K, Na and Cl co-doped TiO2 nanorod arrays on carbon cloth for efficient photocatalytic degradation of formaldehyde under UV/visible LED irradiation†
Wenyu
Diao‡
,
Jun
He‡
,
Qiang
Wang
,
Xi
Rao
 * and
Yongping
Zhang
* and
Yongping
Zhang
 *
*
School of Materials and Energy, Southwest University, Chongqing 400715, China. E-mail: raoxiemail@swu.edu.cn; zhangyyping@yahoo.com
First published on 30th October 2020
Abstract
For applicable air purifiers to decompose formaldehyde, it is important to develop TiO2 based photocatalysts that can be irradiated under visible light and can be anchored on a soft carbon cloth substrate. Herein, we report that rutile TiO2 nanorod arrays were grown on carbon cloth (TiO2@CC) by a seed assisted hydrothermal method and subsequently heated with a KCl and NaCl covering to promote K, Na, and Cl ions doping into the TiO2 nanorods. K, Na, and Cl doping tunes the energy band structure of rutile TiO2, while the carbon fibers facilitate the electron transfer, improving its photocatalytic activity. Thus, the alkali metal (K, Na) and halogen (Cl) co-doped rutile TiO2 nanorod arrays (KNCTC) exhibited better photocatalytic degradation of gaseous formaldehyde (HCHO) under UV and visible light irradiation than pure TiO2. TiO2 nanorod arrays anchored on soft carbon cloth facilitate the design of an air purifier for degrading formaldehyde.
1. Introduction
Nowadays, indoor air pollutants have raised pressing concerns with their adverse effects and the fact that they can even be hazardous to human health. Formaldehyde is one of the leading volatile organic compounds (VOCs) among indoor pollutants emitted from decoration materials and furniture. Commonly used air purification methods include adsorption, biocatalysis and photocatalytic oxidation (PCO).1–4 PCO is an effective approach and the main direction of the latest generation of air purification technologies to decompose chemical pollutants into harmless molecules and even to eliminate gaseous pollutants at low concentrations. To develop applicable and efficient photocatalysts for air purifiers, two primary factors should always be considered: 1) gaining the visible light response of photocatalysts and 2) anchoring the nanostructured catalysts on a soft carbon substrate.Semiconductor photocatalysis has received extensive attention for eliminating environment pollutants and providing green energy, ever since Fujishima and Honda discovered the photocatalytic water splitting phenomenon of TiO2 semiconductor electrodes.5 Such TiO2 based photocatalysts have broad application prospects in sewage treatment, air cleaning and virus sterilization.6–10 TiO2 is considered to be the most promising photocatalyst for the treatment of air pollutants due to its strong oxidation ability of photogenerated carriers. Its band gap varies for different crystal structures. The band gap is 3.2 eV for anatase and 3.0 eV for rutile. Our recent studies have shown that the anatase TiO2 nanowires have good photocatalytic activity under ultraviolet irradiation.9,10 Rutile TiO2 nanorods achieved photocatalytic water splitting under ultraviolet irradiation.11,12 Several strategies have been adopted to improve the visible light response, such as introducing impurities or defects on the surface,11–13 depositing precious metals, other metal oxides, and sulfides,14–18 and doping with metal and nonmetal ions.19–27 In the literature, anion and cation co-doping can improve the structural stability of TiO2.28,29 On the one hand, doping alkali metal ions (such as Li+, Na+, and K+) shifts the absorption edge toward a longer wavelength and decreases the band gap. On the other hand, doping halogen ions (F− and Cl−) narrows the band gap and shifts the adsorption edge toward a higher wavelength. Therefore, alkali and halogen element co-doping could be explored for efficient catalysts for the photocatalytic degradation of formaldehyde.
On the other hand, the vertically oriented growth of 1D nanostructures on flexible carbon fiber cloth has attracted widespread attention. Carbon cloth has an excellent carrier transport performance and high specific surface area, and is easier to recycle and reuse than powdered catalysts.30–34 The growth of vertically oriented 1D nanostructures on the flexible carbon fiber cloth tends to build nanostructure arrays and heterojunctions, enhancing the photocatalytic activity. As for the design of the photocatalytic reactor, nanostructured catalysts anchored on a soft substrate not only provide a large surface area, but also simplify the filling process compared with particulate catalysts, facilitating the design of air purifiers.
Herein, K, Na, and Cl co-doped rutile TiO2 nanorod arrays on carbon cloth (TiO2@CC) were prepared and then were used for the photocatalytic degradation of formaldehyde. Rutile TiO2 nanorod arrays were grown on CC by a seed-assisted hydrothermal method, and subsequently heated with a KCl and NaCl covering to promote K, Na, and Cl doping into the TiO2 nanorods, tuning the band structure of rutile TiO2. Such approaches employed in this work could provide a pathway to grow efficient photocatalysts on a flexible substrate.
2. Experimental section
2.1 Chemicals
Titanium butoxide (C16H36O4Ti, 99%), titanium tetrachloride (TiCl4, 99.5%), absolute ethanol, hydrochloric acid (HCl, 37%), nitric acid (HNO3, 68%) and sulphuric acid (H2SO4, 98%) were purchased from Sinopharm Chemical Reagent Co.2.2 Preparation of rutile TiO2@CC
Rutile TiO2 nanorod arrays were grown on a carbon cloth (CC) substrate by a seed-assisted hydrothermal method.35 Firstly, carbon cloth was immersed in an acid solution (H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 = 3
HNO3 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 24 h, cleaned ultrasonically in deionized water, and then dried at 80 °C. Secondly, the well-cleaned carbon cloth was immersed into a solution (dissolving 220 μL TiCl4 in 10 mL of 2.4 M HCl) for 12 h, then dried at 80 °C. The dried carbon cloth was heated at 400 °C for 30 min, forming TiO2 nanoparticles coated on CC as seeding sites. Thirdly, 1 mL titanium butoxide was added dropwise to 24 mL of 6 M hydrochloric acid. The mixture was stirred ultrasonically until a clear solution was achieved. This clear solution and the CC-coated TiO2 nanoparticles were transferred into a 100 mL Teflon-lined autoclave and heated at 160 °C for 5.5 h. Finally, after the autoclave was cooled down to room temperature, the CC was removed from the autoclave, rinsed with deionized water and ethanol, and dried at 80 °C to obtain TiO2@CC (Fig. S1†). TiO2@CC was annealed in air at 550 °C for 1 h to obtain rutile TiO2 nanorod arrays on carbon cloth, denoted as TC.
