | Conv.b | Selectivityb | |
|---|---|---|---|---|---|---|
| a Reactions conditions: 0.5 mmol substrate, 0.5 mol% cat. (0.0025 mmol metal), 10 mL THF. b Conversions and selectivities were determined by GC using dodecane as the internal standard, and confirmed by GC-MS. | ||||||
| 8/Ru/NH2-rGO |
 (7) (7) |
 (8) (8) |
 (9) (9) |
H2 (30 bar), 1 h, 100 °C | >99% |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9 = 45 9 = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
| 9/Pt5Ru1/NH2-rGO | >99% |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9 = 95 9 = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
||||
| 10/Pt/NH2-rGO | >99% |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9 = 97 9 = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
||||
| 11/Ru/NH2-rGO |
 (10) (10) |
 (11) (11) |
 (12) (12) |
H2 (50 bar), 4 h, 130 °C | >99% |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 13 = 84 13 = 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 12/Pt5Ru1/NH2-rGO |
 (13) (13) |
93% |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 13 = 100 13 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
|||
| 13/Pt/NH2-rGO | 67% |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 12: 13 = 100 12: 13 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
||||
Owing to the potential activity and selectivity of Pt5Ru1/NH2-rGO for hydrogenation reactions, we have also studied here the reduction of an important biomass derived platform molecule such as 5-hydroxymethylfurfural (HMF). The selective hydrogenation of the formyl group to produce 2,5-bis(hydroxymethyl)furan (BHMF) is gaining increasing attention since it is a bio feedstock of high industrial potential.26 A variety of products can be obtained during the catalytic reduction of HMF, as can be seen in Fig. 3a. The hydrogenation products are mainly BHMF and 2,5-bis(hydroxymethyl)tetrahydrofuran (BHMTHF), depending on whether the aldehyde group is only reduced, or the HMF is totally hydrogenated. Moreover, during the catalysis, HDO products such as 5-methylfurfural (5-MF), 2-hydroxymethyl-5-methylfuran (MFA) or 2-hydroxymethyl-5-methyltetrahydrofuran (MTHFA) can also be formed. Comparing the results for the different products after 4 h of reaction (Fig. 3b–d), it can be clearly observed that although Ru/NH2-rGO is the most active catalyst (96% conversion), Pt5Ru1/NH2-rGO shows a similar activity, but with superior selectivity to the desired BHMF (87% of BHMF at 93% conversion). Meanwhile, Ru/NH2-rGO only shows 78% selectivity towards the symmetric diol BHMF, forming also BHMTHF (18%) and MFA (4%). A noticeable amount of the HDO product, MFA, is also observed for Pt5Ru1/NH2-rGO (10%). On the other hand, monometallic Pt/NH2-rGO is clearly the least active system, but the most selective one (94% of BHMF at 83% conversion). Again, we can conclude that the bimetallic system Pt5Ru1/NH2-rGO is almost as active as Ru/NH2-rGO, but maintaining the high selectivity of Pt/NH2-rGO. Doping with Ru the graphene-supported Pt NPs strongly increase the activity of Pt while preserving its high selectivity for the hydrogenation of the carbonyl group.
