Open Access Article
Open Access ArticleVanadium complexes derived from oxacalix[6]arenes: structural studies and use in the ring opening homo-/co-polymerization of ε-caprolactone/δ-valerolactone and ethylene polymerization†
Tian
Xing
a,
Timothy J.
Prior
a,
Mark R. J.
Elsegood
 b,
Nina V.
Semikolenova
b,
Nina V.
Semikolenova
 c,
Igor E.
Soshnikov
c,
Igor E.
Soshnikov
 cd,
Konstantin
Bryliakov
cd,
Konstantin
Bryliakov
 cd,
Kai
Chen
cd,
Kai
Chen
 ae and
Carl
Redshaw
ae and
Carl
Redshaw
 *a
*a
aPlastics Collaboratory, Department of Chemistry, University of Hull, Cottingham Road, Hull, HU6 7RX, UK. E-mail: c.redshaw@hull.ac.uk
bChemistry Department, Loughborough University, Loughborough, Leicestershire, LE11 3TU, UK
cBoreskov Institute of Catalysis, Pr. Lavrentieva 5, Novosibirsk 630090, Russian Federation
dNovosibirsk State University, Pirogova 1, Novosibirsk 630090, Russian Federation
eCollaborative Innovation Center of Atmospheric Environment and Equipment Technology, Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control, School of Environmental Science and Engineering, Nanjing University of Information Science & Technology, Nanjing 210044, P. R. China
First published on 19th November 2020
Abstract
Reaction of Na[VO(tBuO)4] (generated in situ from VOCl3 and NaOtBu) with p-tert-butyltetrahomodioxacalix[6]areneH6 (L1H6) afforded, after work-up (in MeCN), the mixed-metal complex [(VO)2(μ-O)Na2(L1)(MeCN)4]·5(MeCN) (1·5MeCN), whilst the oxo complex {[VO]4L1} (2·6MeCN) was isolated via the use of [VO(OnPr)3]. Reaction of L1H6 with [V(Np-CH3C6H4)(OtBu)3] afforded the complex {[V(Np-CH3C6H4)]2L1} (3·7MeCN·0.5CH2Cl2). Use of similar methodology afforded the imido complexes {[V(Np-RC6H4)]2L1} (R = OMe (4); CF3 (5); Cl (6); F (7)); on one occasion, reaction of [V(Np-CH3C6H4)(OEt)3] with L1H6 afforded the product [VO(L2)]2·4MeCN (8·4MeCN) (L2 = 2-(p-CH3-C6H4NCH)-4-tBu-C6H2O-6-CH2)-4-tBuC6H2OH) in which L1 has been cleaved. For comparative catalytic ring opening polymerization (ROP) studies, the known complexes [VOL3] (L3 = oxacalix[3]arene) (I), [V(Np-CH3-C6H4)L3]2 (II), [Li(MeCN)4][V2(O)2Li(MeCN)(L6H2)2] (L6H6 = p-tert-butylcalix[6]areneH6) (III) and [(VO)2L8H] (L8H8 = p-tert-butylcalix[8]areneH8) (IV) have also been prepared. ROP studies, with or without external alcohol present, indicated that complexes 1 to 8 exhibited moderate to good conversions for ε-Cl, δ-VL and the co-polymerization thereof. Within the imido series, a positive influence was observed when electron withdrawing substituents were present. These systems afforded relatively low molecular weight products and were also inactive toward the ROP of rac-lactide. In the case of ethylene polymerization, complexes 3, 5 and 7 exhibited highest activity when screened in the presence of dimethylaluminium chloride/ethyltrichloroacetate; the activity of 4 was much lower. The products were highly linear polyethylene with Mw in the range 74–120 × 103 Da.
Introduction
The need for new environmentally friendly plastics has been highlighted by on-going global pollution issues, however traditional plastics, when used and disposed of correctly, still have a large part to play in society. Indeed, the recent COVID19 outbreak is a good illustration of our dependence on plastics, where there was widespread demand for the use of plastic-based facemasks.1 Metal catalysts play a central role in the production of both petroleum-based plastics (α-olefin polymerization) and biodegradable polymers formed via the ring opening polymerization (ROP) of cyclic esters.2 For both processes, manipulation of the catalyst properties can be achieved by variation of the metal-bound groups, and this allows for control over both catalytic activity and polymer properties. It is also important that the catalytic metal centre employed is cheap and non-toxic. With this in mind, a number of earth-abundant metals have been employed as the reactive metal centre in both polymerization processes.1,3 Furthermore, results using vanadium-based systems indicate this metal also has potential in this area,4 and it is noteworthy that reports on the effect of imido ligand variation in vanadium-based systems have appeared for studies on oligo-/polymerization of ethylene, ethylene/propylene copolymerization and ethylene/cyclic olefin copolymerization.5 The ligands in the pre-catalyst can take a number of forms, selected in-part for their ability to impose some stability to the catalytically active species. In α-olefin polymerization, an external alkylating co-catalyst is typically employed to form a metal–alkyl [M–R] species, whilst in ROP, the generation of metal alkoxide [M–OR] species is favoured. Calix[n]arenes, which are phenolic macrocycles, have shown potential in a variety of catalytic applications.6 By variation of n, the number of phenolic groups, calix[n]arenes can act as platforms for binding multiple metal centres.7 In our previous work, a series of vanadium-based calix[n]arene complexes were structurally identified, and by changing the calixarene bridging group from a methylene (–CH2–) to a dimethyleneoxa group (–CH2OCH2–), vanadium complexes with superior catalytic performance for both α-olefin homo- and co-polymerization over related methylene-bridged systems were obtained.8 These vanadium studies previously focused on oxacalix[3]arene derivatives, however larger oxacalix[n]arenes are known.9 Herein, we investigate the use of the p-tert-butyltetrahomodioxacalix[6]areneH6-derived systems (Chart 1) in both the ROP of the cyclic esters ε-caprolactone and δ-valerolactone, and the copolymerization thereof, and for the polymerization of ethylene. Results are compared versus known vanadium catalysts bearing either p-tert-butylhexahomotrioxacalix[3]areneH3 or p-tert-butylcalix[6 and 8]areneH6,8-derived ligands (Chart 2).7,8,10 We note that poly(ε-caprolactone), PCL and PVL are favoured polymers given their biodegradability and the fact that they are considered as potential environmentally friendly commodity plastics.11 | ||
| Chart 2 Known pre-catalysts I–IV used herein.7,9 | ||
Results and discussion
Syntheses and solid-state structures
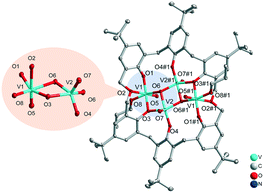
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O5 bond length is typical at 1.588(3) Å,10 linked via near linear (171.8(2)°) V–O11–V bonding. Each vanadium is further coordinated by three phenoxide oxygens of the oxacalix[6]arene, with longer bonding (ca. 1.95 Å) observed to each of the bridging calixarene oxygens (O2 and O8) which are also involved in bonding to a 5-coordinate sodium cation, with the latter (Na1 and Na2) each bonding to two of the phenoxide oxygens and the oxygen of the dimethyleneoxa bridge. Two acetonitrile ligands complete the bonding at each sodium centre.
O5 bond length is typical at 1.588(3) Å,10 linked via near linear (171.8(2)°) V–O11–V bonding. Each vanadium is further coordinated by three phenoxide oxygens of the oxacalix[6]arene, with longer bonding (ca. 1.95 Å) observed to each of the bridging calixarene oxygens (O2 and O8) which are also involved in bonding to a 5-coordinate sodium cation, with the latter (Na1 and Na2) each bonding to two of the phenoxide oxygens and the oxygen of the dimethyleneoxa bridge. Two acetonitrile ligands complete the bonding at each sodium centre.
Reaction of [VO(OnPr)3] (4 equivalents) with L1H6 led, after work-up, to the blue complex {[VO]4L1}·6MeCN (2·6MeCN). The molecular structure is shown in Fig. 2, with selected bond lengths and angles given in the caption. Calixarenes bind four vanadyl centres which form a 3-step V4O4 ladder. Such ladders have been observed previously in titanium calixarene chemistry.13
Two of the vanadyl centres (V1) are distorted octahedral and are bound by a long V–O2 bond (2.3732(14) Å) involving the oxygen of the dimethyleneoxa bridge trans to the vanadyl group, two phenoxides (1.7934(14) and 1.8128(14) Å) and an n-propoxide (V1–O8 1.7869(14) Å). The other vanadyl centre (V2) is trigonal bipyramidal (τ = 0.18),14 and is linked to V1 via a triply bridging oxygen O6 (Fig. 2).