1) for 24 h, cleaned ultrasonically in deionized water, and then dried at 80 °C. Secondly, the well-cleaned carbon cloth was immersed into a solution (dissolving 220 μL TiCl4 in 10 mL of 2.4 M HCl) for 12 h, then dried at 80 °C. The dried carbon cloth was heated at 400 °C for 30 min, forming TiO2 nanoparticles coated on CC as seeding sites. Thirdly, 1 mL titanium butoxide was added dropwise to 24 mL of 6 M hydrochloric acid. The mixture was stirred ultrasonically until a clear solution was achieved. This clear solution and the CC-coated TiO2 nanoparticles were transferred into a 100 mL Teflon-lined autoclave and heated at 160 °C for 5.5 h. Finally, after the autoclave was cooled down to room temperature, the CC was removed from the autoclave, rinsed with deionized water and ethanol, and dried at 80 °C to obtain TiO2@CC (Fig. S1†). TiO2@CC was annealed in air at 550 °C for 1 h to obtain rutile TiO2 nanorod arrays on carbon cloth, denoted as TC.
2.3 Preparation of K, Na and Cl doped TiO2@CC
K, Na and Cl co-doped TiO2 nanorod arrays were achieved by a thermal diffusion method with molten salt. NaCl and KCl with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were ground with a pestle and mortar. The TiO2@CC covered with the NaCl and KCl mixture was annealed in air at 550 °C for 1 h. Then TiO2@CC was sintered with deionized water and dried at 80 °C to obtain K, Na and Cl doped TiO2@CC, denoted as KNCTC.
1 were ground with a pestle and mortar. The TiO2@CC covered with the NaCl and KCl mixture was annealed in air at 550 °C for 1 h. Then TiO2@CC was sintered with deionized water and dried at 80 °C to obtain K, Na and Cl doped TiO2@CC, denoted as KNCTC.
In a similar annealing process, TiO2@CC covered with KCl was annealed at 550 °C to obtain the K and Cl doped TiO2, denoted as KCTC, while TiO2@CC was covered with NaCl to obtain Na and Cl doped TiO2, denoted as NCTC.
2.4 Characterization of rutile TiO2 nanorods
The catalysts were characterized with X-ray diffraction (XRD, Shimadzu 7000), transmission electron microscopy (TEM, Libra 200FE, Zeiss), scanning electron microscopy (SEM, JSM-7800F10100), X-ray photoelectron spectroscopy (XPS, Thermo Fisher ESCALAB 250Xi), Raman spectroscopy (LabRAM HR Evolution), UV-vis diffuse reflection spectroscopy (Agilent Cary 5000), photoluminescence (PL, Hitachi F-7000), and electron paramagnetic resonance (EPR, Bruker EMX nano). Electrochemical tests were performed on an AUTOLAB workstation (PGSTAT 302N) using a three-electrode system at room temperature. An FTO conductive glass film with an area of 1 cm2 was used as the working electrode, the electrolyte was a 0.5 M Na2SO4 solution, and the light source was a 500 W xenon lamp with a 365 nm cut-off filter.2.5 Evaluation of photocatalytic performance
The photocatalytic performance was evaluated by measuring the changes of formaldehyde concentration in a continuous-flow reactor in an ambient environment, as shown in Fig. S2.† Three pieces of TiO2@CC (20 mm × 6.5 mm) were placed into a ϕ3 mm quartz tube. Prior to the degradation reaction, the formaldehyde flow rate was well-controlled to achieve an adsorption–desorption equilibrium. An LED lamp (365 nm or 420 nm) was used as the light source. The efficiency of the photocatalytic degradation of formaldehyde was calculated by comparing the formaldehyde concentration change at the inlet and outlet of the quartz tube. The formaldehyde concentration was continuously monitored by the formaldehyde sensor (Dart Sensor Ltd, UK). The formaldehyde degradation rate was calculated according to the following equation: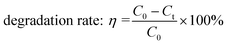 | (1) |
3. Results and discussion
3.1 Structural characterization
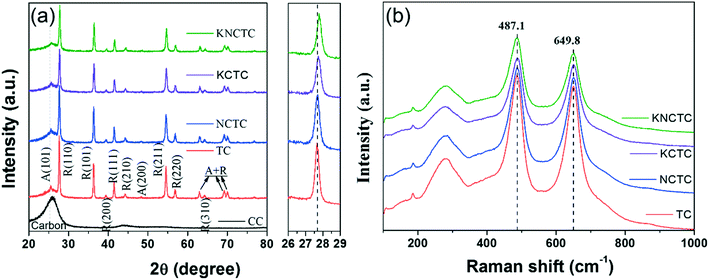
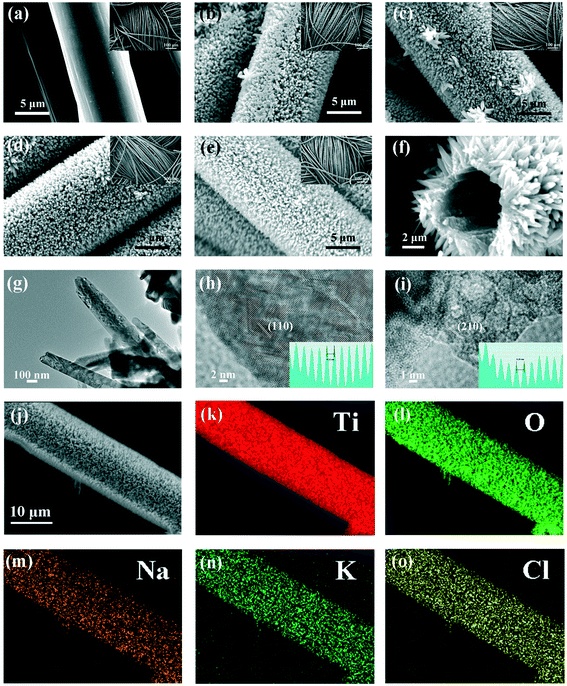