To investigate the stability and recyclability of Pt5Ru1/NH2-rGO NPs a series of experiments were carried out. First, this bimetallic system was analyzed by TEM and HRTEM after catalysis (i.e. hydrogenation of acetophenone). TEM micrographs revealed MNPs with a similar size and metal distribution to the as-synthesized Pt5Ru1/NH2-rGO NPs (2.3 ± 0.7 nm; Fig. S8, ESI†). On the other hand, TEM images of Pt5Ru1/rGO NPs after catalysis showed a significant increase in MNP size (5.8 ± 1.2 nm; Fig. S9, ESI†) and a considerable amount of aggregates. This demonstrates the efficiency of N-doped graphene to stabilize the nanoparticles on the graphene sheets even under harsh reaction conditions (20 h, 130 °C and 50 bar H2), preventing their agglomeration. HRTEM analysis confirmed that Pt5Ru1/NH2-rGO NPs after catalysis (Pt5Ru1/NH2-rGOafter) still maintain their crystalline structure (Fig. S1f, ESI†). In particular, the nanoparticles present an fcc crystalline structure with a lattice spacing of 2.28 Å that corresponds to the (111) plane. To test the recyclability of Pt5Ru1/NH2-rGO, a multiple addition experiment was carried out. More precisely, the hydrogenation of nitrobenzene was performed in a multiple way by using Pt5Ru1/NH2-rGO as a catalyst, and after seven consecutive additions of nitrobenzene, the activity and selectivity of Pt5Ru1/NH2-rGO remain constant (Fig. 4). This result confirms the stability of the catalyst with time under catalytic conditions. TEM analysis of Pt5Ru1/NH2-rGO NPs after the multiple addition experiment showed no significant differences in size, dispersion and distribution (Fig. S10, ESI†), which is in line with the high stability of this catalyst. To prove the heterogeneous nature of Pt5Ru1/NH2-rGO, a “hot filtration” test was carried out during a HMF hydrogenation reaction (130 °C, 50 bar H2, 10 ml THF). After 50 min of reaction, Pt5Ru1/NH2-rGO was removed by thermal filtration and the reactor was re-charged with the starting feed. After 4 h at 130 °C and 50 bar H2, no change in the conversion is observed; it remained at 63% (Table S5, ESI†), while almost full conversion is obtained in the presence of the catalyst (Fig. 3c). This result, together with the absence of metal leaching observed by ICP after the “hot filtration” (see the Experimental part), confirms that the reaction is heterogeneously catalyzed.
Experimental
General considerations and starting materials
All chemical operations were carried out using standard Schlenk tubes, Fischer–Porter bottle techniques or in a glove-box under an argon atmosphere. Solvents were purified before use; THF (Sigma-Aldrich) by distillation under an argon atmosphere through filtration in the column of a purification system (MBraun). Ru(COD)(COT) and Pt(NBE)3 were purchased from Nanomeps, and acetophenone (99%), 1-phenylethanol (98%), 1-cyclohexylethanol (97%), ethylbenzene (99%), ethylcyclohexane (99%), hydroxymethylfurfural (99%), benzaldehyde (99%), nitrobenzene (99%) and dodecane (99%) from Sigma Aldrich. All reagents were used without purification, except for HMF which is purified by filtering with an equimolar silica![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alumina mixture, and stored in a refrigerator.
alumina mixture, and stored in a refrigerator.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 (6
HNO3 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solution was then sonicated for 90 minutes and the samples were digested at 180 °C for 15 hours. After that, it was cooled down until room temperature (r.t.), diluted with 100 mL of water and analyzed by ICP.
1). The solution was then sonicated for 90 minutes and the samples were digested at 180 °C for 15 hours. After that, it was cooled down until room temperature (r.t.), diluted with 100 mL of water and analyzed by ICP.
Synthesis of graphenes
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1630–1612 cm−1 (ν H2O), 1060 cm−1 (ν C0), 1384 cm−1 (ν bending O–H), 1270 cm−1 (ν O–H phenolic).
O), 1630–1612 cm−1 (ν H2O), 1060 cm−1 (ν C0), 1384 cm−1 (ν bending O–H), 1270 cm−1 (ν O–H phenolic).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 h. Elemental analysis: C: 83.6%, H: 0.5%, S: 0.14%.
30 h. Elemental analysis: C: 83.6%, H: 0.5%, S: 0.14%.
Synthesis of monometallic catalysts
Synthesis of bimetallic catalysts
Hydrogenation reactions
The hydrogenation of acetophenone, nitrobenzene, benzaldehyde and hydroxymethylfurfural was carried in a 25 mL engineer autoclave equipped with a mechanical stirrer (750 rpm). Hydrogenations were performed such that the molar ratio metal–substrate was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200. Thus, taking into account that 0.5 mmol of substrate was used and each catalyst has 3% of the corresponding metal, 0.5 mol% of metal-catalyst was added into each reaction (0.0025 mmol). The exact mass of catalysts added during the catalysis is shown in Table S4 (see the ESI†).