Interaction of [V(Np-CH3C6H4)(OtBu)3] with L1H6 led, following work-up, to the isolation of the complex {[V(Np-CH3C6H4)]2L1} (3·7MeCN·0.5CH2Cl2). Single crystals were grown from a saturated acetonitrile solution at ambient temperature and an X-ray structure determination revealed the structure shown in Fig. 3; selected bond lengths and angles are given Table 1. Each vanadium adopts a distorted squared-based pyramidal geometry with the near-linear imido ligand at the trigonal bipyramidal (τ = 0.11),14 and are linked via asymmetric aryloxide bridges (of the dioxacalix[6]arene).
 | ||
| Fig. 3 Molecular structure of {[V(Np-CH3C6H4)]2L1} (3·7MeCN·0.5CH2Cl2). (H atoms and CH2Cl2 molecules are omitted for clarity). | ||
| Bond | 3·7MeCN·0.5CH2Cl2 | 4·4MeCN | 5 | 6 |
|---|---|---|---|---|
| V1–O1 | 1.837(3) | 1.852(15) | 1.786(4) | 1.826(4) |
| V1–O2 | 2.095(3) | — | — | — |
| V1–O3 | — | 1.789(14) | 1.815(4) | 1.782(4) |
| V2–O4 | — | 2.068(15) | 2.015(4) | 2.010(4) |
| V2–O5 | 1.839(3) | 1.841(16) | 1.775(4) | 1.836(4) |
| V2–O7 | — | 1.785(15) | 1.818(4) | 1.794(4) |
| V1–N1 | 1.652(3) | 1.656(18) | 1.645(5) | 1.665(5) |
| V1–O6 | 2.016(3) | — | — | — |
| V1–O7 | 1.786(3) | — | — | — |
| V1–O1–C1 | 123.2(2) | 123.00(12) | 127.2(3) | 122.7(4) |
| V1–O1–C58 | 129.3(2) | — | — | — |
| V1–O8–C58 | — | 128.44(12) | 120.9(3) | 127.4(3) |
| V1–O2–V2 | 108.34(11) | — | — | — |
| V1–O6–V2 | 110.10(11) | — | — | — |
| V2–O4–V1 | — | 108.68(7) | 109.14(19) | 108.7(2) |
| V1–O8–V2 | — | 109.05(7) | 108.65(19) | 108.5(2) |
| V1–N1–C70 | 173.7(3) | — | — | 169.5(5) |
| V1–N1–C71 | — | 176.51(16) | 175.9(5) | — |
| V2–N2–C81 | — | 178.53(17) | 174.5(5) | — |
| V1–N2–C76A | — | — | — | 174.0(4) |
Replacement of the para methyl group by OMe (4), CF3 (5), Cl (6) and F (7) led to the formation of the complexes 4–7 adopting the same general dimeric structure as observed for 3 (for synthetic details see the experimental section). The geometrical parameters (Table 1), show the similarity between these molecules and the p-tolyl analogue. The molecular structures of 4–6 are given in the ESI† (Fig. S1). As reported by Maatta et al.,16 the 51V NMR shifts are very sensitive to the nature of the para substituent in such imido complexes (Fig. 4). In general, the trend observed herein is as observed by Maatta, with the electron donating groups (e.g. p-OMe) at low field versus electron withdrawing groups (e.g. p-CF3) at high field. The exception is the p-F derivative, which surprisingly is found at low field (−173.4 ppm) and we tentatively attribute this to its position in relation to the macrocycle conformation.
 | ||
| Fig. 4 51V NMR chemical shifts for 3–7 and [V(Np-RC6H4)Cl3] (R = Me, OMe, CF3, Cl, F).16 | ||
When the metal precursor employed was [V(Np-CH3C6H4)(OEt)3], reaction with L1H6 led to the formation of small yellow prisms of [VO(L2)]2·4MeCN (8·4MeCN) (L2 = 2-(p-CH3C6H4NCH)-4-tBu-C6H2O-6-CH2)-4-tBuC6H2OH). The molecular structure of 8, as determined using synchrotron radiation, is shown in Fig. 5, with selected bond lengths and angles given in the caption. The molecule sits on a centre of symmetry. Interestingly, the dioxacalix[6]arene has been split in two by a reaction of the bridging oxygens and the p-tolylimido groups. The result is a metallo-macrocycle containing two square-based pyramidal vanadyl centres. The vanadyl V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O4 bond length is typical at 1.5933(4) Å,10 whilst the phenoxide bond lengths at 1.8891(15) and 1.9046(15) Å are comparable with those observed elsewhere in vanadyl calixarene chemistry.7,10 The two uncoordinated phenolic OH groups are involved in H-bonding to vanadium-bound phenoxide groups. There are four solvent molecules (MeCN) of crystallization per molecule, with two residing inside the metallo-macrocycle. We note that calixarene cleavage is rare, with previous reports involving cleavage of a methylene bridge under acidic conditions.18
O4 bond length is typical at 1.5933(4) Å,10 whilst the phenoxide bond lengths at 1.8891(15) and 1.9046(15) Å are comparable with those observed elsewhere in vanadyl calixarene chemistry.7,10 The two uncoordinated phenolic OH groups are involved in H-bonding to vanadium-bound phenoxide groups. There are four solvent molecules (MeCN) of crystallization per molecule, with two residing inside the metallo-macrocycle. We note that calixarene cleavage is rare, with previous reports involving cleavage of a methylene bridge under acidic conditions.18
Ring opening polymerization studies
General: The performance of these complexes to act as catalysts for the ring opening polymerization (ROP) of ε-caprolactone (ε-CL) (Table S1, ESI†), δ-valerolactone (δ-VL) (Table S2, ESI†), both with and without one equivalent of benzyl alcohol (BnOH) per vanadium present, has been investigated.Results in the absence of BnOH were less controlled but for completion are presented in the ESI† (Tables S1 and S2). The co-polymerization of ε-caprolactone and δ-valerolactone (Table 4) has also been investigated. In each case, performances are compared against the known complexes I–IV (Chart 2).
ε-Caprolactone (ε-CL)
Complexes 1–8 and I–IV were screened for their ability to polymerise ε-caprolactone and the results are collated in Table 2. The polymerization screening indicated that the best conditions were 500 equivalents of ε-caprolactone to vanadium at 130 °C. The activity of complex 1 increased with temperature and peaked at 500 equivalents of monomer. Complex 1 was also active at low catalyst loading leading to 82.5% conversion after 8 h for 1000 equivalents of monomer.| Run | Cat. | CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
t/h | T/°C | Conva (%) | M n,GPC × 10–3b | M w × 10–3b | M n,Cal × 10–3c | PDId | TONe | TOFf (h−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy. b M n/w, GPC values corrected considering Mark–Houwink factor (0.56) from polystyrene standards in THF. c Calculated from ([monomer]0/V) × conv (%) × monomer molecular weight (MCL = 114.14) + molecular weight of BnOH. d From GPC. e Turnover number (TON) = number of moles of ε-CL consumed/number of moles V. f Turnover frequency (TOF) = TON/time (h). | |||||||||||
| 1 | 1 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 82.5 | 10.22 | 17.21 | 94.26 | 1.68 | 825 | 103 |
| 2 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 89.2 | 11.89 | 16.48 | 51.01 | 1.39 | 446 | 56 |
| 3 | 1 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 87.9 | 4.59 | 6.49 | 25.19 | 1.41 | 220 | 27 |
| 4 | 1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 86.3 | 2.34 | 3.01 | 9.96 | 1.28 | 86 | 11 |
| 5 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 100 | 44.9 | 5.99 | 7.49 | 25.73 | 1.24 | 225 | 28 |
| 6 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 80 | 25.1 | 1.42 | 1.76 | 14.43 | 1.26 | 126 | 16 |
| 7 | 2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 34.1 | 4.98 | 5.98 | 19.57 | 1.20 | 171 | 21 |
| 8 | 3 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 39.2 | 5.26 | 6.31 | 22.48 | 1.19 | 196 | 25 |
| 9 | 4 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 41.2 | 5.89 | 6.72 | 23.62 | 1.14 | 206 | 26 |
| 10 | 5 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 59.2 | 9.05 | 13.76 | 33.89 | 1.52 | 296 | 37 |
| 11 | 6 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 46.6 | 6.54 | 8.05 | 26.70 | 1.24 | 233 | 29 |
| 12 | 7 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 43.1 | 6.40 | 7.33 | 24.70 | 1.16 | 216 | 27 |
| 13 | 8 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 71.4 | 9.87 | 12.59 | 40.85 | 1.27 | 357 | 45 |
| 14 | 8 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 100 | 49.7 | 6.92 | 7.92 | 28.47 | 1.14 | 249 | 31 |
| 15 | 8 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 80 | — | — | — | — | — | — | — |
| 16 | I | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 32.8 | 4.63 | 5.33 | 18.82 | 1.15 | 164 | 21 |
| 17 | II | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 44.5 | 6.13 | 8.26 | 25.50 | 1.34 | 223 | 28 |
| 18 | III | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 32.5 | 4.62 | 6.78 | 18.65 | 1.47 | 163 | 20 |
| 19 | IV | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 30.8 | 2.76 | 3.12 | 17.68 | 1.12 | 154 | 19 |
| 20 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 99.4 | 11.61 | 16.42 | 56.83 | 1.41 | 497 | 21 |
| 21 | 2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 65.3 | 5.56 | 7.42 | 37.37 | 1.33 | 327 | 14 |
| 22 | 3 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 90.3 | 5.20 | 6.42 | 51.63 | 1.23 | 452 | 19 |
| 23 | 4 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 96.5 | 6.13 | 7.51 | 55.17 | 1.23 | 483 | 20 |
| 24 | 5 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 99.0 | 9.52 | 11.01 | 56.60 | 1.16 | 495 | 21 |
| 25 | 6 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 98.1 | 7.45 | 8.53 | 56.08 | 1.14 | 491 | 20 |
| 26 | 7 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 98.3 | 7.12 | 8.56 | 56.20 | 1.20 | 492 | 20 |
| 27 | 8 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 99.5 | 10.33 | 13.63 | 56.88 | 1.32 | 498 | 21 |
| 28 | I | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 62.5 | 4.36 | 7.63 | 35.77 | 1.75 | 313 | 13 |
| 29 | II | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 76.2 | 6.88 | 7.52 | 43.59 | 1.09 | 381 | 16 |
| 30 | III | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 60.5 | 5.12 | 6.23 | 34.63 | 1.22 | 303 | 13 |
| 31 | IV | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 130 | 58.3 | 3.20 | 4.13 | 33.37 | 1.29 | 292 | 12 |
The observed activity of complex 1 surpassed that of the other complexes screened herein, and this was attributed to the additional presence of the sodium centres.