Fig. 1(a) shows the XRD patterns of TC, NCTC, KCTC, and KNCTC. The main diffraction peaks at the 2θ position of 27.5°, 36.4°, 39.4°, 41.5°, 44.2°, 54.6°, 56.8°, 62.9°, 64.3°, 69.2°, and 69.9° can be readily indexed to the (110), (101), (200), (111), (210), (211), (220), (002), (310), (301), and (112) planes of the rutile phase of TiO2 (JCPDS 87-0710). The weak peaks at 25.5°, 48.3° are attributed to the (101), (200) planes of anatase phase of TiO2 (JCPDS 71-1166). The peaks at 62.9, 69.2, 69.9° may correspond to the (204), (116), and (220) planes of the anatase phase (JCPDS 71-1166). In all the catalysts, the hump peak at 26.1° corresponds to the (002) plane of the carbon cloth substrate, which appears to be weaker than that of pure carbon cloth. The XRD results show that the catalysts are composed mainly of the rutile phase and a small amount of the anatase phase of TiO2. With Na, K, and Cl element doping, the 2θ position for the rutile (110) peak increases slightly, indicating that the lattice constant decreases a little after element doping. It may be inferred that K+ and Na+ substitute Ti4+ and Cl− substitutes O2−. The Raman spectra in Fig. 1(b) show the typical Eg (487.1 cm−1) and A1g (649.8 cm−1) modes corresponding to the P42/mnm group of rutile. The broad band at 281.5 cm−1 is due to the multi-photon scattering of rutile phase TiO2, while the weak peak at 185.5 cm−1 is attributed to anatase phase TiO2.36–38 This indicates that the catalysts are composed mainly of the rutile phase of TiO2, which is consistent with the XRD observations.SEM images in Fig. 2(a–e) show that the TiO2 nanorod arrays are uniformly grown on the carbon cloth, completely covering the surface of carbon fiber. Fig. 2(f) shows the cross-section of KNCTC, in which the thickness of the TiO2 nanorod arrays is about 2 μm and the TiO2 nanorods are a length of about 2 μm and a width of about 100 nm. The TEM image of KNCTC in Fig. 2(g) further shows that some pores exist in the TiO2 nanorods, indicating that it is a porous material. The HRTEM images of KNCTC, as shown in Fig. 2(h) and (i), demonstrate that the distances between the lattice fringes, 0.32 nm and 0.20 nm, can be assigned to the (110) and (210) of rutile TiO2, respectively. The EDS element mapping of KNCTC, as shown in Fig. 2(j–o), indicates that Ti, O, Na, K, and Cl are uniformly distributed on the background of the carbon fiber. The results confirm that uniform rutile TiO2 nanorod arrays are successfully grown on the carbon cloth and K, Na, and Cl elements are doped in the TiO2 nanorods.
X-ray photoelectron spectroscopy (XPS) was performed to study the chemical states of all the catalysts. Fig. 3(a) shows the XPS scan spectra of TC and KNCTC. The Ti 2p and O 1s peaks for TC can be seen, and the additional peaks K 2p, Na 1s, and Cl 2p for KNCTC appear compared with CC, indicating that the K, Na, and Cl elements have been successfully introduced into the catalysts. The atomic ratio of Ti to O is about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.77, demonstrating that the surface of TiO2 is oxygen deficient. In the Ti 2p spectra shown in Fig. 3(b), two peaks at a binding energy of 457.7 eV and 463.4 eV are assigned to the spin–orbital splitting doublet of 2p3/2 and 2p1/2, respectively, corresponding to Ti4+ in TiO2.39,40 With the element doping, the binding energy of Ti 2p shifts by 0.2 eV to a lower binding energy due to the electron transfer from alkali metals Na and K to Ti and O of TiO2; this could also be partly attributed to the further reduction of Ti by the addition of alkali metal ions.41,42 The O 1s peak, as shown in Fig. 3(c), can be fitted to the peaks at 528.9 eV, 530.4 eV, and 531.5 eV, which correspond to oxygen in the Ti–O lattice and physiosorbed H2O and O2, respectively. The Ti–O peak shifts to the lower binding energy of 0.1 eV after element doping, which is attributed to the influence of K, Na alkali metal and Cl element doping on lattice oxygen. The Na 1s spectrum in Fig. 3(d) shows that the Na 1s peak is located at 1070.4 eV, indicating that Na atoms exist in TiO2 in the form of Na+.41,42 The K 2p spectrum as shown in Fig. 3(e) shows that 2p3/2 and 2p1/2 peaks are located at 294.7 eV and 291.9 eV, respectively, indicating that K atoms exist in TiO2 as K+.41,42 The Cl 2p spectrum in Fig. 3(f) shows that the 2p3/2 and 2p1/2 peaks are located at 198.5 eV and 196.9 eV, respectively, indicating that Cl atoms exist in the form of O–Ti–Cl.43–45 The atomic ratios for KNCTC are 1, 1.77, 0.06, 0.26, and 0.02, for Ti, O, K, Na, and Cl, respectively. This shows that element doping induces oxygen deficiency to fulfill the electric neutrality.