200. Thus, taking into account that 0.5 mmol of substrate was used and each catalyst has 3% of the corresponding metal, 0.5 mol% of metal-catalyst was added into each reaction (0.0025 mmol). The exact mass of catalysts added during the catalysis is shown in Table S4 (see the ESI†).
The determined amount of the catalyst in each case was dispersed in 10 mL of THF sonicating for 90 minutes. The mixture was then transferred into the reactor and this one was purged with H2 three times, and finally charged with 35 bar H2. The reactor was then heated at 150 °C, reaching a final pressure of 50 bar. The catalyst is kept under these conditions for two hours, to ensure the reduction and activation of the active metal. Before the substrate (0.5 mmol) is introduced with the help of a 250 μL Hamilton syringe, the temperature is reduced to the desired temperature (100 or 130 °C) to carry out the reaction. After holding the reaction for the time required, we stopped the heater and the reactor was left to reach room temperature. Then, the mechanical stirring was stopped and the H2 pressure removed. Finally, the catalyst was separated by filtration, and the products of the reaction mixture were analyzed by GC using dodecane as the internal standard.
Kinetic experiments
For the kinetic experiments, the autoclave was charged, pressurized and heated under the required conditions and aliquots were taken from the reaction medium and analyzed by GC, using dodecane as the internal standard.Multiple addition experiment
For the multiple addition experiment, the autoclave was charged, pressurized and heated under the required conditions for nitrobenzene hydrogenation (100 °C, 30 bar H2, 10 ml THF). Each hour, an aliquot was taken from the reaction medium and a new starting material (i.e. nitrobenzene) was added to the reactor. The aliquots were analyzed by GC, using dodecane as the internal standard. The activity and selectivity remained over 7 catalytic cycles confirming the stability of the catalyst.“Hot filtration” and leaching experiments
For the “hot filtration” experiment, the autoclave was charged, pressurized and heated under the required conditions for HMF hydrogenation (130 °C, 50 bar H2, 10 ml THF). After 50 min, Pt5Ru1/NH2-rGO was removed by filtration, and the reactor was charged with the mother liquor, pressurized and heated under the same conditions. Then, the conversion of HMF was determined after 4 h and compared to that at 50 min (Table S5, ESI†). No change in the conversion was observed. After thermal filtration, the mother liquor was analysed by ICP and ruthenium and platinum detected were negligible.Conclusions
Bimetallic PtRu NPs of different metal composition (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) have been generated on N-doped reduced graphene oxide (NH2-rGO) by co-decomposition of two organometallic precursors, namely [Pt(NBE)3] and [Ru(COD)(COT)]. The similar decomposition rates of the precursors led to the formation of alloy NPs. These supported-bimetallic NPs, together with their monometallic analogues (Ru/NH2-rGO and Pt/NH2-rGO), were fully characterized by TEM, HRTEM, STEM-EDX, Raman and XPS. Different surface metal compositions depending on the Pt/Ru ratio used during the synthesis process were observed for the bimetallic systems. Interestingly, both the activity and selectivity of these PtRu NPs were highly dependent on the nanoparticle composition and the support employed. To evaluate the activity and selectivity of the catalytic systems prepared, the hydrogenation of acetophenone was used as a model reaction. While monometallic Ru/NH2-rGO NPs showed a high conversion to the over reduced product (1-cyclohexylethanol), Pt/NH2-rGO exhibited great selectivity towards 1-phenylethanol, but with a much lower activity. In-between we can find the reactivity of supported-bimetallic NPs, Pt5Ru1/NH2-rGO being the most interesting catalyst since it is as selective as Pt/NH2-rGO but much more active. The remarkable activity and selectivity of Pt5Ru1/NH2-rGO are due to two factors: (i) the electron-density modification of Pt by Ru that activates the C