All polymers obtained were of low polydispersity (PDI < 1.6), which suggested that these polymerizations occurred without significant side reactions. Interestingly, only low molecular weight polymers were obtained using these oxovanadium/imido vanadium systems.
The screening of complexes 1–8 and I–IV (Table 2) revealed that the vanadium-based L1 or L2-containing complexes namely 1, 5, 6 and 8 herein, exhibited higher activities than the known complexes I–IV under the conditions employed. After 24 h (Table 1), complexes 2, I, II, III, IV afforded relatively lower conversions (<90%), whereas higher conversions (>90%) were reached using complexes 1, 3–7, 8, under similar conditions. From a kinetic study (Fig. 6a), it was observed that the PCL polymerization rate followed the order: 1 > 8 > II > I > III ≈ 3 > 2 > IV. For complexes within the imido-alkoxide family, the kinetic and TON/TOF results (Table 2, Fig. 6b) showed that the catalytic activity followed the order: 5 (CF3) > 6 (Cl) > 7 (F) > 4 (OMe) > 3 (Me), which suggested that the presence of electron withdrawing para substituents favours higher activity. 1H NMR spectra of the PCL indicated the presence of an BnO end group (e.g. Fig. S11, ESI†), which agrees with the MALDI-ToF mass spectra (e.g. Fig. S6, ESI†) and indicates that the polymerization proceeded via a coordination insertion mechanism. The observed molecular weights were lower than the calculated values, whilst the MALDI-ToF mass spectra were consistent with BnO end groups [M = n × 114.12 (CL) + 108.05 (BnOH) + 22.99 (Na+)]. In the absence of BnOH, spectra (Fig. S5 and S10, ESI†) indicated the products were catenulate with chains and cyclic polymers present. In the MALDI-ToF mass spectra, a part family of peaks consistent with the chain polymer (terminated by 2 OH) [M = 17 (OH) + 1(H) + n × 114.14 (CL) + 22.99 (Na+)] and the cyclic polymer [M = 22.99 (Na+) + n × 114.14 (CL)] were observed.
δ-Valerolactone (δ-VL)
Complexes 1–8 and I–IV were also evaluated as catalysts in the presence of one equivalent of BnOH for the ROP of δ-VL (Table 3). Using compound 1, the conditions of temperature and [V]: [δ-VL] were varied. Best observed results were achieved at 130 °C using [V]: [δ-VL] at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 over 8 h. As in the case of the ROP of ε-CL, kinetic studies (Fig. 7 and S3, ESI†) revealed that the catalytic activities followed the order: 1 > 8 > II > I > III ≈ 3 > 2 > IV, whilst the performance of the vanadium-based imido catalysts exhibited the order 5 > 6 ≈ 7 > 4 > 3, which was again suggestive of a positive influence exerted by electron-withdrawing para substituents on the imido group. As for the ROP of ε-Cl, there was evidence of significant transesterification and nearly all observed Mn values were significantly lower than the calculated values. The MALDI-ToF mass spectra (Fig. S8, ESI†) exhibited a major family of peaks consistent with OBn end groups [M = 108.05 (BnOH) + n × 100.12 (VL) + 22.99 (Na+)], and a minor family assigned to cyclic PVL. 1H NMR spectra of the PVL also indicated the presence of an BnO end group (e.g. Fig. S13, ESI†). In the absence of BnOH, spectra (Fig. S7 and S12, ESI†) again indicated the products were catenulate. The MALDI-ToF mass spectra exhibited the peaks consistent with the chain polymer (terminated by 2 OH) [M = 17 (OH)+ 1(H) + n × 100.12 (VL) + 22.99 (Na+)] and cyclic polymer [M = 22.99 (Na+) + n × 100.12 (VL)].
500 over 8 h. As in the case of the ROP of ε-CL, kinetic studies (Fig. 7 and S3, ESI†) revealed that the catalytic activities followed the order: 1 > 8 > II > I > III ≈ 3 > 2 > IV, whilst the performance of the vanadium-based imido catalysts exhibited the order 5 > 6 ≈ 7 > 4 > 3, which was again suggestive of a positive influence exerted by electron-withdrawing para substituents on the imido group. As for the ROP of ε-Cl, there was evidence of significant transesterification and nearly all observed Mn values were significantly lower than the calculated values. The MALDI-ToF mass spectra (Fig. S8, ESI†) exhibited a major family of peaks consistent with OBn end groups [M = 108.05 (BnOH) + n × 100.12 (VL) + 22.99 (Na+)], and a minor family assigned to cyclic PVL. 1H NMR spectra of the PVL also indicated the presence of an BnO end group (e.g. Fig. S13, ESI†). In the absence of BnOH, spectra (Fig. S7 and S12, ESI†) again indicated the products were catenulate. The MALDI-ToF mass spectra exhibited the peaks consistent with the chain polymer (terminated by 2 OH) [M = 17 (OH)+ 1(H) + n × 100.12 (VL) + 22.99 (Na+)] and cyclic polymer [M = 22.99 (Na+) + n × 100.12 (VL)].