1.77, demonstrating that the surface of TiO2 is oxygen deficient. In the Ti 2p spectra shown in Fig. 3(b), two peaks at a binding energy of 457.7 eV and 463.4 eV are assigned to the spin–orbital splitting doublet of 2p3/2 and 2p1/2, respectively, corresponding to Ti4+ in TiO2.39,40 With the element doping, the binding energy of Ti 2p shifts by 0.2 eV to a lower binding energy due to the electron transfer from alkali metals Na and K to Ti and O of TiO2; this could also be partly attributed to the further reduction of Ti by the addition of alkali metal ions.41,42 The O 1s peak, as shown in Fig. 3(c), can be fitted to the peaks at 528.9 eV, 530.4 eV, and 531.5 eV, which correspond to oxygen in the Ti–O lattice and physiosorbed H2O and O2, respectively. The Ti–O peak shifts to the lower binding energy of 0.1 eV after element doping, which is attributed to the influence of K, Na alkali metal and Cl element doping on lattice oxygen. The Na 1s spectrum in Fig. 3(d) shows that the Na 1s peak is located at 1070.4 eV, indicating that Na atoms exist in TiO2 in the form of Na+.41,42 The K 2p spectrum as shown in Fig. 3(e) shows that 2p3/2 and 2p1/2 peaks are located at 294.7 eV and 291.9 eV, respectively, indicating that K atoms exist in TiO2 as K+.41,42 The Cl 2p spectrum in Fig. 3(f) shows that the 2p3/2 and 2p1/2 peaks are located at 198.5 eV and 196.9 eV, respectively, indicating that Cl atoms exist in the form of O–Ti–Cl.43–45 The atomic ratios for KNCTC are 1, 1.77, 0.06, 0.26, and 0.02, for Ti, O, K, Na, and Cl, respectively. This shows that element doping induces oxygen deficiency to fulfill the electric neutrality.
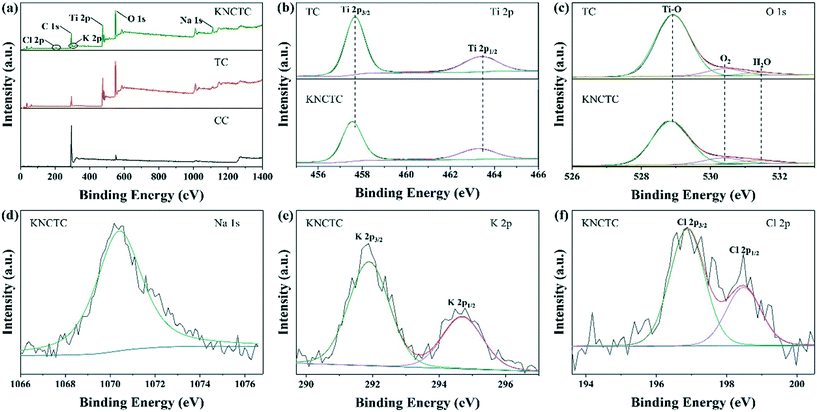 | ||
| Fig. 3 XPS scan spectra (a), Ti 2p (b), O 1s (c), Na 1s (d), K 2p (e), and Cl 2p (f) of TC and KNCTC. | ||
3.2 UV-vis and PL spectra
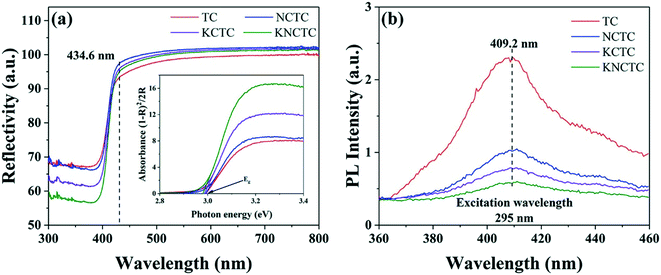
Fig. 4(a) shows the UV-vis reflectance spectra of all catalysts, with a band-edge adsorption around 434.6 nm, and the absorption edge red shifts with the doping of elements. The plots of the transformed Kubelka–Munk function versus the photon energy give the band gap as 2.99 eV, 2.99 eV, 2.99 eV, and 2.98 eV, for TC, NCTC, KCTC, and KNCTC, respectively, indicating that K, Na, and Cl co-doping narrows the band gap. Among those catalysts, the three-element doping narrows the band gap the most and increases visible light absorption. The results imply that element doping can tune the band structure of TiO2 and increase the harvesting of visible light. Fig. 4(b) shows the PL spectra with the excitation wavelength of 295 nm, and the fluorescence band is about 409.2 nm. In Fig. 4(b), obvious fluorescence quenching occurs after element doping, and KNCTC can achieve effective separation of electron–hole pairs.3.3 Electrochemical measurements
Fig. 5(a) shows the transient photocurrent response under the excitation with light wavelength λ > 365 nm. The current response is in consequence of KNCTC > KCTC > NCTC. The photocurrent of KNCTC, KCTC, NCTC, and TC tended to be stabilized, indicating that the transient photocurrent response of KNCTC, KCTC, NCTC, and TC was stable under visible light illumination. The KNCTC electrode has a high transient photocurrent response, which means that more photoelectrons can be generated. The doping of K, Na, and Cl increases the lag after turning off the light, indicating that element doping is beneficial to the effective separation of electron–hole pairs. Electrochemical impedance spectroscopy (EIS) results are shown in Fig. 5(b), including the circuit diagram fitting selection circuit. After element doping, the interface reaction impedance (Rct) decreases, and the impedance is in consequence of KNCTC < NCTC < KCTC < TC. The minimum impedance of KNCTC can achieve effective separation of electron–hole pairs. The Mott–Schottky curves are shown in Fig. 5(c). The flat band potential of TC is −0.32 V, the flat band potential of NCTC is −0.43 V, the flat band potential of KCTC is −0.25 V, and the flat band potential of KNCTC is −0.30 V. The conduction band potentials are −0.32 V, −0.43 V, −0.25 V, and −0.30 V, respectively. Na and Cl doping makes the conduction band negatively shift, and the co-doping of K, Cl and the three elements shifts the conduction band positively. The schematic diagram of the energy band structure is obtained from Fig. 4(a) and 5(c). As shown in Fig. 5(d), the co-doping of the three elements shifts the conduction band positively, which is beneficial to the generation of ˙OH and ˙O2− to achieve formaldehyde degradation.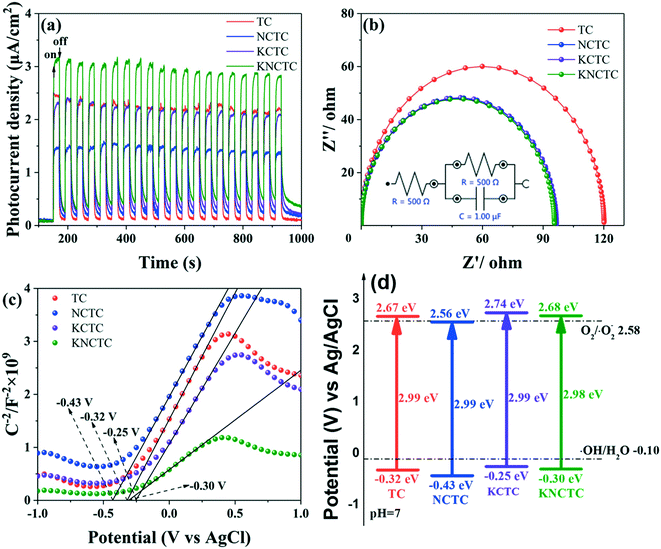 | ||