5) have been generated on N-doped reduced graphene oxide (NH2-rGO) by co-decomposition of two organometallic precursors, namely [Pt(NBE)3] and [Ru(COD)(COT)]. The similar decomposition rates of the precursors led to the formation of alloy NPs. These supported-bimetallic NPs, together with their monometallic analogues (Ru/NH2-rGO and Pt/NH2-rGO), were fully characterized by TEM, HRTEM, STEM-EDX, Raman and XPS. Different surface metal compositions depending on the Pt/Ru ratio used during the synthesis process were observed for the bimetallic systems. Interestingly, both the activity and selectivity of these PtRu NPs were highly dependent on the nanoparticle composition and the support employed. To evaluate the activity and selectivity of the catalytic systems prepared, the hydrogenation of acetophenone was used as a model reaction. While monometallic Ru/NH2-rGO NPs showed a high conversion to the over reduced product (1-cyclohexylethanol), Pt/NH2-rGO exhibited great selectivity towards 1-phenylethanol, but with a much lower activity. In-between we can find the reactivity of supported-bimetallic NPs, Pt5Ru1/NH2-rGO being the most interesting catalyst since it is as selective as Pt/NH2-rGO but much more active. The remarkable activity and selectivity of Pt5Ru1/NH2-rGO are due to two factors: (i) the electron-density modification of Pt by Ru that activates the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and (ii) the cooperative work between the N atoms of graphene and the metal active sites, which dissociates H2 in a heterolytic way, and enhances the selectivity towards the hydrogenation of polar bonds, such as carbonyls. The reactivity of this bimetallic catalyst was also investigated in the hydrogenation of other substrates such as functionalized arenes (i.e. nitrobenzene and benzaldehyde) or hydroxymethylfurfural (HMF), proving that it is possible to control the activity/selectivity of supported-bimetallic PtRu NPs by tuning their metal surface composition. Finally, the stability and recyclability of Pt5Ru1/NH2-rGO was explored by multiple addition and “hot filtration” experiments, demonstrating to be a stable and recyclable catalyst. The remarkable activity, selectivity and stability of these bimetallic catalytic systems make them interesting candidates for future selective catalysis.
O bonds and (ii) the cooperative work between the N atoms of graphene and the metal active sites, which dissociates H2 in a heterolytic way, and enhances the selectivity towards the hydrogenation of polar bonds, such as carbonyls. The reactivity of this bimetallic catalyst was also investigated in the hydrogenation of other substrates such as functionalized arenes (i.e. nitrobenzene and benzaldehyde) or hydroxymethylfurfural (HMF), proving that it is possible to control the activity/selectivity of supported-bimetallic PtRu NPs by tuning their metal surface composition. Finally, the stability and recyclability of Pt5Ru1/NH2-rGO was explored by multiple addition and “hot filtration” experiments, demonstrating to be a stable and recyclable catalyst. The remarkable activity, selectivity and stability of these bimetallic catalytic systems make them interesting candidates for future selective catalysis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Instituto de Tecnología Química (ITQ), Consejo Superior de Investigaciones Científicas (CSIC), and Universitat Politècnica de València (UPV) for the facilities, and Severo Ochoa excellence programme (SEV-2016-0683), “Juan de la Cierva” programme (IJCI-2016-27966) and Primero Proyectos de Investigación (PAID-06-18) for financial support. C. C.-N. gratefully thanks Generalitat Valenciana predoctoral fellowship (GVA: ACIF/2019/076). We also thank the Electron Microscopy Service of the UPV for TEM facilities and A. García Zaragoza for his assistance in catalytic reactions.Notes and references
- M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072 CrossRef CAS.
- A. Corma and H. Garcia, Top. Catal., 2008, 48, 8 CrossRef CAS.
- (a) L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981 CrossRef CAS; (b) L. Liu and A. Corma, Nat. Rev. Mater., 2020 DOI:10.1038/s41578-020-00250-3; (c) L. Liu, M. López-Haro, C. W. Lopes, C. Li, P. Concepcion, L. Simonelli, J. J. Calvino and A. Corma, Nat. Mater., 2019, 18, 866 CrossRef CAS; (d) B. Ni and X. Wang, Adv. Sci., 2015, 2, 1500085 CrossRef; (e) M. R. Axet and K. Philippot, Chem. Rev., 2020, 120, 1085 CrossRef CAS.
- (a) Q.-L. Zhu and Q. Xu, Chem, 2016, 1, 220 CrossRef CAS; (b) Y.-Z. Chen, G. Cai, Y. Wang, Q. Xu, S.-H. Yu and H.-L. Jiang, Green Chem., 2016, 18, 1212 RSC; (c) A. Noujima, T. Mitsudome, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., Int. Ed., 2011, 50, 2986 CrossRef CAS.
- (a) L. Xia, D. Li, J. Long, F. Huang, L. Yang, Y. Guo, Z. Jia, J. Xiao and H. Liu, Carbon, 2019, 145, 47 CrossRef CAS; (b) S. Pisiewicz, D. Formenti, A.-E. Surkus, M.-M. Pohl, J. Radnik, K. Junge, C. Topf, S. Bachmann, M. Scalone and M. Beller, ChemCatChem, 2016, 8, 129 CrossRef CAS; (c) P. Liu, G. Li, W.-T. Chang, M.-Y. Wu, Y.-X. Li and J. Wang, RSC Adv., 2015, 5, 72785 RSC; (d) A. Mollar-Cuni, D. Ventura-Espinosa, S. Martín, Á. Mayoral, P. Borja and J. A. Mata, ACS Omega, 2018, 3, 15217 CrossRef CAS.
- (a) S. A. Giles, Y. Yan and D. G. Vlachos, ACS Catal., 2019, 9, 1129 CrossRef CAS; (b) C. S. Ramirez-Barria, M. Isaacs, C. Parlett, K. Wilson, A. Guerrero-Ruiz and I. Rodríguez-Ramos, Catal. Today, 2019, 357, 8 CrossRef; (c) X. Xie, J. Long, J. Xu, L. Chen, Y. Wang, Z. Zhang and X. Wang, RSC Adv., 2012, 2, 12438 RSC.
- (a) R. V. Siva Prasanna Sanka, K. Balaji, Y. Leterrier, S. Pandey, M. Srivastava, A. Srivastava, W. H. Binder, S. Rana and V. Michaud, Chem. Commun., 2019, 55, 6249 RSC; (b) M. Kuniyil, V. S. J. Kumar, F. S. Adil, R. M. Shaik, M. Khan, E. M. Assal, R. H. M. Siddiqui and A. Al-Warthan, Catalysts, 2019, 9, 469 CrossRef CAS; (c) X. Zhao, J. Xie, X. Liu and X. Liu, Appl. Organomet. Chem., 2019, 33, 4623 CrossRef.
- L. M. Martínez-Prieto, M. Puche, C. Cerezo-Navarrete and B. Chaudret, J. Catal., 2019, 377, 429 CrossRef.
- M. Sankar, N. Dimitratos, P. J. Miedziak, P. P. Wells, C. J. Kiely and G. J. Hutchings, Chem. Soc. Rev., 2012, 41, 8099 RSC.
- (a) F. Gao and D. W. Goodman, Chem. Soc. Rev., 2012, 41, 8009 RSC; (b) N. M. Wilson, P. Priyadarshini, S. Kunz and D. W. Flaherty, J. Catal., 2018, 357, 163 CrossRef.
- (a) L. M. Martínez-Prieto and B. Chaudret, Acc. Chem. Res., 2018, 51, 376 CrossRef; (b) L. M. Martínez-Prieto and P. W. N. M. van Leeuwen, Ligand effects in ruthenium nanoparticle catalysis, in Recent Advances in Nanoparticle Catalysis, Springer Science, 2020 Search PubMed.
- S. Kunz, Top. Catal., 2016, 59, 1671 CrossRef CAS.
- (a) E. Bonnefille, F. Novio, T. Gutmann, R. Poteau, P. Lecante, J.-C. Jumas, K. Philippot and B. Chaudret, Nanoscale, 2014, 6, 9806 RSC; (b) L. M. Martínez-Prieto, J. Marbaix, J. M. Asensio, C. Cerezo-Navarrete, P.-F. Fazzini, K. Soulantica, B. Chaudret and A. Corma, ACS Appl. Nano Mater., 2020, 3, 7076 CrossRef.
- J.-Y. Ruzicka, D. P. Anderson, S. Gaw and V. B. Golovko, Aust. J. Chem., 2012, 65, 1420 CrossRef CAS.
- (a) P. Claus, Top. Catal., 1998, 5, 51 CrossRef CAS; (b) P. Mäki-Arvela, J. Hájek, T. Salmi and D. Y. Murzin, Appl. Catal., A, 2005, 292, 1 CrossRef; (c) X. Qi, M. R. Axet, K. Philippot, P. Lecante and P. Serp, Dalton Trans., 2014, 43, 9283 RSC.
- D. Bouzouita, G. Lippens, E. A. Baquero, P. F. Fazzini, G. Pieters, Y. Coppel, P. Lecante, S. Tricard, L. M. Martínez-Prieto and B. Chaudret, Nanoscale, 2019, 11, 16544 RSC.
- C. Pan, F. Dassenoy, M.-J. Casanove, K. Philippot, C. Amiens, P. Lecante, A. Mosset and B. Chaudret, J. Phys. Chem. B, 1999, 103, 10098 CrossRef CAS.
- S. Navalon, A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Coord. Chem. Rev., 2016, 312, 99 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS.
- L. Lai, L. Chen, D. Zhan, L. Sun, J. Liu, S. H. Lim, C. K. Poh, Z. Shen and J. Lin, Carbon, 2011, 49, 3250 CrossRef CAS.
- D. J. Morgan, Surf. Interface Anal., 2015, 47, 1072 CrossRef CAS.
- F. Sen and G. Gökagaç, J. Phys. Chem. C, 2007, 111, 5715 CrossRef CAS.
- P. W. N. M. van Leeuwen, Homogeneous Catalysis—Understanding the Art, Kluwer Academic Publishers, 2004 Search PubMed.
- See for example: (a) A. Denicourt-Nowicki, B. Leger and A. Roucoux, Phys. Chem. Chem. Phys., 2011, 13, 13510 RSC; (b) D. Duraczynska, A. Drelinkiewicz, E. Bielanska, E. M. Serwicka and L. Litynska-Dobrzynska, Catal. Lett., 2011, 141, 83 CrossRef CAS.
- See for example: (a) M. Fang and R. A. Sánchez-Delgado, J. Catal., 2014, 311, 357 CrossRef CAS; (b) D. Formenti, C. Topf, K. Junge, F. Ragaini and M. Beller, Catal.: Sci. Technol., 2016, 6, 4473 RSC; (c) J. L. Fiorio, R. V. Gonçalves, E. Teixeira-Neto, M. A. Ortuño, N. López and L. M. Rossi, ACS Catal., 2018, 8, 3516 CrossRef CAS.
- R.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499 CrossRef CAS.
- T. Suoranta, M. Niemelä and P. Perämäki, Talanta, 2014, 119, 425 CrossRef CAS.
- L. Lai, L. Chen, D. Zhan, L. Sun, J. Liu, S. H. Lim, C. K. Poh, Z. Shen and J. Lin, Carbon, 2011, 49, 3250 CrossRef CAS.
- (a) W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS; (b) M. Feliz, M. Puche, P. Atienzar, P. Concepción, S. Cordier and Y. Molard, ChemSusChem, 2016, 9, 1963 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cy02379e |
| This journal is © The Royal Society of Chemistry 2021 |