| Run | Cat. | VL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
t/h | T/°C | Conva (%) | M n,GPC × 10–3b | M w × 10–3b | M n,Cal × 10–3c | PDId | TONe | TOFf (h−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy. b M n/w, GPC values corrected considering Mark–Houwink factor (0.57) from polystyrene standards in THF. c Calculated from ([monomer]0/V) × conv (%) × monomer molecular weight (MVL = 100.16) + molecular weight of BnOH. d From GPC. e Turnover number (TON) = number of moles of δ-VL consumed/number of moles V. f Turnover frequency (TOF) = TON/time (h). | |||||||||||
| 1 | 1 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 79.9 | 10.57 | 18.69 | 80.10 | 1.76 | 799 | 100 |
| 2 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 86.3 | 12.38 | 16.31 | 43.31 | 1.31 | 432 | 54 |
| 3 | 1 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 84.6 | 4.26 | 6.45 | 21.28 | 1.51 | 212 | 26 |
| 4 | 1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 82.1 | 2.38 | 3.95 | 8.33 | 1.70 | 82 | 10 |
| 5 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 100 | 42.1 | 6.01 | 7.22 | 21.18 | 1.20 | 211 | 26 |
| 6 | 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 80 | 20.5 | 1.89 | 3.25 | 10.37 | 1.72 | 103 | 13 |
| 7 | 2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 35.9 | 4.28 | 6.51 | 18.08 | 1.52 | 180 | 22 |
| 8 | 3 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 38.4 | 5.23 | 7.24 | 19.33 | 1.38 | 192 | 24 |
| 9 | 4 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 44.3 | 6.90 | 8.49 | 22.28 | 1.23 | 222 | 28 |
| 10 | 5 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 58.1 | 9.43 | 13.25 | 29.19 | 1.40 | 291 | 36 |
| 11 | 6 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 43.9 | 6.27 | 7.53 | 22.08 | 1.20 | 220 | 27 |
| 12 | 7 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 42.1 | 5.94 | 7.56 | 21.18 | 1.27 | 211 | 26 |
| 13 | 8 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 75.5 | 10.61 | 14.64 | 37.90 | 1.38 | 378 | 47 |
| 14 | I | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 37.0 | 4.84 | 5.47 | 18.63 | 1.12 | 185 | 23 |
| 15 | II | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 48.9 | 7.24 | 8.92 | 24.59 | 1.23 | 245 | 31 |
| 16 | III | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 35.6 | 4.21 | 6.21 | 17.93 | 1.48 | 178 | 22 |
| 17 | IV | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 130 | 34.5 | 3.42 | 4.51 | 17.38 | 1.32 | 173 | 22 |
| Run | Cat | CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VL VL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T/°C | CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VLa VLa |
Convb (%) | M n,GPC × 10–3c | M w × 10–3c | PDId |
|---|---|---|---|---|---|---|---|---|
| a Ratio of ε-CL to δ-VL observed in the co-polymer by 1H NMR spectroscopy. b Determined by 1H NMR spectroscopy. c M n/w, GPC values corrected considering Mark–Houwink method from polystyrene standards in THF, Mn/w GPC = [0.56 × Mn/w measured × (1% CL) + 0.57 × Mn/w measured × (1% VL)] × 103. d From GPC. e ε-Caprolactone was firstly added for 24 h, then δ-valerolactone was added and heating for 24 h. f δ-Valerolactone was firstly added for 24 h, then ε-caprolactone was added and heating for 24 h. g ε-Caprolactone and δ-valerolactone were added at the same time and heating for 24 h. | ||||||||
| 1 | 1 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
72.6 | 12.16 | 22.37 | 1.84 |
| 3 | 2 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
25.3 | 1.32 | 2.31 | 1.75 |
| 4 | 3 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
45.2 | 4.68 | 6.23 | 1.33 |
| 5 | 4 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
47.5 | 5.29 | 6.88 | 1.30 |
| 6 | 5 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
59.5 | 7.21 | 8.45 | 1.17 |
| 7 | 5 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
63.7 | 7.44 | 8.95 | 1.20 |
| 8 | 5 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
35.8 | 4.22 | 7.12 | 1.69 |
| 9 | 6 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
57.9 | 6.01 | 7.52 | 1.25 |
| 10 | 6 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
55.4 | 6.21 | 7.69 | 1.24 |
| 11 | 6 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
38.5 | 5.98 | 8.15 | 1.36 |
| 12 | 7 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 50 : 50 |
58.4 | 6.12 | 7.14 | 1.17 |
| 13 | 8 | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
60.8 | 6.85 | 8.69 | 1.27 |
| 15 | I | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 54 |
61.1 | 6.95 | 8.69 | 1.25 |
| 16 | II | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
58.1 | 3.67 | 4.98 | 1.36 |
| 17 | III | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
49.5 | 4.53 | 7.21 | 1.59 |
| 18 | IV | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 65 |
38.4 | 4.21 | 6.49 | 1.54 |
Kinetics
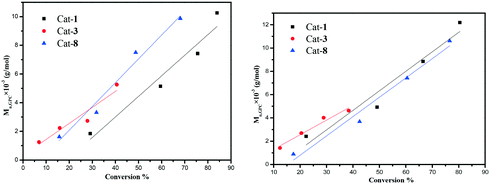
The kinetic results are consistent with a zero-order dependence in monomer. Whilst this kind of behaviour is uncommon, systems that behave in a similar manner have been reported.19 The dependence of the Mn and molecular weight distribution on the monomer conversion in the reactions catalyzed by 1, 3 and 8 with BnOH was also investigated (Fig. 8). For the ROP of ε-CL, the Mn was shown to increase linearly with the conversion, which suggested that the polymerization was well controlled (Fig. 8, left). A similar outcome was also observed in the reaction involving δ-VL (Fig. 8, right).Co-polymerization of ε-CL and δ-VL
The complexes exhibited moderate conversions, with the mixed-metal complex 1 performing best (72.6%), with 5 (p-CF3) and the known complex I also producing conversions >60%. Under the conditions employed, the systems 5, 6, 7 and 8 showed a preference for CL incorporation (50–66%), and in the case of 5 and 6, this was despite the initial addition of δ-VL. Complex 4 exhibited the highest preference (67%) for VL incorporation. In general, the systems appeared to be relatively well behaved with PDIs in the range 1.17–1.84; NMR spectra were consistent with the presence of BnO and OH end groups (Fig. S15, ESI†). The composition of the copolymer was further investigated by 13C NMR spectroscopy. In fact, diagnostic resonances belonging to CL–VL, CL–CL, VL–VL and VL–CL dyads can be observed in the region between δ 63.91 and 64.13 ppm (Fig. 16, ESI†). Based on the current results, the number-average sequence length was found to be 7.16 and 5.30 for CL and VL, respectively, consistent with a randomness degree R of 0.33, which suggests the copolymers possess a “blocking” tendency (Fig. S16, eqn (S1)–(S3), ESI†).20ROP of r-lactide
To enhance the thermal properties of the polymers obtained herein, we also investigated the ROP of the r-lactide. Unfortunately, none of the systems herein proved to be effective as catalysts for the ROP of r-lactide either in solution at high temperatures (130 °C) or as melts.Ethylene polymerization
The imido complexes 3–7 were screened for their ability to polymerize ethylene in the presence of the co-catalysts R2AlCl (R = Me, Et) and the re-activator ethyltrichloroacetate (ETA), see Table 5, showing much higher polymerization activities if Me2AlCl was used as activator (cf. entries 1 and 2 of Table 5). In the presence of Me2AlCl+ETA, the catalytic activities followed the trend 3 (p-Me) ≈ 5 (p-CF3) > 7 (p-F) > 6 (p-Cl) > 4 (p-OMe). Complexes 3–7 exhibited high ethylene consumption rates during 10–20 min of polymerization and virtually lost the activity after 30 min (Fig. 9a). Complex 4 showed the lowest reactivity toward ethylene in the series, which may be explained by the presence of strong electron-donating OMe substituents, reducing its electrophilicity. All systems except 4 were relatively well-controlled with PDIs < 2.5 (Fig. 9b) and afforded highly linear (according to 13C NMR spectroscopy, there were no detectable branches, Fig S25, ESI†) high molecular weight polyethylene. The ethylene polymerization activities found here for complexes 3–7, are very high, several times higher than those reported for vanadium polyphenolate and phenoxyimine catalysts under comparable conditions.21| Run | Cat | V loading/μmol | Co-catalyst | PE yield/g | Activitya | M n × 10−3 | M w × 10−3 | M z/Mw | PDIb |