| Fig. 5 Photocurrent response (a), EIS (b), Mott–Schottky plots (c) and schematic band structure (d) of TC, NCTC, KCTC, and KNCTC. | ||
3.4 Photocatalytic performance
The photocatalytic degradation of formaldehyde was carried out in a quartz tube reactor under room temperature and humidity of 60%, and the inlet concentration of formaldehyde was set to 0.4 ppm. Fig. 6(a) shows the photocatalytic degradation performance of catalysts under 365 nm LED radiation with light intensity of 1.5 W. After the adsorption equilibrium is reached, the light is turned on to degrade the formaldehyde. The outlet concentration of formaldehyde gradually decreases to the minimum value, indicating that the catalyst reaches the limit of the formaldehyde degradation in a short time interval. Then, the outlet formaldehyde concentration rises a little after a period of degradation and eventually reaches equilibrium saturation. After the light is turned on, the maximum formaldehyde degradation rates are 72%, 78%, 86%, and 96% for TC, NCTC, KCTC, and KNCTC, respectively. Then, the degradation rate increases a little after a period of time. Finally, the degradation rate eventually reaches the equilibrium saturation of 58%, 60%, 70%, and 86% after 13 h of light irradiation. As the KNCTC exhibits the highest degradation rate among all the catalysts, the element doping is evidenced to be beneficial to the degradation of formaldehyde. All the catalysts show a similar degradation behavior under 1.7 W 420 nm LED irradiation, as shown in Fig. 6(b). The maximum formaldehyde degradation rates reach 36%, 74%, 92%, and 100%, for TC, NCTC, KCTC, and KNCTC, respectively. Eventually the degradation reaches an equilibrium saturation of 32%, 68%, 80%, and 90% for TC, NCTC, KCTC, and KNCTC, respectively. KNCTC has the highest degradation rate among all the catalysts under visible light. Thus, element doping is also beneficial to enhancing the degradation ability of formaldehyde under visible light. Element doping can induce a supplementary energy level, facilitating the generation of electrons/holes under visible light irradiation.46Fig. 6(c) shows the formaldehyde degradation stability of KNCTC under 365 nm LED, with light intensity of 1.5 W. The formaldehyde degradation rate reaches a limit of 88% and an equilibrium saturation of 84% after turning on the light for 1 h and 20 h. Moreover, the formaldehyde can be completely decomposed by switching to a strong light intensity of 4.4 W. The degradation of formaldehyde does not decrease after 20 cycles in 48 h, indicating that the catalyst is quite stable and can be used continuously.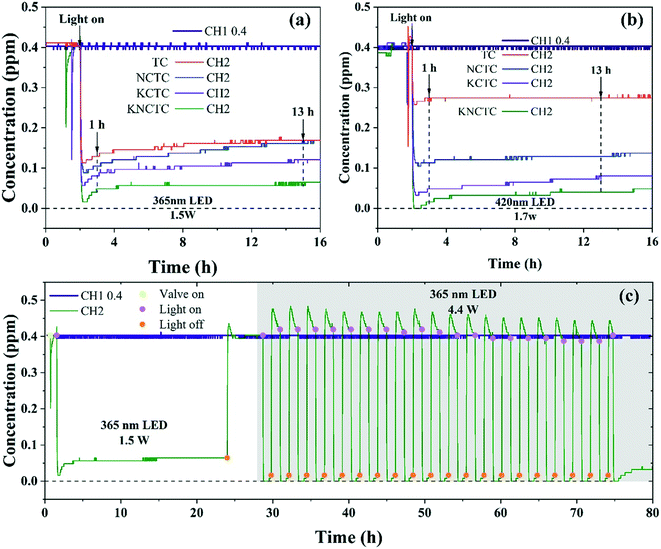 | ||
| Fig. 6 Photocatalytic degradation of formaldehyde under 365 nm LED (a) and 420 nm LED (b) of TC, NCTC, KCTC, and KNCTC. The stability test (c) of KNCTC. | ||
3.5 Photocatalytic mechanism
In order to understand the photocatalytic performance of KNCTC, DMPO (5,5-dimethyl-1-pyrrole N-oxide) was used as a free radical trapping agent, and the active free radicals generated on the catalyst surface were identified by EPR technology. Fig. 7 shows the EPR measurement results of KNCTC in DMPO–˙O2− in a methanol dispersion system and DMPO–˙OH in a water dispersion system. Upon Xe lamp (λ > 365 nm) irradiation, the two samples in the methanol dispersion system show obvious 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 peaks corresponding to ˙O2−, and those in the water dispersion system show 1