|---|---|---|---|---|---|---|---|---|---|
| a In 106 g PE mol V−1 bar−1 h−1. b PDI = Mw/Mn. | |||||||||
| 1 | 3 | 1.0 | Et2AlCl | 0.7 | 1.4 | — | — | — | — |
| 2 | 3 | 0.5 | Me2AlCl | 7.4 | 14.8 | 110 | 245 | 1.8 | 2.2 |
| 3 | 4 | 0.5 | Me2AlCl | 1.2 | 2.4 | 72 | 520 | 5.0 | 7.2 |
| 4 | 5 | 0.5 | Me2AlCl | 7.2 | 14.2 | 74 | 180 | 2.1 | 2.4 |
| 5 | 6 | 0.5 | Me2AlCl | 4.5 | 9.0 | 120 | 290 | 1.9 | 2.4 |
| 6 | 7 | 0.5 | Me2AlCl | 6.7 | 13.4 | 74 | 185 | 2.0 | 2.5 |
 | ||
| Fig. 9 (a) Activity vs. time plot for entries 4–6 of Table 5. (b) GPC traces of polyethylenes in entries 2 and 4–6 of Table 5. | ||
Conclusions
In conclusion, the use of p-tert-butyltetrahomodioxacalix[6]areneH6, L6H6, with [NaVO(OtBu)4] or [VO(OnPr)3] affords the mixed-metal complex [(VO)2(μ-O)Na2(L1)(MeCN)4] or the tetranuclear complex {[VO]4L1}, respectively. Use of imido-alkoxide precursors [V(Np-RC6H4)(OR/)3] leads to the formation of {[V(Np-RC6H4)]2L1} type complexes. Under the conditions employed, the calixarene ring system can break in situ, and result in two 2-(p-CH3C6H4NCH)-4-tBu-C6H2O-6-CH2)-4-tBuC6H2OH (L2), which can bind to the vanadium to form a metallocyclic complex of the form [VO(L2)]2. All complexes were active for the ROP of the cyclic esters ε-CL and δ-VL, with and without benzyl alcohol (BnOH) present, but not for r-LA. The co-polymerization of ε-CL with δ-VL was also possible. Low molecular weight products were obtained but with good control. For the imido complexes in the presence of BnOH, kinetic studies indicated the rate order 5 (CF3) > 6 (Cl) > 7 (F) > 4 (OMe) > 3 (Me) for ε-CL which suggested that the presence of electron withdrawing para substituents favour higher activity, and a similar order for δ-VL; in the absence of BnOH, structure/activity trends were less evident. Observed conversion rates were superior to related oxacalix[3]arene species (I and II) as well as methylene (–CH2–) bridged calix[6 and 8]arene complexes III and IV under similar conditions. This suggests the presence and large flexibility of the dioxacalix[6]arene scaffold is beneficial in ROP. For ethylene polymerization (with DMAC/ETA), the imido complexes 3 and 5–7 showed very high catalytic activities of the order 0.9–1.5 × 107 g PE (mol V)−1 bar−1 h−1, which followed the trend 3 (p-Me) ≈ 5 (p-CF3) > 7 (p-F) > 6 (p-Cl), whilst the activity of 4 (p-OMe) was much lower and was thought to be due to its reduced electrophilicity. The product in each case was highly linear polyethylene.Experimental
General
The known compounds L1H6, L3H3, [V(Np-RC6H4)(OiPr)3] (R= OMe, CF3, Cl), [V(Np-CH3C6H4)(OtBu)3] and [V(Np-CH3C6H4)(OEt)3], [VOL3] (L3 = oxacalix[3]arene) (I), [V(Np-CH3C6H4)L3]2 (II), [Li(MeCN)4][V2(O)2Li(MeCN)(L6H2)2] (L6H6 = p-tert-butylcalix[6]areneH6) (III), [(VO)2L8H] (L8H8 = p-tert-butylcalix[8]areneH8) (IV) and [VOL4]2 (L4 = 2,6-bis(3,5-tert-butyl-2-hydroxybenzyl)-4-tert-butylphenol) (V) were prepared by the literature methods.7,9,15,16,22 All reactions were conducted under an inert atmosphere using standard Schlenk techniques. Toluene was dried from sodium, acetonitrile was distilled from calcium hydride, diethylether was distilled from sodium benzophenone, and all solvents were degassed prior to use. IR spectra (nujol mulls, KBr or NaCl windows) were recorded on a Nicolet Avatar 360 FT IR spectrometer.1H NMR spectra were recorded at room temperature on a Varian VXR 400 S spectrometer at 400 MHz or a Gemini 300 NMR spectrometer or a Bruker Avance DPX-300 spectrometer at 300 MHz. 1H and 13C NMR spectra of polyethylene samples (1,2-dichlorobenzene, 100 °C) were recorded on a Bruker Avance 400 MHz NMR spectrometer at 400.130 and 100.613 MHz, respectively. 1H NMR spectra were calibrated against the residual protio impurity of the deuterated solvent.
Crystal structures were determined from data collected at the UK National Crystallography Service (1–6) and the SRS at Daresbury (8). Full details are given in the ESI.† Crystal data are summarised in Table 6.
| Compound | 5 | 6 | 8·4MeCNb | |
|---|---|---|---|---|
| a Ref. 24. b For 8: data collected on a Bruker APEX 2 CCD diffractometer at Daresbury SRS station 9.8 (λ = 0.6710 Å). c τ = (β − α)/60.14 | ||||
| Formula | C82H90F6N2O8V2 | C80H90Cl2N2O8V2 | C96H108N8O8V2 | |
| Formula weight | 1447.43 | 1380.31 | 1711.96 | |
| Crystal system | Monoclinic | Monoclinic | Triclinic | |
| Space group | P21/c | P21/n |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
|
| Unit cell dimensions | ||||
| a (Å) | 20.0005(13) | 19.7039(9) | 12.0966(2) | |
| b (Å) | 19.9315(8) | 20.0862(7) | 12.3373(2) | |
| c (Å) | 22.023(2) | 20.6921(10) | 17.8971(3) | |
| α (°) | 90 | 90 | 90.8728(8) | |
| β (°) | 116.080(10) | 113.114(5) | 107.9966(7) | |
| γ (°) | 90 | 90 | 99.2458(8) | |
| V (Å3) | 7885.5(11) | 7532.0(6) | 2501.28(7) | |
| Z | 4 | 4 | 1 | |
| Temperature (K) | 100(2) | 100(2) | 150(2) | |
| Wavelength (Å) | 0.71075 | 1.54184 | 0.71073 | |
| Calculated density (g cm−3) | 1.219 | 1.217 | 1.137 | |
| Absorption coefficient (mm−1) | 0.305 | 3.160 | 0.243 | |
| Transmission factors (min./max.) | 0.555 and 1.000 | 0.738 and 1.000 | 0.974 and 0.990 | |
| Crystal size (mm3) | 0.12 × 0.02 × 0.01 | 0.150 × 0.030 × 0.005 | 0.11 × 0.06 × 0.04 | |
| θ(max) (°) | 25.0 | 66.13 | 28.3 | |
| Reflections measured | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 213 213 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 287 287 |
|
| Unique reflections | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 936 936 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175 175 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 217 217 |
|
| R int | 0.1918 | 0.1107 | 0.036 | |
| Reflections with F2 > 2σ(F2) | 6929 | 5544 | 8563 | |
| Number of parameters | 886 | 791 | 591 | |
| R 1 [F2 > 2σ(F2)] | 0.096 | 0.085 | 0.059 | |
| wR2 (all data) | 0.192 | 0.247 | 0.185 | |
| GOOF, S | 1.02 | 1.02 | 1.03 | |
| Largest difference peak and hole (e Å−3) | 1.14 and −0.71 | 0.420 and −0.378 | 0.87 and −0.28 | |
| τ | 0.43 | 0.38 | 0.28 | |
Elemental analyses were performed by the elemental analysis service at the University of Hull. Matrix assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry was performed in a Bruker autoflex III smart beam in linear mode, and the spectra were acquired by averaging at least 100 laser shots. 2,5-Dihydroxybenzoic acid was used as the matrix and THF as solvent. Sodium chloride was dissolved in methanol and used as the ionizing agent. Samples were prepared by mixing 20 μL of matrix solution in THF (2 mg mL−1) with 20 μL of matrix solution (10 mg mL−1) and 1 μL of a solution of ionizing agent (1 mg mL−1). Then 1 mL of these mixtures was deposited on a target plate and allowed to dry in air at ambient temperature.
Weight-average (Mw) and number-average (Mn) molecular weights, molecular weight distributions (MWD), and polydispersities (Mw/Mn) were measured on a waters-150 chromatograph at 150 °C, with trichlorobenzene as solvent.