1 peaks corresponding to ˙O2−, and those in the water dispersion system show 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 peaks corresponding to ˙OH. This indicates that KNCTC produces ˙O2− and ˙OH to degrade formaldehyde. The EPR experiments show that the hydroxyl radical (˙OH) and superoxide radical (˙O2−) are the main reactive oxygen species (ROS) involved in the photocatalysis. The ROS are generated through the interaction of photoinduced charge carriers with H2O and O2 adsorbed on the catalyst surface. Under visible light irradiation, the photoinduced electrons are trapped by O2 to yield ˙O2−, and ˙O2− reacts with water to form –OH. HCHO is then oxidized into a formate species with the surface –OH. Holes in the VB directly oxidize –OH and/or water to produce ˙OH. Subsequently, the formate is further oxidized to CO2 and H2O by ˙OH.9 On the other hand, TiO2 nanorod arrays on carbon cloth have several advantages: 1) the photoinduced electrons are rapidly injected into the conduction band and transported along the carbon fibers because of their high electrical conductivity; 2) TiO2 nanorod arrays on carbon cloth have a large surface area and can be easily assembled in the quartz tube reactor; 3) TiO2 nanorod arrays on soft carbon cloth could be integrated into curtain fabrics for indoor air purification.
1 peaks corresponding to ˙OH. This indicates that KNCTC produces ˙O2− and ˙OH to degrade formaldehyde. The EPR experiments show that the hydroxyl radical (˙OH) and superoxide radical (˙O2−) are the main reactive oxygen species (ROS) involved in the photocatalysis. The ROS are generated through the interaction of photoinduced charge carriers with H2O and O2 adsorbed on the catalyst surface. Under visible light irradiation, the photoinduced electrons are trapped by O2 to yield ˙O2−, and ˙O2− reacts with water to form –OH. HCHO is then oxidized into a formate species with the surface –OH. Holes in the VB directly oxidize –OH and/or water to produce ˙OH. Subsequently, the formate is further oxidized to CO2 and H2O by ˙OH.9 On the other hand, TiO2 nanorod arrays on carbon cloth have several advantages: 1) the photoinduced electrons are rapidly injected into the conduction band and transported along the carbon fibers because of their high electrical conductivity; 2) TiO2 nanorod arrays on carbon cloth have a large surface area and can be easily assembled in the quartz tube reactor; 3) TiO2 nanorod arrays on soft carbon cloth could be integrated into curtain fabrics for indoor air purification.
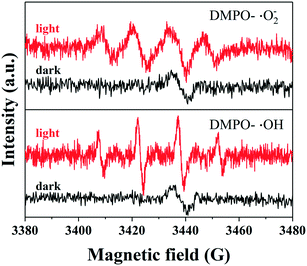 | ||
| Fig. 7 EPR spectra in methanol dispersion for DMPO–˙O2− and aqueous dispersion for DMPO–˙OH for KNCTC in the dark and under Xe light irradiation (λ > 365 nm). | ||
4. Conclusions
In summary, rutile TiO2 nanorod arrays were grown on carbon cloth by a seeding assisted hydrothermal technique, and were then doped with K, Na, and Cl by thermal diffusion. The photocatalytic degradation results showed that element doped TiO2 arrays exhibited an excellent visible light response. Especially, KNCTC showed the enhanced photocatalytic degradation of formaldehyde with good stability under UV and visible LED irradiation. Such rutile TiO2@CC realized the immobilized integration of catalysts on a flexible substrate with good catalytic performance, providing a more efficient and convenient design for air purifiers than nanoparticles.Author contributions
The manuscript was written through contributions of all the authors. All the authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51801164), Fundamental Research Funds for Central Universities (XDJK2020C005), Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2018080).References
- Z. Zhang, Z. Jiang and W. Shangguan, Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review, Catal. Today, 2016, 264, 270–278 CrossRef CAS.
- R. Ahmed, Z. Ahmad, A. U. Khan, N. R. Mastoi, M. Aslam and J. Kim, Photocatalytic system as an advanced environmental remediation: Recent developments, limitations and new avenues for applications, J. Environ. Chem. Eng., 2016, 4, 4143–4164 CrossRef.
- J. Yu, X. Li, Z. Xu and W. Xiao, NaOH-Modified Ceramic Honeycomb with Enhanced Formaldehyde Adsorption and Removal Performance, Environ. Sci. Technol., 2013, 47, 9928–9933 CrossRef CAS.
- Y. Li, X. Wu, J. Li, K. Wang and G. Zhang, Z-scheme g-C3N4@CsxWO3 heterostructure as smart window coating for UV isolating, Vis penetrating, NIR shielding and full spectrum photocatalytic decomposing VOCs, Appl. Catal., B, 2018, 229, 218–226 CrossRef CAS.
- A. Fujishima and K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nature, 1972, 238, 37–38 CrossRef CAS.
- Photocatalytic Purification and Treatment of Water and Air, ed. D. F. Ollis and H. Al-Ekabi, Elsevier, Amsterdam, 1993 Search PubMed.
- Z. Shayegan, C.-S. Lee and F. Haghighat, TiO2 photocatalyst for removal of volatile organic compounds in gas phase – A review, Chem. Eng. J., 2018, 334, 2408–2439 CrossRef CAS.