Preparation of [(VO)2(μ-O)Na2(L1)(MeCN)4]·5MeCN (1·5MeCN)
To in situ Na[VO(OtBu)4] (generated from 0.98 mmol from VOCl3 and 4tBuONa) in Et2O (30 mL) was added L1H6 (0.50 g, 0.49 mmol) and at −78 °C. The mixture was allowed to warm to ambient temperature and then the volatiles were removed in vacuo. Toluene (30 mL) was then added and the system was refluxed for 12 h. On cooling, volatiles were removed in vacuo, and the residue was extracted into warm MeCN (30 mL). On prolonged standing at 0 °C, green prisms of 1 formed. Yield 0.46 g, 34%. Anal. cald for C86H109N9Na2O11V2: C, 64.85; H, 6.90; N 7.92%; found C, 65.22; H, 7.5%; 7.23%. IR (nujol mull, KBr): 3901w, 3422w, 1747w, 1260s, 1227w, 1199m, 1059s, 1021s, 965m, 903w, 868m, 831w, 796s, 708w, 658m, 641m, 623m, 602w, 553w, 453w. 1H NMR (CDCl3) δ: 8.00–7.5 (m, 12H, arylH), 5.71 (d, J = 8.1, 4H, –OCH2), 4.90 (d, J = 8.1, 4H, OCH2), 4.05 (d, J = 8.0, 4H, –CH2–), 3.53 (d, J = 8.0, 4H, –CH2), 2.05 (s, CH3CN), 1.35–1.24 (m, 54H, C(CH3)3). 51V NMR (CDCl3): δ: −675.3 ppm (ω1/2 272 Hz). Mass spec (EI): 1592 [M]+.Synthesis of [(VO)4L1] (2·6MeCN)
[VO(OnPr)3] (0.49 g, 2.0 mmol) and L1H6 (0.50 g, 0.49 mmol) were combined in toluene (20 mL). After refluxing for 12 h, volatiles were removed in vacuo, and the residue was extracted into MeCN (20 mL). Prolonged standing at 0 °C afforded 2·6MeCN as dark blue prisms. Yield 0.32 g, 49%. Anal. cald for C74H96O16V4 (sample dried in vacuo for 12 h): C, 61.49; H, 6.70%. Found C, 61.49; H, 6.53%. IR (nujol mull, KBr): 1651w, 1599m, 1461s, 1377s, 1363m, 1304w, 1260s, 1206m, 1092s, 1052s, 1016s, 986m, 922w, 873m, 851m, 799 s. 1H NMR (CDCl3) δ: 6.88–7.35 (m, 12H, arylH), 6.07 (d, J = 4.8 Hz, 2H, –OCH2–), 5.77 (m, 2H, –OCH2–), 5.51 (d, J = 4.8 Hz, 2H, –OCH2–) 5.15 (m, 4H, –OCH2CH2CH3), 4.86 (d, J = 4.8 Hz, 2H, –OCH2–), 4.20 (m, 4H, –CH2–), 3.40 (d, J = 12.4 Hz, 4H, –CH2–), 2.32 (m, 4H, –OCH2CH2CH3), 1.13–1.49 (m, 54H, C(CH3)3), 1.02 (m, 6H, –OCH2CH2CH3). 51V NMR (CDCl3) δ: −411.7 (ω1/2 524 Hz), −461.5 ppm (ω1/2 735 Hz). Mass spec (EI): 1351 [M + Na–2OnPr]+.Preparation of {[V(Np-CH3C6H4)]2L1} (3·7MeCN·0.5CH2Cl2)
To [V(Np-CH3C6H4)(OtBu)3] (0.37 g, 0.99 mmol) and L1H6 (0.50 g, 0.49 mmol) was added toluene (30 mL) and then the system was refluxed for 12 h. On cooling, the volatiles were removed in vacuo, and the residue was extracted into warm MeCN (30 mL). On prolonged standing at 0 °C, an orange crystalline material formed, yield 0.61 g, 47%. Single orange prisms of 3·7MeCN·0.5CH2Cl2 were grown from a saturated MeCN solution containing CH2Cl2 (2 mL) at 0 °C. Anal. cald for C82H96N2O8V2: C, 73.41; H, 7.21; N, 2.12%; found C, 73.32%; H, 7.41%, N, 2.24%. IR (nujol mull, KBr): 3236w, 2727w, 2349w, 2288w, 2248w, 1614m, 1592m, 1556w, 1300m, 1261s, 1073s, 1020s, 990m, 937m, 878m, 840s, 819s, 763s, 754w, 722w, 696w, 639w, 584w. 1H NMR (CDCl3) δ: 7.78–6.85 (m, 12H, aryl–H), 6.46 (d, J = 8.4 Hz, 4H, –N–C6H4–), 6.16 (d, J = 8.4 Hz, 4H, –N–C6H4–), 4.86–5.10 (d, J = 7.6 Hz, 4H, OCH2–), 4.53–4.66 (m, 4H, –OCH2–), 4.34–4.25 (m, 4H, –CH2) 3.66–4.00 (d, J = 12.4 Hz, 4H, –CH2), 2.35 (m, 6H, p-tolyl–CH3), 2.05 (s, 3H, MeCN) 1.49 (m, 54H, C(CH3)3) 51V NMR (CDCl3) δ: −312.2 (ω1/2 650 Hz) ppm. Mass spec (EI): 1362 [M + Na]+, 1379 [M + K]+.Synthesis of {[V(Np-(OMe)C6H4)]2L1} (4·4MeCN)
As for 2, but using [V(Np-(OMe)C6H4)(OiPr)3] (0.35 g, 1.0 mmol) and L1H6 (0.50 g, 0.49 mmol) affording 4·4MeCN as orange/red prisms. Single orange prisms were grown from a saturated MeCN (30 mL) solution at 0 °C; yield 0.43 g, 50%. Anal. cald for C82H96N2O10V2 (sample dried in vacuo for 12 h): C, 71.80; H, 7.06; N, 2.04%. Found C, 71.78; H, 7.14; N, 2.38%. IR (nujol mull, KBr): 3237w, 1613w, 1585m, 1554w, 1540w, 1486m, 1459s, 1383m, 1362w, 1297w, 1260s, 1206m, 1159 m 1075s, 1023s, 910w, 872m, 800s, 754w, 688m. 1H NMR (CDCl3) δ: 6.87–7.54 (m, 12H, arylH), 6.49 (d, J = 8.0 Hz, 4H, –NC6H4–OMe), 5.88 (d, J = 8.0 Hz, 4H, –NC6H4–OMe), 5.11 (d, J = 6.8 Hz, 4H, –OCH2–), 4.95 (d, J = 6.8 Hz, 4H, –OCH2–), 4.47–4.67 (m, 4H, –CH2–),3.64 (d, J = 5.2 Hz, 4H, –CH2–), 3.51 (s, 6H, p-C6H4–OCH3), 2.01 (s, 3H, MeCN), 1.35–1.15 (m, 54H, C(CH3)3). 51V NMR (CDCl3) δ: −178.1 (ω1/2 470 Hz) ppm. Mass spec (EI): 1470 [M + Na]+.Synthesis of {[V(Np-(CF3)-C6H4)]2L1} (5)
As for 2, but using [V(Np-(CF3)C6H4)(OiPr)3] (0.38 g, 1.0 mmol) and L1H6 (0.50 g, 0.49 mmol) affording 5 as orange/brown prisms. Single orange prisms were grown from a saturated MeCN (30 mL) solution at 0 °C (yield 0.26 g, 36%). Anal. Cald for C82H90F6N2O8V2: C, 68.04; H, 6.27; N, 1.94%. Found: C, 67.36; H, 6.45; N, 2.32%. IR (nujol mull, KBr): 1730w, 1598m, 1557m, 1509m, 1456s, 1383s, 1363w, 1324s, 1260s, 1201w, 1163m, 1059s, 1067s, 1071s, 956w, 877m, 849m, 799s, 723 m. 1H NMR (CDCl3) δ: 6.84–7.82 (m, 12H, arylH), 6.51 (d, J = 8.8 Hz, 4H, –C6H4–CF3), 6.19 (m, 4H, –C6H4–CF3), 5.02–4.91 (m, 4H, –OCH2–), 4.52 (d, J = 5.2 Hz, 4H, –OCH2–), 4.27–4.16 (m, 4H, –CH2–), 3.72 (m, 4H, –CH2–), 1.95 (s, 3H, MeCN), 1.35–1.06 (m, 54H, C(CH3)3). 51V NMR (CDCl3) δ: −408.1 ppm (ω1/2 474 Hz). 19F NMR (CDCl3): δ = −62.69 ppm. Mass spec (EI): 1441 [M].Synthesis of {[V(Np-Cl-C6H4)]2L1} (6)
As for 2, but using [V(Np-Cl-C6H4)(OiPr)3] (0.35 g, 1.0 mmol) and L1H6 (0.50 g, 0.49 mmol) affording 6 as dark brown prisms from a saturated MeCN (30 mL) solution at 0 °C (yield 0.35 g, 51%). Anal. cald for C80H90Cl2N2O8V2·MeCN C, 69.29; H, 6.89; N, 2.96%. Found C, 67.72; H, 7.43; N 2.25%.23 IR (nujol mull, KBr): 2359m, 1737w, 1593m, 1554m, 1513m, 1461s, 1377m, 1294m, 1260m, 1200 m, 1117s, 1088w, 822w, 795w, 726w. 1H NMR (CDCl3) δ: 6.88–7.69 (m, 12H, arylH), 6.50 (d, J =10.0 Hz, 4H, –C6H4–Cl), 6.35 (d, J = 10.0 Hz, 4H, –C6H4–Cl), 4.86–5.09 (m, J = 10.0 Hz, 4H, –OCH2–), 4.68 (d, J = 10.0 Hz, 4H, –OCH2–), 4.31–4.53 (m, 4H, –CH2–),3.67 (d, J = 12.8 Hz, 4H, –CH2–), 1.13–1.35 (m, 54H, C(CH3)3). 51V NMR (CDCl3) δ: −335.3 ppm (ω1/2 120 Hz). Mass spec (EI): 1386 [M]+.Synthesis of {[V(Np-F-C6H4)]2L1} (7·MeCN)
As for 2, but using [V(Np-F-C6H4)(OnPr)3] (0.34 g, 0.99 mmol) and L1H6 (0.50 g, 0.49 mmol) affording 7·MeCN as red/brown crystals from a saturated MeCN (30 mL) solution at 0 °C. Yield: 0.57 g, 86%. Anal. cald for C80H90F2N2O8V2·MeCN C, 70.92; H, 6.75; N, 3.03%. Found C, 70.72; H, 6.89; N 3.38%. IR (nujol mull, KBr): 2360w, 2341w, 1682w, 1510 m, 1411w, 1299w, 1260s, 1228m, 1202s, 1155w, 1144w, 1096s, 1064s, 1017s, 988m, 912w, 872w, 832s, 821s, 806s, 722m, 668w, 581w, 460w. 1H NMR (CDCl3) δ: 6.84–7.81 (m, 12H, arylH), 6.49 (d, J = 6.8 Hz, 4H, –NC6H4–Cl), 5.91 (d, J = 6.8 Hz, 4H, –NC6H4–Cl), 4.91–4.98 (d, J = 8.8 Hz, 4H, –OCH2–), 4.49 (d, J = 8.8 Hz, 4H, –OCH2–), 4.13–4.26 (m, 4H, –CH2–), 3.69 (d, J = 5.2 Hz, 4H, –CH2–), 1.36–1.09 (m, 54H, C(CH3)3). 19F NMR (CDCl3) δ: 108.30 ppm. 51V NMR (CDCl3): δ: −173.4 ppm (ω1/2 265 Hz).Synthesis of [VO(L2)]2 (L2 = 2-(p-tolylNCH)-4-tBu-C6H2O-6-CH2)-4-tBuC6H2OH) (8·4MeCN)