- A. E. Burakova, E. V. Galunina, I. V. Burakovaa, A. E. Kucherovaa, S. Agarwalb, A. G. Tkacheva and V. K. Guptab, Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review, Ecotoxicol. Environ. Saf., 2018, 148, 702–712 CrossRef.
- H. Dou, D. Long, X. Rao, Y. Zhang, Y. Qin, F. Pan and K. Wu, Photocatalytic Degradation Kinetics of Gaseous Formaldehyde Flow Using TiO2 Nanowires, ACS Sustainable Chem. Eng., 2019, 7, 4456–4465 CrossRef CAS.
- W. Diao, H. Cai, L. Wang, X. Rao and Y. Zhang, Efficient photocatalytic degradation of gas-phase formaldehyde by Pt/TiO2 nanowires in a continuous flow reactor, ChemCatChem, 2020, 12, 5420–5429 CrossRef CAS.
- L. Li, J. Yan, T. Wang, Z. Zhao, J. Zhang, J. Gong and N. Guan, Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production, Nat. Commun., 2015, 6, 5881 CrossRef.
- Z. Zhao, X. Zhang, G. Zhang, Z. Liu, D. Qu, X. Miao, P. Feng and Z. Sun, Effect of defeacts on photocatalytic activity of rutile TiO2 nanorods, Nano Res., 2015, 8, 4061–4071 CrossRef CAS.
- X. M. Yu, B. Kim and Y. K. Kim, Highly enhanced photoactivity of anatase TiO2 nanocrystals by controlled hydrogenation induced surface defects, ACS Catal., 2013, 3, 2479–2486 CrossRef CAS.
- X. Wang, M. Sun, M. Murugananthan, Y. Zhang and L. Zhang, Electrochemically self-doped WO3/TiO2 nanotubes for photocatalytic degradation of volatile organic compounds, Appl. Catal., B, 2020, 260, 118205 CrossRef CAS.
- S. Suarez, I. Jasson, B. Ohtani and B. Sanchez, From titania nanoparticles to decahedral anatase particles: Photocatalytic activity of TiO2/zeolite hybrids for VOCs oxidation, Catal. Today, 2019, 326, 2–7 CrossRef CAS.
- W. Lin, X. Xie, X. Wang, Y. Wang, D. Segets and J. Sun, Efficient adsorption and sustainable degradation of gaseous acetaldehyde and o-xylene using rGO-TiO2 photocatalyst, Chem. Eng. J., 2018, 349, 708–718 CrossRef CAS.
- P. S. Basavarajappa, S. B. Patil, N. Ganganagappa, K. R. Reddy, A. V. Raghu and C. V. Reddy, Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis, Int. J. Hydrogen Energy, 2020, 45, 7764–7778 CrossRef CAS.
- J. Diaz-Angulo, J. Lara-Ramos, M. Mueses, A. Hernández-Ramírez, G. Li Puma and F. Machuca-Martínez, Enhancement of the oxidative removal of diclofenac and of the TiO2 rate of photon absorption in dye-sensitized solar pilot scale CPC photocatalytic reactors, Chem. Eng. J., 2020, 381, 122520 CrossRef CAS.
- V. Kumaravel, S. Mathew, J. Bartlett and S. C. Pillai, Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances, Appl. Catal., B, 2019, 244, 1021–1064 CrossRef CAS.
- C. Zhang, F. Liu, Y. Zhai, H. Ariga, N. Yi, Y. Liu, K. Asakura, M. Flytzani-Stephanopoulos and H. He, Alkali-metal-promoted Pt/TiO2 opens a more efficient pathway to formaldehyde oxidation at ambient temperatures, Angew. Chem., Int. Ed., 2012, 51, 9628–9632 CrossRef CAS.
- R. Alvarez Roca, F. Guerrero, J. A. Eiras and J. D. S. Guerra, Structural and electrical properties of Li-doped TiO2 rutile ceramics, Ceram. Int., 2015, 41, 6281–6285 CrossRef CAS.
- E. Shin, S. Jin, J. Kim, S. J. Chang, B. H. Jun, K. W. Park and J. Hong, Preparation of K-doped TiO2 nanostructures by wet corresion and their sunlight-driven photocatalytic performance, Appl. Surf. Sci., 2016, 379, 33–38 CrossRef CAS.
- C. Zhang, Y. Li, Y. Wang and H. He, Sodium-promoted Pd/TiO2 for catalytic oxidation of formaldehyde at ambient temperature, Environ. Sci. Technol., 2014, 48, 5816–5822 CrossRef CAS.
- S. Shalini, N. Prabavathy, R. Balasundaraprabhu, T. Satish Kumar, P. Walke, S. Prasanna and D. Velayuthapillai, Effect of Na doping on structure, morphology and properties of hydrothermally grown one dimensional TiO2 nanorodstructures, J. Mater. Sci.: Mater. Electron., 2017, 28, 3500–3508 CrossRef CAS.
- C. D. Valentin and G. Pacchioni, Trend in non-metal doping of anatase TiO2: B, C, N and F, Catal. Today, 2013, 206, 12–18 Search PubMed.
- H. Xu, Z. Zheng, L. Zhang, H. Zhang and F. Deng, Hierarchical chlorine-doped rutile TiO2 spherical clusters of nanorods: Large-scale synthesis and high photocatalytic activity, J. Solid State Chem., 2008, 181, 2516–2522 CrossRef CAS.
- A. Miyoshi, K. Kato, T. Yokoi, J. J. Wiesfeld, K. Nakajima, A. Yamakara and A. Yamakara, Nano vs. bulk rutile TiO2:N,F in Z-scheme overall water splitting under visible light, J. Mater. Chem. A, 2020, 8, 11996–12002 RSC.
- D. Zhang and M. Yang, Band structure engineering of TiO2 nanowires by n-p codoping for enhanced visible-light photoelectrochemical water-splitting, Phys. Chem. Chem. Phys., 2013, 15, 18523–18529 RSC.