[V(Np-tolyl)(OEt)3] (0.29 g, 0.99 mmol) and L1H6 (0.50 g, 0.49 mmol) were refluxed in toluene (30 mL) for 12 h. Following removal of volatiles in vacuo, the residue was dissolved in hot MeCN (30 mL), filtered and left to stand (1–2 days) at 0 °C to afford 8 as orange-brown prisms (0.43 g, 29% yield). Anal. cald for C96H108N4O8V2 (sample dried in vacuo for 12 h, –4MeCN): C, 74.49; H, 7.03; N 3.62%; found C, 73.60; H, 7.12; N, 2.88%. IR (nujol mull, KBr): 3231w, 1613m, 1593m, 1555w, 1505w, 1459s, 1377s, 1363m, 1299w, 1261s, 1209s, 1073s, 1019s, 991, 965m, 878s, 871s, 839m, 818m, 802s, 765w, 764m, 753 m, 737m. 1H NMR (CDCl3) results of complex 7. 1H NMR (CDCl3) δ: 9.01 (s, 2H, –OH), 6.84–7.68 (m, 12H, arylH), 6.45 (d, J = 8.4 Hz, 4H, –NC6H4–Me), 6.16 (d, J = 8.4 Hz, 4H, –NC6H4–Me), 4.85–5.10 (d, J = 9.6 Hz, 4H, –N–CH2–), 4.53–4.65 (d, J = 9.6 Hz, 4H, –N–CH2–), 4.25–4.34 (m, 4H, –CH2–), 3.65 (d, J = 12.8 Hz, 4H, –CH2–), 2.33 (m, 12H, –CH3), 2.01 (s, 3H, MeCN), 1.11–1.36 (m, 54H, C(CH3)3) 51V NMR (CDCl3) δ: −513.2 ppm (ω1/2 512 Hz). Mass spec (EI): 1556 [M + Na+–3MeCN].Procedure for ROP of ε-caprolactone/δ-valerolactone
A toluene solution of pre-catalyst (0.010 mmol, 1.0 mL toluene) was added into a Schlenk tube in the glove-box at room temperature. The solution was stirred for 2 min, and then the appropriate equivalent of BnOH (from a pre-prepared stock solution of 1 mmol BnOH in 100 mL toluene) and the appropriate amount of ε-CL or δ-VL along with 1.5 mL toluene was added to the solution. For example, for Table 2, entry 1, a toluene solution of pre-catalyst 1 (0.010 mmol, 1.0 mL toluene) was added into a Schlenk tube, then 2 mL BnOH solution (1 mmol BnOH/100 mL toluene) and 20 mmol ε-CL along with 1.5 mL toluene was added to the solution. The reaction mixture was then placed into an oil/sand bath pre-heated at 130 °C, and the solution was stirred for the prescribed time (8 or 24 h). The polymerization mixture was quenched on addition of an excess of glacial acetic acid (0.2 mL) into the solution, and the resultant solution was then poured into methanol (200 mL). The resultant polymer was then collected on filter paper and was dried in vacuo.Kinetic studies
The polymerizations were carried out at 130 °C in toluene (2 mL) using 0.010 mmol of complex. The molar ratio of monomer to initiator was fixed at 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and at appropriate time intervals, 0.5 μL aliquots were removed (under N2) and were quenched with wet CDCl3. The percent conversion of monomer to polymer was determined using 1H NMR spectroscopy.
1, and at appropriate time intervals, 0.5 μL aliquots were removed (under N2) and were quenched with wet CDCl3. The percent conversion of monomer to polymer was determined using 1H NMR spectroscopy.
Procedure for ethylene polymerization
Ethylene polymerization experiments were performed in a steel 500 mL autoclave. The reactor was evacuated at 80 °C, cooled down to 20 °C and then charged with the freshly prepared solution of the co-catalyst in heptane/toluene. Pre-catalysts were introduced into the reactor in sealed glass ampoules, containing 0.5 or 1.0 μmol of appropriate V-complex in 0.5 mL of solvent. After setting up the desired temperature and ethylene pressure, the reaction was started by breaking the ampoule with the pre-catalyst. During the polymerization, ethylene pressure (2 bar), temperature (70 °C) and stirring speed (2000 rpm) were maintained constant. After 30 min (during which time the ethylene consumption rate declined to nearly zero level), the reactor was opened to the atmosphere and the polymeric product was dried in a fume-hood to a constant weight.Polymerization conditions. For entry 1 of Table 5: V loading 1.0 μmol (dissolved in CH2Cl2), co-catalyst Et2AlCl + ETA (molar ratio V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2AlCl
Et2AlCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ETA = 1
ETA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000
1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) in 50 mL of toluene + 100 mL of heptane, T pol 70 °C, P C2H4 = 2 bar, for 30 min. For entries 2–8 of Table 5: V complex was dissolved in toluene, Co-catalyst Me2AlCl + ETA (molar ratio V
500) in 50 mL of toluene + 100 mL of heptane, T pol 70 °C, P C2H4 = 2 bar, for 30 min. For entries 2–8 of Table 5: V complex was dissolved in toluene, Co-catalyst Me2AlCl + ETA (molar ratio V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Me2AlCl
Me2AlCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ETA = 1
ETA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000
1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) in 100 mL of toluene + 100 mL of heptane; T pol 70 °C, P C2H4 = 2 bar, for 30 min.
1000) in 100 mL of toluene + 100 mL of heptane; T pol 70 °C, P C2H4 = 2 bar, for 30 min.
Crystallography
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the China Scholarship Council (CSC) for a PhD Scholarship to TX. The EPSRC Mass Spectrometry Service (Swansea, UK) and the EPSRC National X-ray Crystallography Service (Southampton) are thanked for data collection. CR also thanks the EPSRC (grant EP/S025537/1) for financial support and the British Council for funding a workshop in Novosibirsk. We also thank Dr Orlando Santoro for useful discussion. NVS and IES thank the Ministry of Science and Higher Education of Russia (#AAAA-A17-117041710085-9), and Dr. M. A. Matsko for the GPC measurements.References
- T. Czigány and F. Ronkay, eXPRESS Polym. Lett., 2020, 14, 510–511 CrossRef.
- For reviews, see (a) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147–6176 CrossRef CAS; (b) M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC; (c) C. M. Thomas, Chem. Soc. Rev., 2010, 39, 165–173 RSC; (d) A. Arbaoui and C. Redshaw, Polym. Chem., 2010, 1, 801–826 RSC; (e) Y. Sarazin and J.-F. Carpentier, Chem. Rev., 2015, 115, 3564–3614 CrossRef CAS and references therein.
- (a) M. Cozzolino, V. Leo, C. Tedesco, M. Mazzeo and M. Lamberti, Dalton Trans., 2018, 47, 13229–13238 RSC; (b) C. Redshaw, Catalysts, 2017, 7, 165–176 CrossRef; (c) B. Antelmann, M. H. Chisholm, S. S. Iyer, J. C. Huffman, D. Navarro-Llobet, M. Pagel, W. J. Simonsick and W. Zhong, Macromolecules, 2001, 34, 3159–3175 CrossRef CAS; (d) W. Braune and J. Okuda, Angew. Chem., Int. Ed., 2003, 42, 64–68 CrossRef CAS.
- (a) F. Ge, Y. Dan, Y. Al-Khafaji, T. J. Prior, L. Jiang, M. R. J. Elsegood and C. Redshaw, RSC Adv., 2016, 6, 4792–4802 RSC; (b) C. Redshaw, Dalton Trans., 2016, 45, 9018–9030 RSC; (c) H. Ishikura, R. Neven, T. Lange, A. Galetová, B. Blom and D. Romano, Inorg. Chim. Acta, 2021, 515, 120047 CrossRef CAS.
- (a) Y. Onishi, S. Katao, M. Fujiki and K. Nomura, Organometallics, 2008, 27, 2590–2596 CrossRef CAS; (b) A. Arbaoui, D. Homden, C. Redshaw, J. A. Wright, S. H. Dale and M. R. J. Elsegood, Dalton Trans., 2009, 8911–8922 RSC; (c) S. Zhang and K. Nomura, J. Am. Chem. Soc., 2010, 132, 4960–4965 CrossRef CAS; (d) K. Nomura, A. Igarashi, S. Katao, W. Zhang and W.-H. Sun, Inorg. Chem., 2013, 52, 2607–2614 CrossRef CAS; (e) X.-Y. Tang, A. Igarashi, W.-H. Sun, A. Inagaki, J. Liu, W. Zhang, Y.-S. Li and K. Nomura, Organometallics, 2014, 33, 1053–1060 CrossRef CAS; (f) N. Diteepeng, X. Tang, X. Hou, Y.-S. Li, K. Phomphrai and K. Nomura, Dalton Trans., 2015, 44, 12273–12281 RSC; (g) K. Nomura, M. Oshima, T. Mitsudome, H. Harakawa, P. Hao, K. Tsutsumi, G. Nagai, T. Ina, H. Takaya, W.-H. Sun and S. Yamazoe, ACS Omega, 2017, 2, 8660–8673 CrossRef CAS; (h) G. Zanchin, L. Vendier, I. Pierro, F. Bertini, G. Ricci, C. Lorber and G. Leone, Organometallics, 2018, 37, 3181–3195 CrossRef CAS; (i) G. Zanchin, F. Bertini, L. Vendier, G. Ricci, C. Lorber and G. Leone, Polym. Chem., 2019, 10, 6200–6216 RSC.
- D. M. Homden and C. Redshaw, Chem. Rev., 2008, 108, 5086–5130 CrossRef CAS.
- C. Redshaw, M. Walton, K. Michiue, Y. Chao, A. Walton, P. Elo, V. Sumerin, C. Jiang and M. R. J. Elsegood, Dalton Trans., 2015, 44, 12292–12303 RSC.
- C. Redshaw, M. Rowan, L. Warford, D. M. Homden, A. Arbaoui, M. R. J. Elsegood, S. H. Dale, T. Yamato, C. P. Casas, S. Matsui and S. Matsuura, Chem. – Eur. J., 2007, 13, 1090–1107 CrossRef CAS.
- B. Masci, in Calixarenes, ed. M.-Z. Asfari, V. Böhmer, J. Harrowfield, J. Vicens and M. Saadioui, Springer, Netherlands, 2001, ch. 12 Search PubMed.
- C. Redshaw, M. Walton, D. S. Lee, C. Jiang, M. R. J. Elsegood and K. Michiue, Chem. – Eur. J., 2015, 21, 5199–5210 CrossRef CAS.
- See for example, (a) M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef CAS; (b) Y. F. Al-Khafaji and F. H. Hussein, in Green and Sustainable Advanced Materials: Processing and Characterization, ed. S. Ahmed and C. M. Hussain, Wiley, 2018, ch. 6 Search PubMed.
- M. Bochmann, G. Wilkinson, G. B. Young, M. B. Hursthouse and K. M. A. Malik, J. Chem. Soc., Dalton Trans., 1980, 1863–1871 RSC.
- D. M. Miller-Shakesby, S. Nigam, D. L. Hughes, E. Lopez-Estelles, M. R. J. Elsegood, C. J. Cawthorne, S. J. Archibald and C. Redshaw, Dalton Trans., 2018, 47, 8992–8999 RSC; W. Clegg, M. R. J. Elsegood, V. C. Gibson and C. Redshaw, J. Chem. Soc., Dalton Trans., 1998, 3037–3039 RSC; N. Li, J.-J. Liu, J.-W. Sun, B.-X. Dong, L.-Z. Dong, S.-J. Yao, Z. Xin, S.-L. Li and Y.-Q. Lan, Green Chem., 2020, 22, 5325–5332 RSC; X.-X. Yang, W.-D. Yu, X.-Y. Yi and C. Liu, Inorg. Chem., 2020, 59, 7512–7519 CrossRef CAS.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC.
- T. Moriuchi and T. Hirao, Coord. Chem. Rev., 2011, 255, 2371–2377 CrossRef CAS.
- (a) D. D. Devore, J. D. Lichtenhan, F. Takusagawa and E. A. Maatta, J. Am. Chem. Soc., 1987, 109, 7408–7416 CrossRef CAS; (b) E. A. Maatta, Inorg. Chem., 1984, 23, 2560–2561 CrossRef CAS.
- M. Lutz, H. Hagen, A. M. M. Schreurs and G. Van Koten, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 1636–1639 CrossRef.
- (a) P. Slavík and P. Lhoták, Tetrahedron Lett., 2018, 59, 1757–1759 CrossRef; (b) P. Slavík, H. Dvořáková, M. Krupička and P. Lhoták, Org. Biomol. Chem., 2018, 16, 838–843 RSC.
- See for example, (a) K. Ding, M. O. Miranda, B. Moscato-Goodpaster, N. Ajellal, L. E. Breyfogle, E. D. Hermes, C. P. Schaller, S. E. Roe, C. J. Cramer, M. A. Hillmyer and W. B. Tolman, Macromolecules, 2012, 45, 5387–5396 CrossRef CAS; (b) M. Normand, V. Dorcet, E. Kirillov and J.-F. Carpentier, Organometallics, 2013, 32, 1694–1709 CrossRef CAS; (c) Y. Huang, W. Wang, C.-C. Lin, M. P. Blake, L. Clark, A. D. Schwarz and P. Mountford, Dalton Trans., 2013, 42, 9313–9324 RSC; (d) A. Thevenon, C. Romain, M. S. Bennington, A. J. P. White, H. J. Davidson, S. Brooker and C. K. Williams, Angew. Chem., Int. Ed., 2016, 55, 8680–8685 CrossRef CAS.
- (a) Q. Hu, S.-Y. Jie, P. Braunstein and B.-G. Lia, Chin. J. Polym. Sci., 2020, 38, 240–247 CrossRef CAS; (b) M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef CAS; (c) T. Wu, Z. Wei, Y. Ren, Y. Yu, X. Leng and Y. Li, Polym. Degrad. Stab., 2018, 155, 173–182 CrossRef CAS; (d) M. T. Hunley, N. Sari and K. L. Beers, ACS Macro Lett., 2013, 2, 375–379 CrossRef CAS; (e) Z. Sun, Y. Zhao, O. Santoro, M. R. J. Elsegood, E. V. Bedwell, K. Zahra, A. Walton and C. Redshaw, Catal. Sci. Technol., 2020, 10, 1619–1639 RSC.
- I. E. Soshnikov, N. V. Semikolenova, A. A. Shubin, K. P. Bryliakov, V. A. Zakharov, C. Redshaw and E. P. Talsi, Organometallics, 2009, 28, 6714–6720 CrossRef CAS; I. E. Soshnikov, N. V. Semikolenova, K. P. Bryliakov, A. A. Shubin, V. A. Zakharov, C. Redshaw and E. P. Talsi, Macromol. Chem. Phys., 2009, 210, 542–548 CrossRef; I. E. Soshnikov, N. V. Semikolenova, K. P. Bryliakov, V. A. Zakharov, C. Redshaw and E. P. Talsi, J. Mol. Catal. A: Chem., 2009, 303, 23–29 CrossRef.
- (a) B. Masci, J. Org. Chem., 2001, 66, 1497–1499 CrossRef CAS; (b) B. Dhawan and C. D. Gutsche, J. Org. Chem., 1983, 48, 1536–1539 CrossRef CAS.
- Despite repeated attempts, the elemental analysis results for this acetonitrile solvate were the closest we could obtain.
- SAINT and APEX 2 software for CCD diffractometers, Bruker AXS Inc., Madison, USA, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1998549–1998555 (1·5MeCN, 2·6MeCN, 3·7MeCN·0.5CH2Cl2, 4·4MeCN, 5, 6, 8·4MeCN) contain the supplementary crystallographic data. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cy01979h |
| This journal is © The Royal Society of Chemistry 2021 |