- H. Zhuang, Y. Zhang, Z. Chu, J. Long, X. An, H. Zhang, H. Lin, Z. Zhang and X. Wang, Synergy of metal and nonmetal dopants for visible-light photocatalysis: a case-study of Sn and N co-doped TiO2, Phys. Chem. Chem. Phys., 2016, 18, 9636–9644 RSC.
- S. Matsumoto, A. Ohtaki and K. Hori, Carbon fiber as an excellent support material for wastewater treatment biofilms, Environ. Sci. Technol., 2012, 46, 10175–10181 CAS.
- Y. Zhang, G. Duoerkun, Z. Shi, W. Cao, T. Liu, J. Liu, L. Zhang, M. Li and Z. Chen, Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater, J. Colloid Interface Sci., 2020, 571, 213–221 CrossRef CAS.
- W. Guo, C. Xu, X. Wang, S. Wang, C. Pan, C. Lin and Z. L. Wang, Rectangular bunched rutile TiO2 nanorod arrays grown on carbon fiber for dye-sensitized solar cells, J. Am. Chem. Soc., 2012, 134, 4437–4441 CrossRef CAS.
- Y. Yang, M. Qiu and L. Liu, TiO2 nanorod array@carbon cloth photocatalyst for CO2 reduction, Ceram. Int., 2016, 42, 15081–15086 CrossRef CAS.
- Z. Shi, Y. Zhang, T. Liu, W. Cao, L. Zhang, M. Li and Z. Chen, Synthesis of BiOBr/Ag3PO4 heterojunctions on carbon-fiber cloth as filter-membrane-shaped photocatalyst for treating the flowing antibiotic wastewater, J. Colloid Interface Sci., 2020, 575, 183–193 CrossRef CAS.
- N. Li, W.-Y. Xia, J. Wang, Z.-L. Liu, Q.-Y. Li, S.-Z. Chen, C.-W. Xu and X.-H. Lu, Manganese oxides supported on hydrogenated TiO2 nanowire array catalysts for the electrochemical oxygen evolution reaction in water electrolysis, J. Mater. Chem. A, 2015, 3, 21308–21313 RSC.
- B. Pitna Laskova, L. Kavan, M. Zukalova, K. Mocek and O. Frank, In situ Raman spectroelectrochemistry as a useful tool for detection of TiO2 (anatase) impurities in TiO2 (B) and TiO2 (rutile), Monatsh. Chem., 2016, 147, 951–959 CrossRef CAS.
- T. Mazza, E. Barborini, P. Piseri, P. Milani, D. Cattaneo, A. L. Bassi, C. E. Bottani and C. Ducati, Raman spectroscopy characterization of TiO2 rutile nanocrystals, Phys. Rev. B, 2007, 75, 045416 CrossRef.
- A. Li, Z. Wang, H. Yin, S. Wang, P. Yan, B. Huang, X. Wang, R. Li, X. Zong, H. Han and C. Li, Understanding the anatase-rutile phase junction in charge separation and transfer in a TiO2 electrode for photoelectrochemical water splitting, Chem. Sci., 2016, 7, 6076–6082 RSC.
- Z. Hao, Q. Chen, W. Dai, Y. Ren, Y. Zhou, J. Yang, S. Xie, Y. Shen, J. Wu, W. Chen and G. Q. Xu, Oxygen-Deficient Blue TiO2 for Ultrastable and Fast Lithium Storage, Adv. Energy Mater., 2020, 10, 1903107 CrossRef CAS.
- H. Dou, Y. Qin, F. Pan, D. Long, X. Rao, G. Q. Xu and Y. Zhang, Core–shell g-C3N4/Pt/TiO2 nanowires for simultaneous photocatalytic H2 evolution and RhB degradation under visible light irradiation, Catal. Sci. Technol., 2019, 9, 4898–4908 RSC.
- Y. Li, C. Zhang, H. He, J. Zhang and M. Chen, Influence of alkali metals on Pd/TiO2 catalysts for catalytic oxidation of formaldehyde at room temperature, Catal. Sci. Technol., 2016, 6, 2289–2295 RSC.
- R.-T. Guo, Q.-S. Wang, W.-G. Pan, W.-L. Zhen, Q.-L. Chen, H.-L. Ding, N.-Z. Yang and C.-Z. Lu, The poisoning effect of Na and K on Mn/TiO2 catalyst for selective catalytic reduction of NO with NH3: A comparative study, Appl. Surf. Sci., 2014, 317, 111–116 CrossRef CAS.
- P. Wang, Q.-S. Wang, X.-X. Ma, R.-T. Guo and W.-G. Pan, The influence of F and Cl on Mn/TiO2 catalyst for selective catalytic reduction of NO with NH3: A comparative study, Catal. Commun., 2015, 71, 84–87 CrossRef CAS.
- X.-K. Wang, C. Wang, W.-Q. Jiang, W.-L. Guo and J.-G. Wang, Sonochemical synthesis and characterization of Cl-doped TiO2 and its application in the photodegradation of phthalate ester under visible light irradiation, Chem. Eng. J., 2012, 189–190, 288–294 CrossRef CAS.
- A. Jiamprasertboon, M. J. Powell, S. C. Dixon, R. Quesada-Cabrera, A. M. Alotaibi, Y. Lu, A. Zhuang, S. Sathasivam, T. Siritanon, I. P. Parkin and C. J. Carmalt, Photocatalytic and electrically conductive transparent Cl-doped ZnO thin films via aerosol-assisted chemical vapour deposition, J. Mater. Chem. A, 2018, 6, 12682–12692 RSC.
- V. Etacheri, C. D. Valentin, J. Schneider, D. Bahnemann and S. C. Pillai, Visible-light activation of TiO2 photocatalysts: advances in theory and experiments, J. Photochem. Photobiol. C, 2015, 25, 1–29 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cy01918f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |