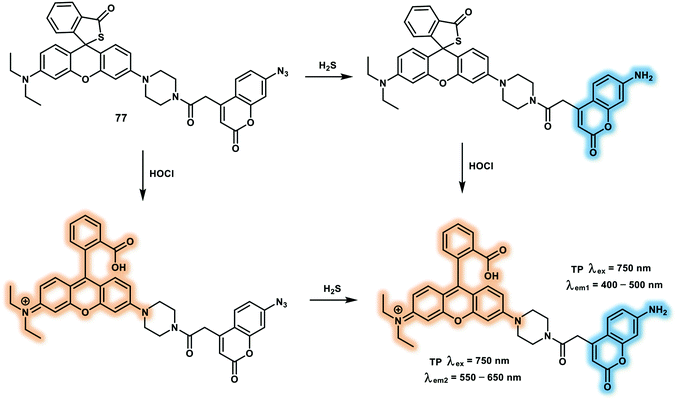
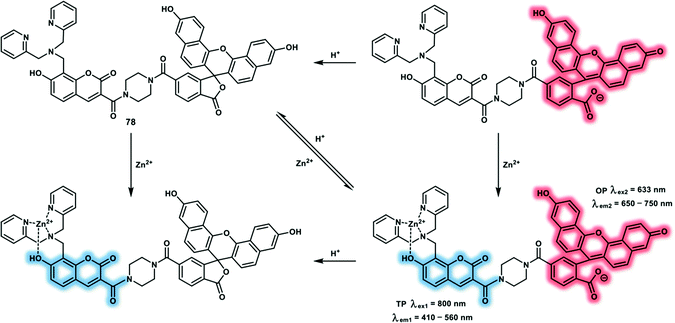
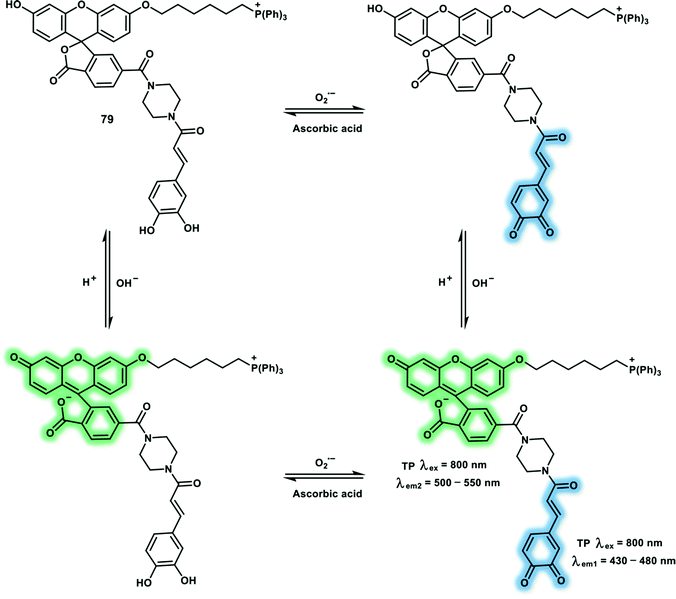
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two-photon small-molecule fluorescence-based agents for sensing, imaging, and therapy within biological systems
Luling
Wu†
 *ab,
Jihong
Liu†
ab,
Ping
Li
*ab,
Jihong
Liu†
ab,
Ping
Li
 *a,
Bo
Tang
*a,
Bo
Tang
 *a and
Tony D.
James
*a and
Tony D.
James
 *abc
*abc
aCollege of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Institutes of Biomedical Sciences, Shandong Normal University, Jinan, 250014, P. R. China. E-mail: lw960@bath.ac.uk; lip@sdnu.edu.cn; tangb@sdnu.edu.cn; t.d.james@bath.ac.uk
bDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK
cSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, P. R. China
First published on 21st January 2021
Abstract
In this tutorial review, we will explore recent advances for the design, construction and application of two-photon excited fluorescence (TPEF)-based small-molecule probes. The advantages of TPEF-based probes include deep tissue penetration and minimal photo-damage. We discuss the underlying two-photon (TP) fluorophores including hemicyanine and design strategies such as Förster resonance energy transfer (FRET). Moreover, we emphasize applications for the detection or imaging of cations, anions, small neutral molecules, biomacromolecules, cellular microenvironments, subcellular localization and dual-responsive systems. Examples of photodynamic therapy (PDT) using TP irradiation are also illustrated.
Key learning points(1) The review will provide a simple guide for the design principles involved in the construction of two-photon excitation-based sensors and imaging agents.(2) The review will focus on biological applications, including sensing and imaging in a cellular environment. The review should provide a foundation and guidelines enabling the reader to develop their own novel two-photon excitation-based sensing systems including dual-responsive probes. (3) In addition, we anticipate that the review will facilitate a deep understanding of two-photon excitation-based systems for photodynamic therapy. |
Introduction
Small-molecule probes are powerful tools to monitor specific cellular processes via fluorescence detection and/or imaging.1 These molecular probes change their fluorescence emission when they respond to a specific analyte or environment. They have been widely used in diverse fields such as cellular imaging, drug screening and medical therapy. Small-molecule probes-based detection methods have specific advantages including: measurable fluorescence emission with good sensitivity and selectivity, spatial and temporal resolution in cell and animal imaging, and readily accessible fluorophores.Combining one-photon (OP) probes and one-photon microscopy (OPM) is the most common tool for targeting various biomolecules and organelles. Common fluorophores including coumarin, BODIPY, fluorescein and rhodamine have been used for the construction of OP probes using appropriate design strategies.2 These fluorophores are usually excited by OPM using UV-visible excitation wavelengths (350–650 nm). Therefore, OP probes have restrictions for practical application. The main causes include: (1) short-wavelength excitation light that limits the penetration depth in biological samples; (2) photobleaching which makes the probe inappropriate for long-time imaging; (3) photo damage to biological samples; and (4) auto-fluorescence from biological species that interfere with the fluorescence signal. These problems of OPM can be overcome by two-photon excited fluorescence (TPEF) to different degrees. Notably, fluorophores exhibiting TPEF usually exhibit one-photon excited fluorescence (OPEF). The excitation spectra of most commonly used OP fluorophores is in the range of 400 to 500 nm, whereas the wavelengths used to excite the same fluorophore with two-photon microscopy (TPM) is between 800 and 1000 nm. As such TPM reduces photobleaching and increases penetration depth and provides higher spatial resolution than OPM.3,4
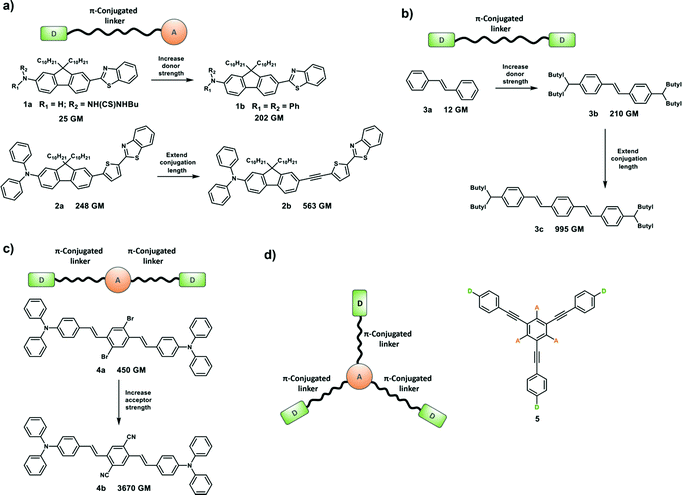
Two-photon absorption (TPA) was first proposed by Maria Göppert-Mayer as part of her doctoral dissertation in 1931,5 which was then experimentally confirmed by Kaiser and Garret in 1961 after the invention of high-energy pulsed lasers.6 However, organic molecules or materials with large two-photon absorption cross section (δ) values are required to facilitate practical applications of TPA, because high-power laser beams can damage the sample. Which means that molecules with large δ would allow TP excitation at a lower laser power, minimizing photodamage to the sample. The strength of TPA can be described using δ which is dependent on the molecular structure. δ is quoted in the units of Göppert-Mayer (GM), where 1 GM is 10−50 cm4 s per photon per molecule. TPA is a photophysical phenomenon by which a molecule or material absorbs two photons at the same time to reach an excited state through an intermediate virtual state (Scheme 1). TPA can be described as degenerate or nondegenerate depending on whether the two photons have the same or different frequencies (energies), respectively.
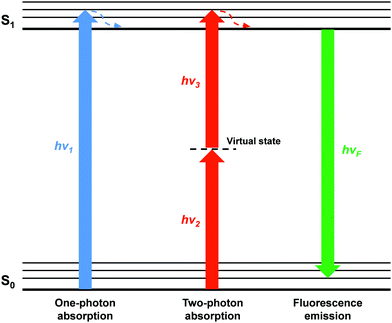 | ||
| Scheme 1 Energy diagram of one-photon and two-photon excitation. The fluorescence is emitted from the first electronic excited state regardless of the excitation mode. | ||
Optimizing δ is important in order to increase the optical sensitivity of fluorophores for TPM. Molecular δ at a given optical frequency (ω) is proportional to the imaginary part of the second hyperpolarizability, Im[γ(ω)].
| δ(ω) ∝ Im[γ(ω)] |
Design strategies for TP probes can be the same as those employed for OP probes.7 Generally, a TP probe is composed of three functional units: (1) TP fluorophore. Fluorescence emission intensity and wavelength of the TP probe will change when bound or having reacted with the target; (2) recognition receptor, which is designed to specifically bind or react with the target; and (3) linker, which connects the fluorophore and recognition receptor. Importantly, both TP probes and OP probes are fluorescent sensors that rely on the same types of sensing outputs including a change of intensity and/or emission wavelengths. Therefore, as with the development of OP probes, the construction of TP probes requires the assembly of appropriate receptors or recognition groups linked to a fluorophore. For example, oxygen-rich receptors are often used for sensing Mg2+ and Ca2+ while nitrogen-containing receptors such as the pyridine moiety are used for Zn2+ (e.g. probe 27) and Cd2+. Similarly the boronic ester group can be used to report peroxynitrite (ONOO−) or hydrogen peroxide (H2O2) (e.g. probe 30).
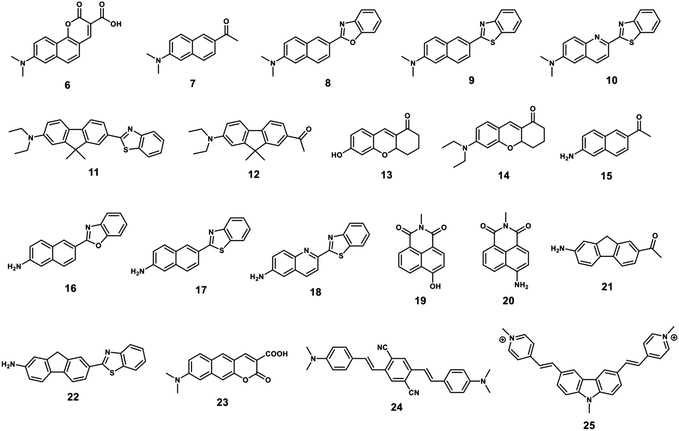
A TP fluorophore for biological applications requires the following properties optimised for biological usage: (1) good water solubility, which can be improved by introducing water-solubilizing groups; (2) a large δ × Φf to generate bright TPM images, which can be improved by modifying the donor–acceptor strength or length of the intramolecular conjugation; (3) suitable photo-stability for the system being monitored, longer-duration imaging, can be facilitated by introducing rigid conjugated cyclic linkers. Compounds 6–14 with optimized structures meet these criteria and are suitable for the construction of practical TP probes (Fig. 1). In addition, compounds 15–25 have desirable photo-physical properties (e.g. large δ × Φf value) and have functional groups suitable for appropriate modification in order to generate suitable TP probes (Fig. 1).
Photodynamic therapy (PDT) is a method of treating cancer based on a photochemical reaction involving a photosensitizer, light irradiation and molecular oxygen to generate cytotoxic species. The formation of singlet oxygen during type II photochemical reactions is the main cause of the observed cell damage. In particular the use of longer wavelength excitation light (i.e. 800–1100 nm) in TP PDT is very important in order to improve both tissue penetration depth and reduce damage to surrounding healthy tissue when compared to OP PDT using UV or visible light excitation.
This review will summarize recent progress in the development of TP small-molecule probes. The goal is to provide an insight into the importance of this continually expanding area. Specifically, this review will describe the design principles required for the construction of TP small-molecule probes and provide guidance on function. Characteristic TP fluorophores published in the literature and their utility will be described. Reaction mechanisms are an important factor in the design of fluorescence-based sensors and imaging agents. Fluorescence mechanisms including ICT,8 Förster resonance energy transfer (FRET)9 and excited-state intramolecular proton-transfer (ESIPT)10 will be discussed. The relationship between the receptor and the change in photophysical properties will be illustrated when a TP probe interacts with analyte(s). Additionally, the potential biological applications of TP small-molecule probes will be highlighted. Finally, the future research directions and opportunities for the development of TP probes will be outlined. Since, this treatise is not a comprehensive review. We must apologize to some groups whose work has not been included due to restrictions in the number of references.
1. Two-photon (TP) excitation-based fluorescent sensors for cations
As structural or catalytic required cofactors, cellular metal ions play important roles in regulating cell function and participate in many cellular processes. Metal ion homeostasis can be controlled by several families of proteins including diffusible cytoplasmic metallochaperone proteins and integral transmembrane transporters, while several neurodegenerative diseases are related to disruption of metal ions balance. As such it is vital to elucidate the biological function of metal ions in cells and living organisms, and to develop efficient TP probes for the detection and imaging of cellular metal ions in vivo. A large number of small-molecule fluorescent probes for metal ions have been developed using TP fluorophores which have significant δ and selective metal ion receptors. A variety of TP probes for the detection of metal ions have been constructed based on mechanisms which are widely used for the development of OP probes, such as ICT, through-bond energy transfer (TBET) and photoinduced electron transfer (PeT). Targeted metal ions trigger a turn-on TPEF emission or wavelength shift (hypsochromic or bathochromic shift) of TPEF spectra, which facilitates bio-imaging using TPM. This section will include some representative TP probes for biologically important cations (ferrous iron, zinc ions, and palladium ion), including working mechanism and biological applications.Labile heme (LH), a tetrapyrrole macrocycle containing a central ferrous iron (Fe2+) with a metal-chelate structure, acts as cofactors for many cellular proteins that control metabolic functions. As a protein prosthetic group, heme plays an important role in gene regulation, hormone metabolism and cell growth. For instance, tumorigenesis is associated with high expression levels of heme. Probe 26 is the first example of a LH-responsive small-molecule based fluorescent probe, that was constructed by conjugating the 4-amino-1,8-naphthalimide fluorophore and the endoperoxide bridge through a carbamate linker (Scheme 3).11 The design strategy was inspired by the bioactivation mechanism of artemisinin. In the presence of LH (2 μM), the absorption peak of 26 in a PBS/DMSO solution at 375 nm shifted to 440 nm, concomitant with a 13-fold fluorescence enhancement at 540 nm. Moreover, 26 displayed high sensitivity for the detection of LH, without interference from hemin, hemoproteins and zinc protoporphyrin. With the excellent fluorescent properties of 26, the levels of endogenous LH in HEK293 cells were monitored. Subsequently, 26 was used to monitor LH fluctuations during the hemolysis process, TP confocal microscopy images of mice liver revealed that phenylhydrazine-induced hemolysis of mice displays up-regulated LH levels between 500–600 nm with TP excitation at 780 nm, indicating the ability of 26 to monitor hemolysis.
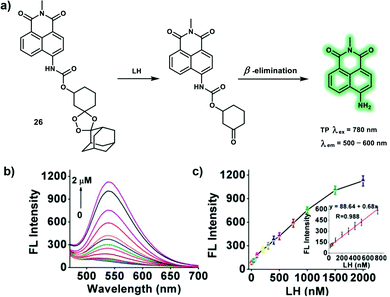 | ||
Scheme 3 (a) Proposed mechanism of TP probe 26 in response to LH. (b) Fluorescence emission spectra of 26 (2 μM) with different concentrations of LH (0–2 μM) in aqueous solution (PBS/DMSO = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 10 mM, pH 7.4). (c) Fluorescence intensity at 540 nm vs. LH concentrations. Inset: linear plot of the fluorescence intensity versus LH concentrations (20–750 nM). Reproduced with permission from ref. 11. Copyright (2020) American Chemical Society. 1, v/v, 10 mM, pH 7.4). (c) Fluorescence intensity at 540 nm vs. LH concentrations. Inset: linear plot of the fluorescence intensity versus LH concentrations (20–750 nM). Reproduced with permission from ref. 11. Copyright (2020) American Chemical Society. | ||
As a trace element in our body, zinc ions (Zn2+) play an important role in gene transcription, cell proliferation, protein metabolism, regulate immunity and anti-oxidation. Recent research has been directed towards the elucidation of the importance of biological Zn2+. Vesicular Zn2+ was found to be involved in neurotransmission functions of the brain. While Zn2+ concentrations in living systems have significant impact on function and cellular zinc homeostasis can be controlled by Zrt-Irt-like proteins (ZIP) and zinc transporters (ZnT). Hence, monitoring changes of Zn2+ levels is of great importance for the study Zn2+-related diseases, such as Alzheimer's disease, Parkinson's disease, and prostate cancer. A novel TP probe 27 for the detection of lysosomal Zn2+ was designed by conjugating a morpholine group and N,N-di-(2-picolyl)ethylenediamine (DPEN) ligand to a naphthalimide dye (Scheme 4).12 Upon addition of Zn2+ (0–50 μM), the PeT processes were suppressed, resulting in a remarkable fluorescence enhancement of 27 at pH = 5.0. Probe 27 displayed a negligible fluorescence at pH 7.4 even after binding with Zn2+, indicating that the probe was capable of specifically recognizing lysosomal Zn2+ rather than cytosolic Zn2+. Probe 27 displays strong binding affinity with an association constant (Ka) of 1.17 × 105 M−1. Intracellular Zn2+ in lysosomes was detected by TPM under excitation at 900 nm in NIH 3T3 cells. Moreover, probe 27 could be an effective tool to image endogenous Zn2+ in the deep tissues of a mouse brain via TP microscopic imaging.
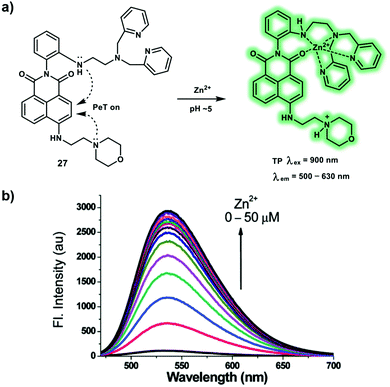 | ||
| Scheme 4 (a) Detection of zinc ions under acidic conditions with TP probe 27, through the blocking of two PeT processes. (b) Fluorescence titration of probe 27 (10 μM) with Zn(ClO4)2 in a pH 5.0 MES buffer containing 1% EtOH. Reproduced with permission from ref. 12. Copyright (2016) The Royal Society of Chemistry. | ||
Probe 28 is a ratiometric TP probe for Zn2+ based on ICT. Probe 28 was constructed by conjugating the 2,2′-dipyridyl group (electron acceptor) and benzene group (electron donor) with alkene linker, which behaves as D–π–A–π–D (Scheme 5).13 Increased conjugation length of the alkene group and stronger electron donating ability of the benzene group facilitated probe 28 to achieve a high δ with a value of 516 ± 77 GM at 700 nm excitation, which increased to 958 ± 144 GM after coordination of probe 28 with Zn2+. In addition, probe 28 displayed an enhanced Φf with values from 0.59 to 0.71 in the absence and presence of Zn2+, respectively. The apparent dissociation constant for probe 28 was calculated to be 3.66 ± 0.42 μM in MeOH/buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) solution. TP fluorescence emission spectra indicated that the addition of Zn2+ (80 μM) induced a red-shift of emission from 465 nm to 550 nm upon excitation at 800 nm. The experimental results indicated that probe 28 behaved as a ratiometric TP fluorescent probe for the determination of Zn2+. The enhanced fluorescence emission ratio Ired/Igreen (red channel: 495–585 nm; green channel: 460–495 nm) was dose-dependent towards probe 28 (between 0 and 25 μM) in live SHSY-5Y cells, which was observed via TP confocal fluorescence imaging. In addition, probe 28 was used to monitor increased Zn2+ levels in mouse brains with Alzheimer's disease and 3 day-old zebrafish, confirming that it could be used as a robust TP probe to map endogenous levels of Zn2+.
19) solution. TP fluorescence emission spectra indicated that the addition of Zn2+ (80 μM) induced a red-shift of emission from 465 nm to 550 nm upon excitation at 800 nm. The experimental results indicated that probe 28 behaved as a ratiometric TP fluorescent probe for the determination of Zn2+. The enhanced fluorescence emission ratio Ired/Igreen (red channel: 495–585 nm; green channel: 460–495 nm) was dose-dependent towards probe 28 (between 0 and 25 μM) in live SHSY-5Y cells, which was observed via TP confocal fluorescence imaging. In addition, probe 28 was used to monitor increased Zn2+ levels in mouse brains with Alzheimer's disease and 3 day-old zebrafish, confirming that it could be used as a robust TP probe to map endogenous levels of Zn2+.
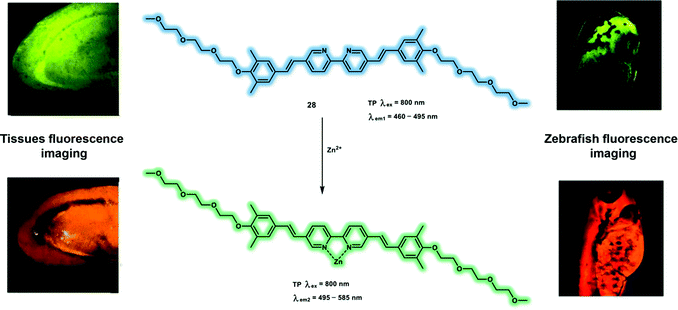 | ||
| Scheme 5 TP response of probe 28 for imaging Zn2+ in brain tissue and zebrafish. Reproduced with permission from ref. 13. Copyright (2017) American Chemical Society. | ||
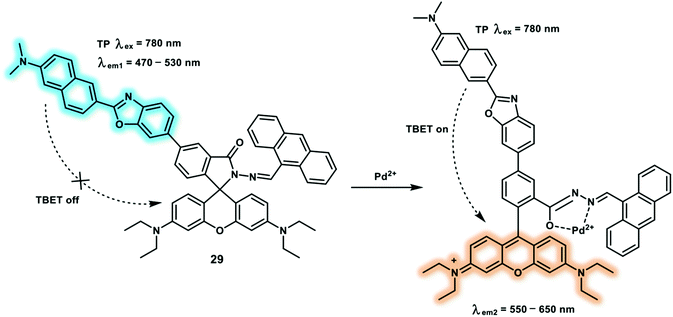
As a member of platinum group elements, palladium has been largely used in aerospace, automobile manufacturing and catalytic industry. Palladium can disturb cellular functions which is attributed to the formation of palladium complexes between palladium ions (Pd2+) and DNA, RNA, proteins and biomacromolecules. As a result of palladium toxicity, governments strictly restrict the residual palladium levels in pharmaceutical products (5–10 ppm) to avoid health risks. A naphthalene-rhodamine based TP fluorescent probe 29 has been reported for the ratiometric detection of Pd2+ (Scheme 6).14 The TP fluorophore naphthalene derivative was selected as the energy donor and rhodamine spirolactam was chosen as the energy acceptor to develop a TBET platform. This platform exhibited two well-separated fluorescence emission maxima (wavelength difference of 100 nm) and excellent energy transfer efficiency (90%). When binding Pd2+, the spirolactam of the rhodamine B opened and resulted in enhanced fluorescence at 595 nm and concomitant fluorescence decrease at 495 nm. Using this ratiometric probe, it was possible to image Pd2+ in HeLa cells and nude mice liver tissue via dual-channel mode (green channel was collected between 470–530 nm and the red channel was collected between 550–650 nm). The images collected from the two fluorescence channels using TPM indicated that probe 29 could be used to selectively assess changes of Pd2+ in living cells and mice liver tissue (90–270 μm) with TP excitation.
2. Two-photon (TP) excitation-based fluorescent sensors for anions
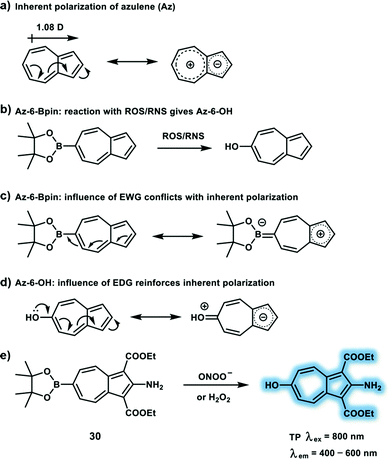
Owing to the important role of anions in physiological and pathological processes, significant attention has been paid to the development of TP fluorescent probes for tracking endogenous anions in vivo, such as ONOO−, hypochlorite (ClO−/HOCl) and superoxide (O2˙−). However, because of the high reactivity, short lifetime and transient conversion characteristics of some anions, real-time monitoring of anion fluctuations using anion-sensitive TP probes remain challenging. Recently, a number of reaction-based small-molecule fluorescent TP probes for anions were reported, which include oxidative reactions, reductive and nucleophilic reactions. In this section, we will summarize representative TP excitation-based fluorescent sensors for several representative reactive nitrogen species (RNS), reactive oxygen species (ROS) and reactive sulfur species (RSS), including recognition, and response mechanisms.As a RNS, ONOO− is generated from the diffusion limited combination of O2˙− and nitric oxide (NO) at a rate of 1.9 × 1010 M−1 s−1. ONOO− and its derived radicals (hydroxyl radical (˙OH), nitrogen dioxide (˙NO2), and carbonate radical (CO3˙−)) can oxidize and nitrate many biomolecules, including DNA, thiols, protein tyrosine residues, lipids and nucleic acids. Tyrosine nitration caused by ONOO− acts as a mediator in cell apoptosis and death pathways. ONOO− overproduction has been implicated in many pathological processes including inflammation, neurodegenerative disease, ischemia-reperfusion injury and Alzheimer's disease.15 A large number of TP probes for ONOO− containing highly reactive groups such as boronic acid, α-ketoamide, phenol and selenides have been developed based on the high oxidative nature and nucleophilicity of ONOO−. Probe 30 (Scheme 7e) was developed as a turn-on TP probe for ONOO− and H2O2, using an azulene fluorophore and boronate ester (responsive group) (Scheme 7b).16 The electron-withdrawing nature of the boronic ester leads to a decrease in the inherent polarization of azulene, which causes a reduction in the ICT character of azulene (Scheme 7a and c). However, after oxidation by ONOO−/H2O2, the as generated hydroxyl group reinforces the inherent polarization of the fluorophore and increases the ICT character (Scheme 7a and d). Probe 30 displays a turn-on response at 483 nm after oxidation in a mixed aqueous buffer/methanol system and exhibits high selectivity for ONOO− over other ROS including HOCl, ˙OH, and O2˙−; high photostability; and low cytotoxicity. The detection limit of probe 30 for ONOO− was calculated to be 21.7 nM, and the largest value of δ × Φf of the product (30 + ONOO−) was calculated to be 3.2 GM at 810 nm excitation. TPM images of probe 30 in phorbol myristate acetate (PMA) or lipopolysaccharide/gamma interferon (LPS/IFN-γ) induced RAW264.7 macrophages revealed that endogenously elevated ROS/RNS levels could be monitored by probe 30 in biological systems. Finally, 30 was further used for TPEF imaging of H2O2 and ONOO− in rat hippocampus slices recorded by TPM at 800 nm excitation wavelength. While, using PMA or SIN-1 (source of exogenous ONOO−) pretreated slices exhibited about 2-fold fluorescence increase compared to control slices.
To achieve in vivo ratiometric imaging of ONOO−, a novel FRET-based TP probe 32 for the ratiometric detection of ONOO− has been developed.17 Probe 32 consists of a coumarin derivative as a TP fluorophore and compound 31 as the recognition group for ONOO−via a FRET strategy (Scheme 8). Probe 32 displays a 93-fold fluorescence ratio (I473nm/I651nm) increase in the presence of ONOO− (7.5 μM). The detection limit was calculated to be 11.3 nM. In addition, probe 32 exhibited excellent selectivity for ONOO− over other biological ROS and RNS, and in particular could avoid interference by H2O2 (0–100.0 equiv.). The red fluorescence channel of probe 32 merged well with the green fluorescence channel of Mito-Tracker Green, with an overlap coefficient of 0.80, which indicated that probe 32 could be used to detect mitochondrial ONOO− at subcellular levels. Using dual-channel TPEF imaging, a dramatic enhancement of the fluorescence signal ratio (Iblue/Ired) in LPS/IFN-γ pretreated HepG2 cells was observed. An obvious ratiometric signal (Iblue/Ired) enhancement was observed in LPS-induced inflamed live mice using TP confocal microscopy upon excitation at 800 nm.
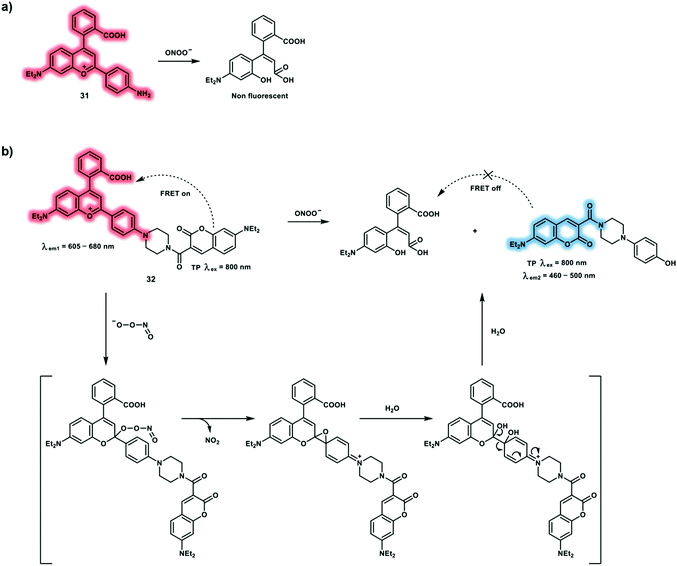 | ||
| Scheme 8 (a) Reaction between compound 31 and ONOO−. (b) FRET-based TP probe 32 and its proposed reaction mechanism for the ratiometric detection of ONOO−. The non-fluorescent products in Fig. 7 of ref. 9 (Chem. Soc. Rev., 2020, 49, 5110–5139) were wrong, as such we have corrected them here in Scheme 8. | ||
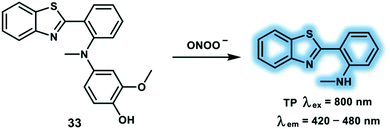
Inspired by the fluorescence mechanism of 2-(2′-hydroxyphenyl)benzothiazole via an ESIPT process, Hu et al. have developed a turn-on TP probe 33 for the detection of ONOO− (Scheme 9).18 The ESIPT effect was blocked by substituting the hydroxyl group with N-methyl-p-hydroxyaniline. In the presence of ONOO−, the phenol group of N-methyl-p-hydroxyaniline could be oxidized, and the N–C bond was cleaved with proton donor (N–H) formation, restoring the ESIPT process. A dramatic 600-fold fluorescence intensity enhancement at 470 nm was observed upon addition of ONOO− (2 equiv.) to probe 33 in PBS (10 mM, pH 7.4). The in vitro experiments demonstrated that probe 33 exhibited high sensitivity and selectivity towards ONOO−; good photostability; excellent pH stability; good blood–brain barrier penetration and low cytotoxicity. A linear relationship between the fluorescence intensity and ONOO− (0–10 μM) was observed. The maximum δ of probe 33 towards ONOO− was calculated to be 3.6 GM using excitation at 820 nm. Using probe 33 in conjunction with TPM, facilitated the measurement of the dynamic elevation of ONOO− during ischemia progression in rose bengal-treated or laser irradiation-induced live mouse brain using a TP excitation wavelength of 800 nm.
As an effective antimicrobial oxidant of neutrophils, hypochlorous acid (HOCl) is mainly produced by myeloperoxidase catalyzed peroxidation of H2O2 and chloride ion (Cl−) in phagolysosomes. HOCl is of particular importance for antimicrobial action in the innate immune system and can oxidatively modify various protein functional groups (sulfhydryl and amino groups) to generate chloramine and aldehyde products, and improve immunity. It is reported that excess HOCl is associated with many diseases, such as rheumatoid arthritis, Parkinson's disease, Alzheimer's disease and cancers. Hence, it is necessary to develop HOCl-responsive probes in order to understand the biological effects. Many fluorescent probes have been reported for the detection of HOCl, which exploit its oxidizing capability towards recognition units such as p-methoxyphenol, dibenzoylhydrazine, oxime, thiol and selenide. Since, TPM facilitates higher spatial resolution and greater tissue penetration, numerous TP probes allowing TPEF imaging of HOCl in live systems have been developed. The reaction mechanism of 36 towards HOCl is shown in Scheme 10b. Probe 36 is oxidized by HOCl to form sulfoxide 37 which undergoes further oxidization to form an unstable sulfone which is hydrolyzed to generate 38. Finally, 38 is partially chlorinated by excess HOCl to produce 39. Probes 34 and 35 were developed for the detection of mitochondrial and lysosomal HOCl, respectively (Scheme 10a).19 Triphenylphosphine and morpholine were used as subcellular targeting groups for 34 and 35, respectively. Both 34 and 35 used acedan as the TP fluorophore and oxathiolane as reaction site towards HOCl. Deprotection of the oxathiolane to a ketone led to a strong “push–pull” system, as well as simultaneous fluorescence enhancement. The subcellular targeting abilities of probes 34 and 35 were evaluated in HeLa and LPS/IFN-γ-stimulated macrophage cells using Mito-Tracker Red and Lyso-Tracker Red, respectively. The application of the probes for tracking the in situ production of HOCl in the LPS-induced inflamed tissue of mice paws using TPEF imaging, indicated that the overproduction of HOCl could be detected under inflammation conditions.
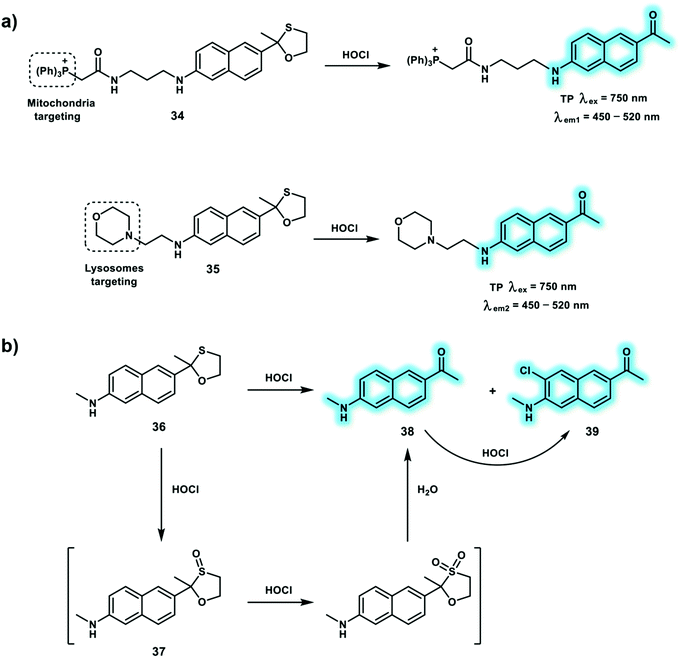 | ||
| Scheme 10 (a) TP probes 34 and 35 for the detection of HOCl in mitochondria and lysosomes, respectively. (b) Proposed sensing mechanism for HOCl by 36. | ||
The ratiometric TP probe 40 was developed for the detection of HOCl using the same recognition receptors as probes 34 and 35, using a different TP fluorophore (quinolone) (Scheme 11).20 Oxidation and deprotection of the oxathiolane group by HOCl led to an enhanced ICT effect, with a red-shifted fluorescence. Moreover, the reaction product with a typical “push–pull” structure facilitated TP excitation, enabling the detection of HOCl fluctuations by TPM. The fluorescence response of probe 40 towards HOCl was investigated in 10 mM PBS (pH = 7.4, containing 5% DMF) buffer solution. The addition of HOCl resulted in a decrease of the emission at 492 nm and concomitant increase in emission at 562 nm. The reaction mechanism between probe 40 and HOCl was confirmed using mass spectroscopic analysis. Addition of 25 μM HOCl caused a maximal fluorescence ratio (I562nm/I492nm) value of 4.0. While, the maximum δ × Φf value of 40 was determined to be 25 GM. The detection limit of probe 40 for HOCl was calculated to be 89 nM. In addition, detection of endogenous RAW264.7 cells using TP excitation at 820 nm. More importantly, overproduced HOCl during wound healing was monitored using probe 40 and ratiometric TPEF imaging.
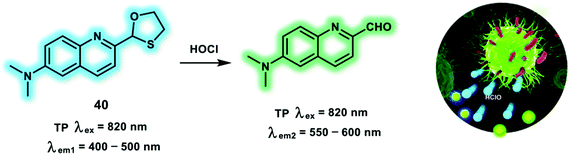 | ||
| Scheme 11 Quinolone-based TP probe 40 for the ratiometric detection of HClO. Reproduced with permission from ref. 20. Copyright (2018) The Royal Society of Chemistry. | ||
Yoon et al. have developed a TP probe 41 for the detection of HOCl based on B–H bond oxidation and it was used to visualize exogenous and endogenous HOCl in living systems by TPM.21 The pyrene of probe 41 serves as a TP fluorophore while the N-heterocyclic carbene (NHC) boranes were used as specific responsive groups towards HOCl (Scheme 12). A low polarity environment promoted probe 41 to form colloidal aggregates and emit pyrene excimer fluorescence. Then HOCl triggered an oxidative hydrolysis reaction of the B–H bond, leading to imidazolium salt formation, deaggregation and monomer fluorescence. Treatment of probe 41 with HOCl resulted in a decrease of emission intensity at 477 nm, accompanied by an increase in emission intensity at 374 nm. In addition, probe 41 exhibited excellent sensitivity and selectivity with a detection limit of 3 μM towards HOCl over other ROS and RNS such as ONOO− and H2O2. These results prompted the use of probe 41 to visualize HOCl using TPM. Using NaOCl or LPS/IFN-γ/PMA in RAW264.7 cells resulted in a reduced fluorescence ratio (Igreen/Iblue) under 710 nm excitation, demonstrating the possibility to monitor exogenous and endogenous HOCl fluctuations in live cells. Similar reduced fluorescence emission ratios (Igreen/Iblue) were observed in PMA-stimulated rat hippocampal slices using TPM.
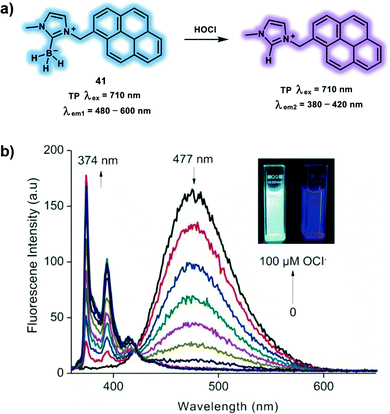 | ||
| Scheme 12 Pyrene-based TP probe 41 for the ratiometric detection of HOCl. (b) Fluorescence titration of 41 (10 μM) with NaOCl (0–100 μM) in PBS (10 mM, pH 7.4) after 1 min at room temperature, with 350 nm excitation. Inset: Photographs of 41 before (left) and after (right) the addition 10 equiv. of NaOCl under UV irradiation (365 nm). Reproduced with permission from ref. 21. Copyright (2018) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
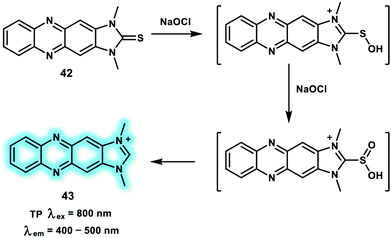
Hypochlorite anion (ClO−) equilibrates with HOCl (pKa of 7.46) under physiological conditions at micromolar concentrations. However, abnormal levels of ClO− are related to various disorders and diseases. In biological systems, ClO− is mainly produced by the myeloperoxidase catalyzed reaction of Cl− + H2O2 → HOCl. Inspired by good water solubility and stable photophysical properties of imidazolium salts, the Yoon group developed a turn-on TP probe 42 derived from imidazoline-2-thiones for the detection of ClO− (Scheme 13).22 Probe 42 was non-fluorescent in PBS solution, then upon the addition of ClO− (0–10 μM), a new emission peak at around 505 nm emerged due to the formation of an imidazolium salt. The detection limit of probe 42 for ClO− was calculated to be 0.071 μM. TP photophysical properties of probe 42 indicated maximum δ × Φf values of probe 42 and product 43 in ethanol at 800 nm excitation to be 0.4 and 8.4 GM, respectively. Similarly, 43 exhibited a higher Φf than probe 42. RAW264.7 cells stained with probe 42 and pre-stimulated with exogenous NaOCl (200 μM) displayed an over 3-fold fluorescence increase compared to the control group under TP excitation at 800 nm, Upon addition of the stimulants (LPS, IFN-γ and PMA), similar enhanced fluorescence signals were observed. Significantly, using probe 42, elevated ClO− levels in PMA stimulated rat hippocampal slice could be monitored at a depth of up to 100 μm through TPEF imaging.
Sulfur dioxide (SO2) can be regarded as a common environmental pollutant, usually equilibrating with sulfite (SO32−) and bisulfite (HSO3−) in living systems. Due to its excellent preservative and antimicrobial properties, SO32− has been widely used in foods, beverages and medicines. Exposure to SO32− mainly through consuming food containing additives and contact in the workplace may induce a variety of adverse effect, including allergic skin reactions (dermatitis, urticaria) and respiratory symptoms (asthma, rhinitis). SO32− cytotoxicity has been reported to be mediated by enhanced ROS formation, which can lead to lipid peroxidation, DNA and protein damage. Excessive SO2 inhalation is connected with neurological disorders, for instance, stroke, migraine headaches, and brain cancer. Hence, there is an urgent need to develop effective imaging tools for monitoring sulfite (SO32−) and bisulfite (HSO3−) levels in living systems. As such a near-infrared (NIR) TP probe 44, which is capable of the ratiometric detection of lysosomal bisulfite species, was developed (Scheme 14).23 The one-step condensation reaction between 8-hydroxyjulolidine and o-hydroxynaphthaldehyde formed benzopyronin derivative 44, where the morpholine group served as a lysosome-targeting group. Then bisulfite can trigger a 1,6-conjugate addition to benzopyronin dye, shortening the conjugated system, leading to a blue shifted fluorescence emission from 704 nm to 512 nm. The HSO3− reaction mechanism was confirmed using 1H NMR and 1H–13C heteronuclear single quantum coherence (HSQC) NMR analysis. Emission intensity ratios at 512 and 704 nm (I512nm/I704nm) are linear with bisulfite concentration ranges from 0 to 50 μM with a detection limit of 0.09 μM. Therefore, probe 44 was able to quantify bisulfite levels ratiometrically. The δ values of probe 44 and probe 44 + HSO3− were determined to be 37 and 23 GM, respectively, under TP excitation at 750 nm. The incubation of living HeLa cells with probe 44 and NaHSO3 displayed an increased green fluorescence signal (500–550 nm) together with a decreased red fluorescence signal (625–665 nm) under TP excitation at 750 nm, which demonstrated that probe 44 could be used for real-time monitoring of intracellular bisulfite changes using TPM.
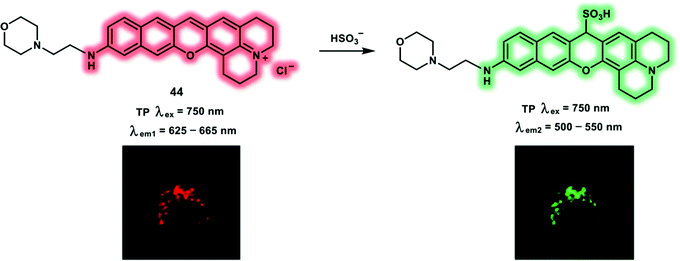 | ||
| Scheme 14 Benzopyronin-based TP probe 44 for the ratiometric detection of lysosomal HSO3−. Reproduced with permission from ref. 23. Copyright (2019) American Chemical Society. | ||
The most important primary ROS produced in living systems is O2˙− which plays an important role in maintaining the cellular redox homeostasis. As the precursor of most ROS/RNS (H2O2, ˙OH, ONOO−), O2˙− is generated by one-electron reduction of oxygen, which occurs mainly in the mitochondrial electron transport chain. However, excessive intracellular levels of O2˙− contribute to ischemia reperfusion, degenerative disorders and many other oxidative-stress-induced diseases. In order to precisely understand the biological roles of O2˙−, well-established fluorescence imaging approaches have been used to detect native O2˙− levels in living cells and in vivo. These probes are based on either the redox reaction or non-redox mechanism, for instance, protection/deprotection strategy and nucleophilic reactions, allowing selective recognition of O2˙− in systems where multiple ROS co-exist. Illuminated by previous work using the phosphinate group which is susceptible to highly nucleophilic O2˙− and ONOO−, a novel turn-on phosphinothioate-based TP probe 45 was developed for sensing O2˙− (Scheme 15).24 With this research the phosphinothioate moiety was used as a specific reactive site towards O2˙− for the first time. Notably, probe 45 was easy to synthesize through one-step reaction and was particularly sensitive and selective towards O2˙−. The emission intensity at 470 nm of probe 45 exhibited a linear relationship towards O2˙− concentrations (0–20 equiv.) due to the formation of fluorescent compound 46. The response of probe 45 towards O2˙− was investigated by evaluating the kinetics and resulted in the determination of a pseudo-first-order rate constant (kobs) of 1.0 × 10−2 min−1. A 2-fold TPEF intensity enhancement was observed in PMA-induced RAW264.7 macrophages confirming the feasibility of using probe 45 to detect endogenous O2˙− levels in living cells. Finally, probe 45 was used to image endogenous O2˙− in fresh rat hippocampal tissue with a TP excitation at 740 nm. Similar to the fluorescence behavior in cells, significant fluorescence enhancement was observed in rat hippocampal slices under PMA-stimulation.
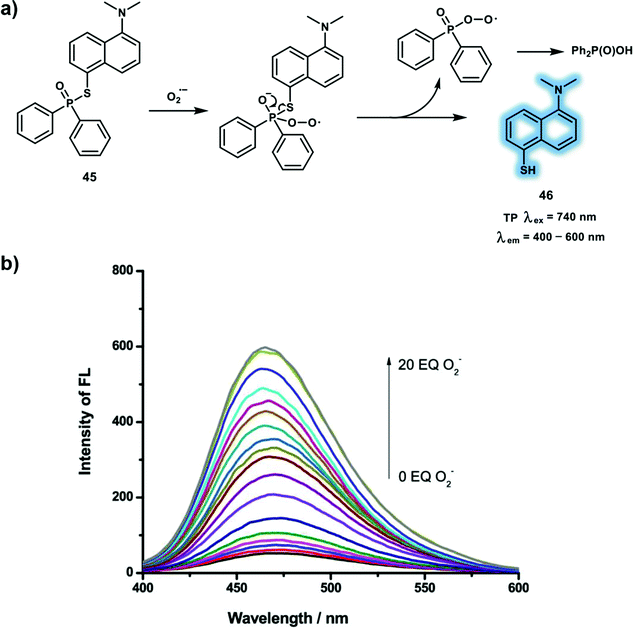 | ||
Scheme 15 (a) Sensing mechanism of phosphinothioate-based TP probe 45 for the detection of O2˙−. (b) Fluorescence spectra of probe 45 in the mixture of PBS/DMF (v/v = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was obtained upon additions of O2˙− (0–200 μM); λex = 345 nm; slit, 5/5 nm. Reproduced with permission from ref. 24. Copyright (2019) American Chemical Society. 3) was obtained upon additions of O2˙− (0–200 μM); λex = 345 nm; slit, 5/5 nm. Reproduced with permission from ref. 24. Copyright (2019) American Chemical Society. | ||
3. Two-photon (TP) excitation-based fluorescent sensors for small neutral molecules
Owing to pivotal role neutral molecules play in maintaining normal biological function and regulating multiple critical biological processes, it is essential to monitor neutral molecules in living systems. To date, many TP probes have been developed for neutral molecules in complicated biological systems, exhibiting high sensitivity and the selective detection of neutral molecules in vitro and in vivo. In this section, some representative TP probes for biologically important small neutral molecules will be summarized, and the working mechanisms and biological applications of the TP probes will be discussed.Nitroxyl (HNO), is a one electron reduced form of nitric oxide (NO), that is involved in many important biological functions. It has been demonstrated that HNO has a cardioprotective role in acute heart failure. In addition, HNO serves as a signaling molecule that promotes vasorelaxation and suppresses vascular smooth muscle cell proliferation. The pKa value of HNO is 11.4, and therefore HNO predominates over the nitroxyl anion (NO−) at physiological pH. Due to its high reactivity and facile dimerization and dehydration to nitrous oxide (N2O), Angeli's salt (AS, a source of HNO) have been widely used to explore HNO in vivo. A turn-on probe 47 has been developed based on the TP fluorophore 6-hydroxyl-quinoline-2-benzothiazole for the detection of HNO (Scheme 16).25 With probe 47, 2-(diphenylphosphino)benzoate was used as the reactive site towards HNO. In the presence of HNO, probe 47 undergoes an aza-ylide reaction by forming 48, which then undergoes Staudinger ligation to generate 49 (Scheme 16a), resulting in a fluorescence enhancement at 550 nm in PBS buffer solution (Scheme 16b and c). Probe 47 exhibited advantageous properties including high sensitivity (detection limit of 0.19 μM) and rapid response (20 min). Subsequently, the probe was used to track exogenous and endogenous HNO levels using TPM. Using living RAW264.7 cells with probe 47 an obvious increased green fluorescence signal upon TP excitation at 730 nm after incubation with AS was observed. In addition endogenous HNO was monitored in living HeLa cells upon addition of L-ascorbate and sodium nitroprusside (a source of NO), which forms HNO in biological media. These results indicated that probe 47 can detect fluctuations of HNO in living cells using TPM.
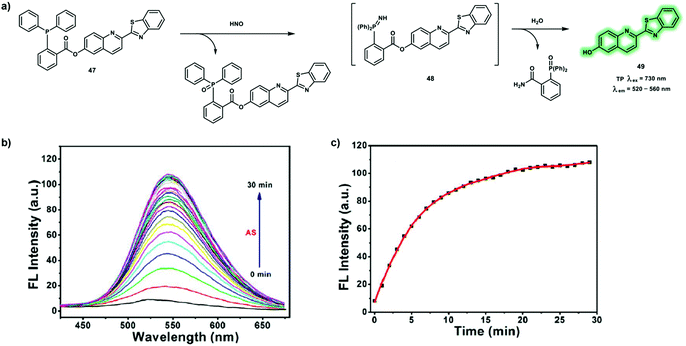 | ||
| Scheme 16 (a) Sensing mechanism of TP probe 47 for the detection of HNO. (b and c) Response time of probe 47 toward HNO. λex = 363 nm, slit: 5/5 nm. Reproduced with permission from ref. 25. Copyright (2018) American Chemical Society. | ||
NO is a gaseous biological signaling molecule, which is produced from NO synthase by catalyzing the reaction of L-arginine, molecular oxygen and reduced nicotinamide-adenine-dinucleotide phosphate (NADPH). It was found that NO acts as a key signaling molecule for the regulation of many physiological and pathological processes. In particular, NO could function as a neurotransmitter, which acts on adjacent neurons by diffusion in the central nervous system. In addition, the cardiovascular protective ability of NO has been demonstrated. A dysfunction in NO homeostasis has been implicated in many disorders and diseases, such as Alzheimer's disease, pulmonary fibrosis, ischemia-reperfusion injury, and cancer. To understand the biological role of NO, numerous fluorescent probes derived from o-phenylenediamine and transition-metal have been developed. Due to the extended π-conjugated system and easily polarized electronic structure Nile Red, a far-red emissive TP probe 50 has been developed for NO (Scheme 17).26 Probe 50 was constructed using o-phenylenediamine as a NO recognition site and Nile Red as red-emissive TP fluorophore. Initially, the fluorescence of Nile Red was blocked by the o-phenylenediamine unit via a PeT process. When probe 50 comes into contact with NO the o-phenylenediamine is transformed into a triazole which disrupts the PeT process, resulting in a far-red emission (maximum at 650 nm). The maximum δ × Φf value of the reaction product was calculated to be 38 GM using an excitation of 820 nm, facilitating the use of probe 50 for tracking NO under TP excitation. The fast response of probe 50 towards NO within 180 s with a low detection limit of 46 nM was observed. The non-toxic probe 50 was then used for the TPEF imaging of exogenous and endogenous NO in HeLa and RAW264.7 cells respectively, using 820 nm excitation. Additionally, NO in mouse liver tissues at a depth of 168 μm was imaged using probe 50. The resultant fluorescence intensity exhibited a dose-dependent response towards NO in inflamed mouse tissues.
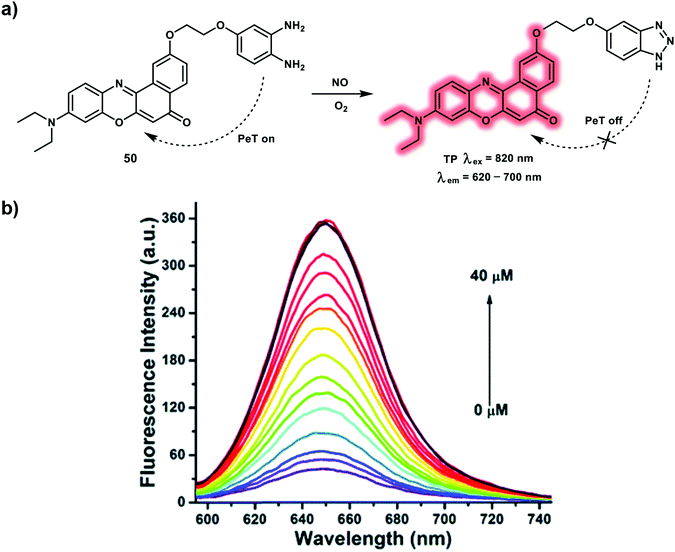 | ||
| Scheme 17 (a) TP probe 50 for the detection of NO. (b) Fluorescence spectra of 5.0 μM of 50 with NO (0–40 μM) after 180 s in 10 mM PBS (pH = 7.4, containing 10% DMF). Reproduced with permission from ref. 26. Copyright (2016) The Royal Society of Chemistry. | ||
H2O2, a key regulator of cellular signaling, is derived from O2˙− through NADPH oxidase complex activation. H2O2 participates in various biological responses and stimulates cell proliferation, differentiation, migration and cell death. H2O2 mediates cellular signaling processes by oxidizing critical thiols within redox-sensitive proteins. Increasing evidence points to excessive H2O2 generation being involved with many diseases, for instance, cardiovascular diseases, neurodegenerative disorders, and cancer. Therefore, developing an efficient H2O2-responsive fluorescent probe is of great significance for elucidating the biological function of H2O2. Carbon monoxide (CO) is regarded as a promoter for vasorelaxation. Probe 51 is a TP flavonol-boronate-based fluorescent sensor, which enables specific H2O2 response and precise CO release in a photo-controllable manner (Scheme 18).27 Probe 51 incorporates a boronate group to specifically recognize H2O2 and upon the addition of H2O2 the protected hydroxyl group of 51 was liberated. Triggering an ESIPT process, causing an increased emission at 585 nm. A 19-fold enhancement of fluorescence intensity at 585 vs. 485 nm (I585nm/I485nm) in the presence of 5 equiv. of H2O2 was observed, with a detection limit of 66 nM. After continuous irradiation using a 405 nm light source with power 15 mW cm−2, the fluorescence emission at 585 nm decreased dramatically, indicating CO photo-release. Incubation of probe 51 with PMA in vascular smooth muscle cells (VSMCs), resulted in PMA dose-dependent H2O2 fluctuations, as such probe 51 could be used to map H2O2 levels in living cells. Furthermore, during light irradiation with a TP laser at 800 nm, the fluorescence signals ratio (I420–510nm/I520–620nm) reduced, indicating CO photo-release. Time-dependent H2O2 fluctuations and TP-induced CO release were monitored in larval zebrafish. Upon angiotensin II administration, up-regulated H2O2 levels associated with oxidative stress in VSMCs were observed. More importantly, vasodilatation effect of CO was also visualized in vessel of zebrafish.
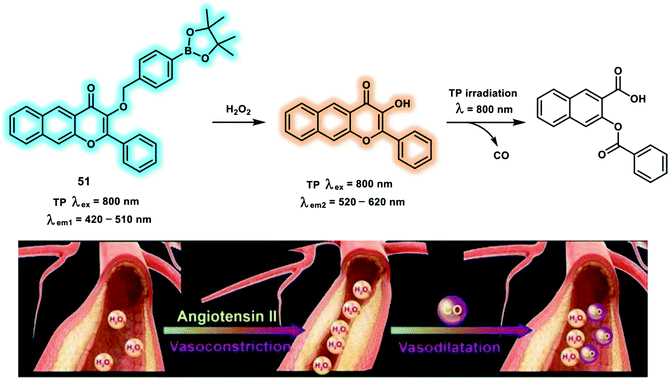 | ||
| Scheme 18 The chemical structure of TP probe 51 and the proposed CO photo-release mechanism. Reproduced with permission from ref. 27. Copyright (2018) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
A ratiometric probe 52 with a novel oxonium response site for H2O2, was constructed for the simultaneous NIR and TP fluorescence imaging of H2O2 in living systems. A large π-conjugated system was constructed based on chromenylium and coumarin groups. Probe 52 exhibited a large NIR emission at 693 nm and a small emission at 472 nm, facilitating NIR fluorescence imaging (Scheme 19).28 Upon the addition of H2O2, probe 52 exhibited a fluorescence enhancement at 472 nm along with a decrease at 693 nm (Schemes 19b and c). This was rationalized by oxidative breakdown of the oxonium and the sensing mechanism is based on Baeyer–Villiger reaction (Scheme 19a). The detection limit of H2O2 established by probe 52 was calculated to be 3.15 μM. The maximum δ of 53 was reported to be 164.7 GM at 760 nm, enabling the possibility of the simultaneous detection of H2O2 using NIR and TP fluorescence. Using probe 52, exogenous and endogenous H2O2 were imaged in living HeLa cells and RAW264.7 macrophages, respectively, using NIR and TP fluorescence channels. Furthermore, probe 52 was used to image H2O2 fluctuations in zebrafish using both the OP and TP modes.
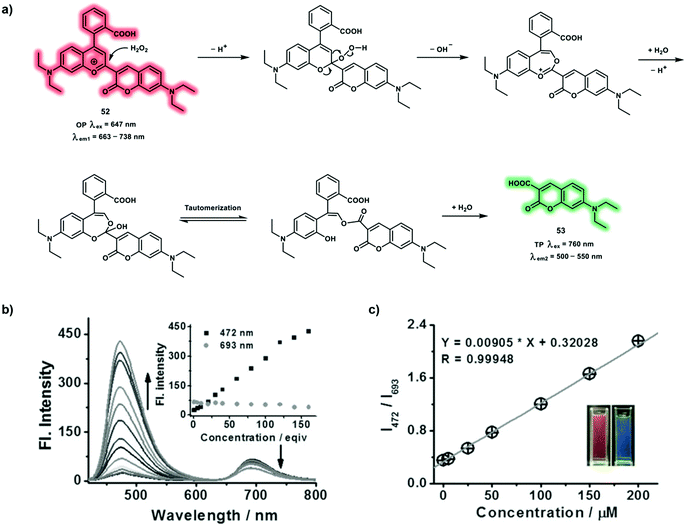 | ||
| Scheme 19 (a) Sensing mechanism of probe 52 for the detection of H2O2 with simultaneous NIR and TP emissions. (b) Fluorescence spectra of 52 (5 μM) in the presence of different concentrations of H2O2 under λex at 410 nm in PBS, inset: the fluorescence intensities at 472 and 693 nm as a function of H2O2 concentration. (c) Linear relationship between I472nm/I693nm and H2O2 concentration in the range, inset: photographs of 52 in the absence (left) and presence (right) of H2O2 (120 equiv.) under 365 nm ultraviolet radiation. Reproduced with permission from ref. 28. Copyright (2018) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
As a fundamental constituent amino acid of proteins, cysteine (Cys) plays important roles in protein synthesis and new tissue growth in the body. Total Cys levels in plasma are around 200 μM. Low levels of Cys in living systems has been correlated with liver damage, lethargy, edema and skin lesions. The polarized sulfur atom of Cys is prone to post-translational oxidative modifications, which control various biological processes, such as protein turnover, metal binding, catalysis and signal transduction. Over the past few years, diverse Cys-responsive fluorescent probes have been developed using the redox properties and nucleophilicity of sulfydryl within Cys. Since, the structure and reactivity of Cys is like homocysteine (Hcy) and glutathione (GSH), the differentiation of Cys, Hcy and GSH in living systems still remains a challenge. The commercial compound 54 has been reported for the selective detection of Cys after screening experiments (Scheme 20).29 Probe 54 went through aromatic nucleophilic substitution with the thiol group of Cys, producing the cyclized product 55 with large fluorescence enhancement. The strong electron-donating amino group on the aromatic core and inflexible cyclized structure of 55, resulting in the selective detection of Cys with TPEF using probe 54. A significant fluorescence intensity enhancement at 500 nm was observed under excitation at 420 nm. Probe 54 exhibited fluorescence emission centered at 450 nm when exposed to Hcy or GSH under 350 nm excitation. Moreover, addition of cetyltrimethylammonium bromide (CTAB) to the product of 54 and Hcy induced a fluorescent colour change from blue to green, which was due to the intramolecular cyclisation. Probe 54 exhibited good performance for the imaging of Cys levels in living cells, displaying a bright green fluorescence with TP excitation.
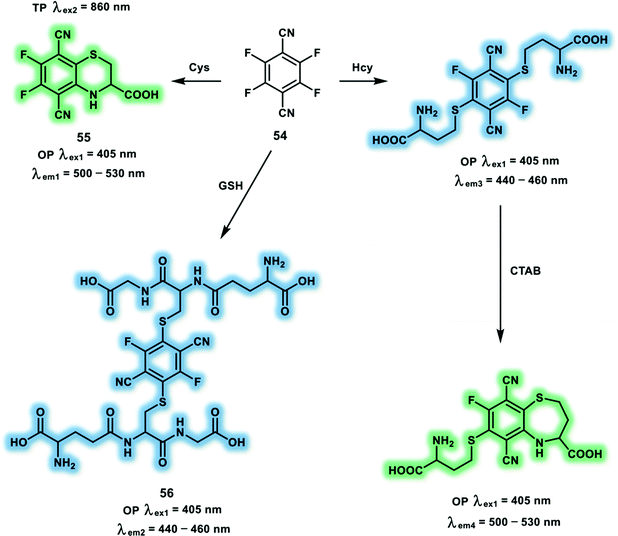 | ||
| Scheme 20 Probe 54 for the selective detection of Cys. Note: fluorescence of the products 55 and 56 are not interfered by CTAB. | ||
A TP fluorescent probe 57 was constructed based on a TP coumarin 120 fluorophore for the specific detection of Cys without interference from other biothiols (Scheme 21).30 With probe 57, the thiobenzoate was chosen as the specific recognition site. Probe 57 exhibits negligible fluorescence at 443 nm because of the weakened electron-donating ability of the amino group. In the presence of Cys (100 μM), nucleophilic reaction and five-membered ring formation results in enhanced electron-donating ability and increased push–pull electronic effect, leading to 25-fold fluorescence increase at 443 nm under excitation with OP (340 nm). After reaction with Cys, the δ changed from 7.39 to 18.01 GM, with an increased Φf from 0.16 to 0.49. Furthermore, no significant fluorescence change was observed upon the addition of Hcy or GSH, indicating that the probe could differentiate Cys over Hcy and GSH. The specificity was confirmed using high-performance liquid chromatography. Using probe 57 a 2.1-fold fluorescence decrease was observed in the brains of mice susceptible to chronic unpredictable mild stress (CUMS) using TPEF imaging, demonstrating that Cys levels in the CUMS mice was reduced.
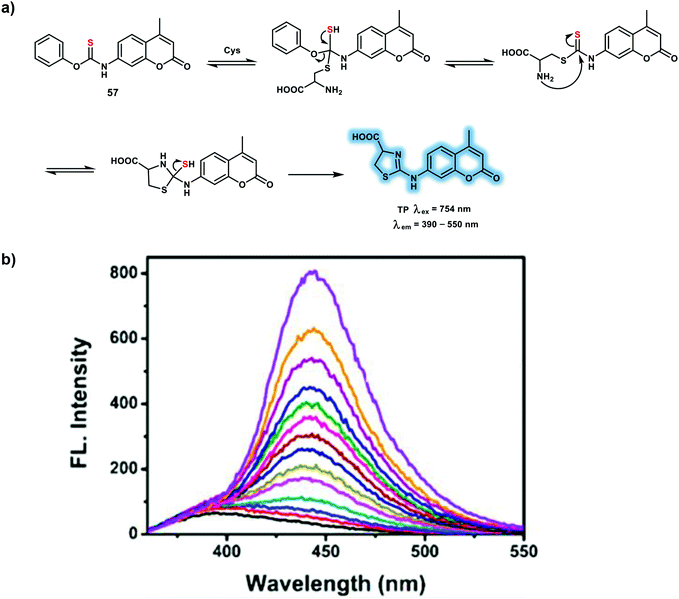 | ||
| Scheme 21 (a) TP probe 57 for the detection of Cys. (b) Changes in the fluorescence of 57 (20 μM) after the addition of various concentrations of Cys (0–100 μM). Reproduced with permission from ref. 30. Copyright (2019) American Chemical Society. | ||
Carbon monoxide (CO) is a gaseous signaling molecule in living systems, which plays important roles in cytoprotection due to its vasodilating, anti-inflammatory properties during oxidative stress and ischemia-reperfusion injury. Endogenous sources of CO are generated from heme oxygenase-mediated heme oxidative breakdown. The toxic effect of CO is caused by binding to hemoglobin strongly and forming carboxyhemoglobin, thereby leading to reduction in the oxygen-carrying capacity of the blood. The affinity of hemoglobin for CO is around 210 to 250 times that for oxygen. It has been reported that CO has a significant correlation with some diseases, such as vascular injury, ventilator-induced lung injury, ischemia-reperfusion and cancer. Probes for CO have been developed using palladium-containing complexes to track CO in biological systems. Probe 58 is a TP excitation NIR emissive probe suitable for the detection of CO that was derived from Nile Red dye as the fluorogen, with heteroatoms acting as ligands for the Pd (Scheme 22).31 The quenched fluorescence of the Nile Red dye by Pd atoms could be restored through the reaction of CO with Pd. Therefore, in presence of 100 equiv. CO, a 60-fold fluorescence enhancement at 660 nm was observed in PBS (10 mM PBS, pH 7.4, with 5% DMSO as cosolvent) with excitation at 580 nm. The advantageous features of probe 58 includes high sensitivity with a detection limit down to 50 nM, a large fluorescence enhancement, high selectivity and TP excitation with NIR emission. Interestingly, the merits of probe 58 enabled the tracking of endogenous CO in zebrafish embryos and exogenous CO in mouse tissues using TPEF imaging. Excessive production of CO was visualized in zebrafish embryos under conditions of hypoxia using TP excitation at 760 nm. Stronger NIR fluorescence was observed for CORM-2 (CO donor)-treated liver tissue slices at a depth of up to 130 μm using TPM.
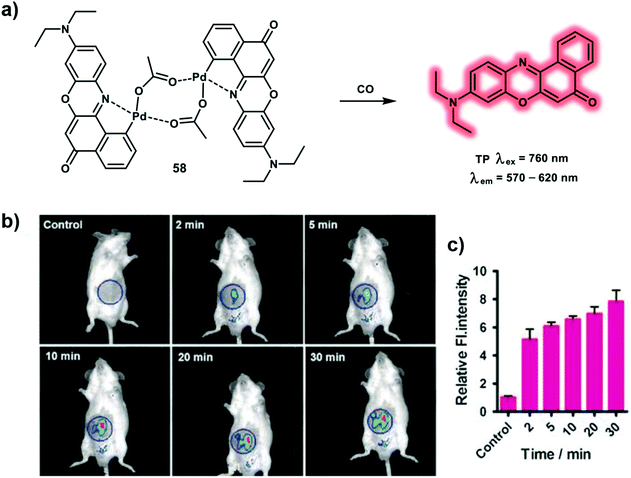 | ||
| Scheme 22 (a) Nile Red-based TP probe 58 for the detection of CO with NIR emission. (b) Time-dependent fluorescence images for CO in mice. Control group: 100 μL of 10 μM 58 was injected. Other groups: 100 μL of 10 μM 58 was injected, then 100 μL of 100 μM CORM-2 was injected and imaged. (c) The relative average fluorescence intensity change in the blue circle area. Error bars represent ±S.D., n = 3. λex = 580 nm, λem = 660 nm. Reproduced with permission from ref. 31. Copyright (2017) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Palladium-based fluorescent probes have been developed for the detection of CO, however these probes have many drawbacks such as delayed fluorescent response times, and they contain highly toxic heavy metal salts. Probe 59 is a TP probe based on ruthenium(II) vinyl complex (Scheme 23a).32 With probe 59, a TP fluorophore 5-(3-thienyl)-2,1,3-benzothiadiazole (TBTD) was used as fluorescence reporter and an oligoethylene glycol moiety was used to improve water solubility. Probe 59 displayed a weak fluorescence emission at 500 nm under 355 nm excitation, that was attributed to quenching of the TBTD fluorescence by the ruthenium(II) center (heavy atom effect). Exposure to CO gas resulted in binding with the ruthenium(II) complex and release of the TBTD fluorophore via indicator displacement (i.e. the system is an IDA), resulting in a fluorescence enhancement at 500 nm in PBS (pH 7.4)–acetone 99.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 v/v solution (Scheme 23b). A dose-dependent CORM-3 and hemin TPEF intracellular response was observed for RAW264.7 cells with a 715 nm excitation. Probe 59 was used to image CO in a LPS-induced air pouch inflammation mouse model using a multiphoton microscopy.
0.1 v/v solution (Scheme 23b). A dose-dependent CORM-3 and hemin TPEF intracellular response was observed for RAW264.7 cells with a 715 nm excitation. Probe 59 was used to image CO in a LPS-induced air pouch inflammation mouse model using a multiphoton microscopy.
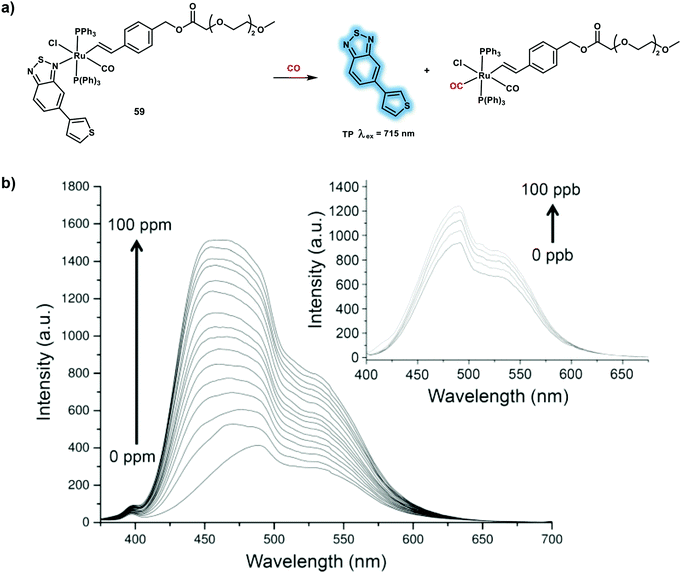 | ||
Scheme 23 (a) Ruthenium(II) vinyl complex-based TP probe 59 for the detection of CO. (b) Turn-on fluorescence response (λex = 355 nm) of 59 (10 μM) in PBS (pH 7.4)–acetone 99.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 v/v solution upon addition of increasing quantities of CO (0–100 ppm and 0–100 ppb). Reproduced with permission from ref. 32. Copyright (2017) American Chemical Society. 0.1 v/v solution upon addition of increasing quantities of CO (0–100 ppm and 0–100 ppb). Reproduced with permission from ref. 32. Copyright (2017) American Chemical Society. | ||
Adenosine triphosphate (ATP), serves as a fast neurotransmitter or neuromodulator and plays an important role in mediating synaptic transmission and synaptic efficacy. In addition, ATP can participate in immunogenic cell death as a signaling molecule. Deficiencies in ATP levels are associated with hypoglycaemia, ischemia injury and neuronal disorders. Thus, monitoring ATP levels in living systems is important in order to help evaluate biological mechanisms, and signaling pathways that are mediated by ATP. Ahn et al. reported on the monitoring a lysosomal membrane fusion processes in living cells using a novel ratiometric TP fluorescent probe, which enables the quantitative tracking of lysosomal ATP fluctuations. A TP probe 60 was constructed by linking a BODIPY dye and rhodamine 6G using a tetramine chain for the ratiometric detection of lysosomal ATP (Scheme 24).33 Under excitation at 403 nm, the probe exhibited BODIPY blue fluorescence emission at 454 nm. Then after binding with ATP, an apparent yellow fluorescence of rhodamine 6G at 557 nm and a slight increased blue fluorescence at 454 nm was observed, which was attributed to the restored FRET process from the BODIPY dye to rhodamine 6G and suppressed PeT. The fluorescence response of probe 60 towards ATP was evaluated using the fluorescence ratio (I454nm/I557nm) at acidic pH, suggesting that probe 60 was capable of monitoring lysosomal ATP. Probe 60 exhibited high sensitivity towards ATP due to strong ionic interactions and π–π stacking interactions. TPM images of probe 60-stained mouse brain corpus callosum (CC) indicated that lysosomal ATPs were distributed mainly in the CC area. Moreover, two different lysosomal changes in ATP levels were observed during “kiss-and-run” and the “full-collapse membrane fusion” process using probe 60 and TPM.
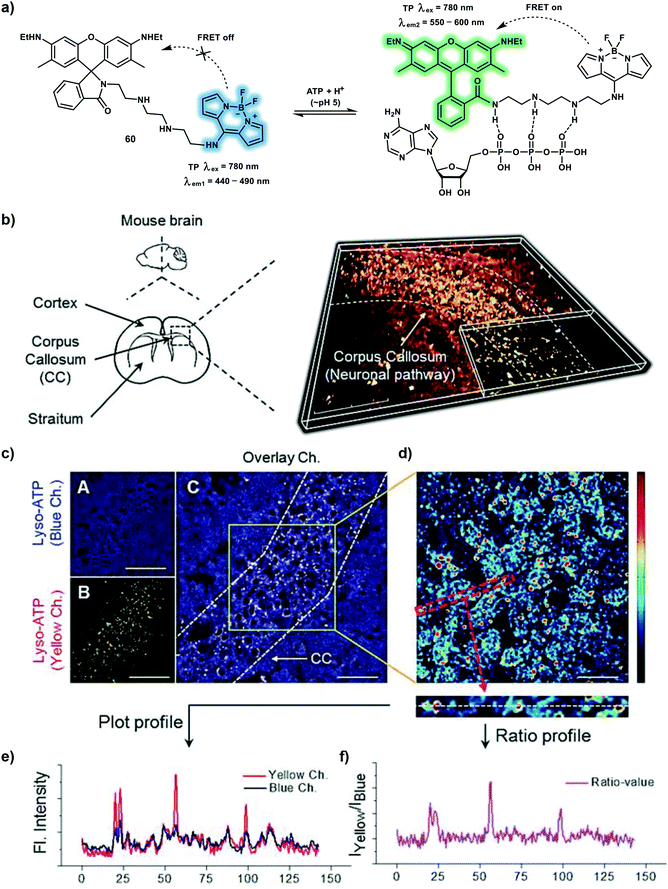 | ||
| Scheme 24 (a) A FRET-based TP probe 60 for the ratiometric detection of lysosomal ATP. (b) 3-Dimensional TP image of brain slice of CC constructed based on the yellow channel of 60 (30 μM). (c) Cross-section images of the blue channel (A), yellow channel (B), and overlay (C). (d) Ratiometric image representing the ratio of the yellow to the blue channel from (c). (e) Profile of blue and yellow channels obtained from (d). (f) Ratio values of (e). Scale bars = 80 μm (b), 40 μm (c), and 20 μm (d). Reproduced with permission from ref. 33. Copyright (2018) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
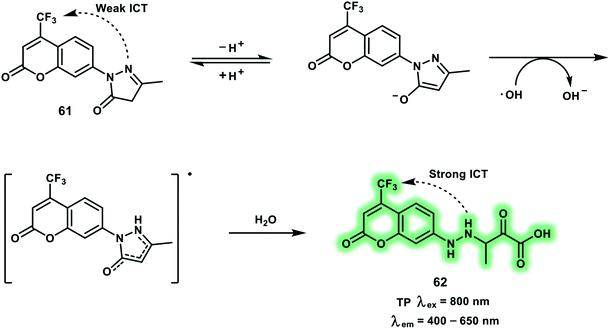
Hydroxyl radical (˙OH) is regarded as the primary cause of damage due to oxidative stress, where it attacks biomacromolecules and induces irreparable damage. Endogenous ˙OH is generated from the Fenton reaction and Haber–Weiss process using H2O2. The ˙OH then damages cells through direct oxidation of lipids, proteins, and DNA. It has been reported that ˙OH concentration imbalances are related to diseases, such as breast cancer, neurodegeneration and depression. The TP turn-on probe 61 based on coumarin (Cou151) bearing a trifluoromethyl group as the TP reporter was developed to image in situ brain ˙OH (Scheme 25).34 With probe 61, 3-methyl-pyrazolone was chosen as the specific reaction site for ˙OH. The one-electron oxidation reaction between ˙OH and 3-methyl-pyrazolone, cleaved the electron-withdrawing ring and restored the push–pull electron effect of the coumarin ring. Probe 61 exhibited an absorption band centered at around 350 nm and a weak fluorescence at 500 nm. After reaction with ˙OH, a remarkable fluorescence enhancement with increased Φf (from 0.037 to 0.25) and δ (from 5.0 GM to 42.6 GM) was observed, which was attributed to enhanced ICT within product 62. The fluorescence intensity of probe 61 at 500 nm exhibited a linear relationship towards ˙OH concentrations from 0 to 20 μM and a low detection limit of 2.4 nM. The trifluoromethyl group enabled probe 61 to cross the blood–brain barrier allowing an increase of ˙OH in the brain of the mice under restraint stress to be monitored using TPEF imaging. Subsequently, up-regulated ˙OH levels of mice susceptive to CUMS were visualized under TP excitation at 800 nm.
4. Two-photon (TP) excitation-based fluorescent sensors for biomacromolecules
Abnormal enzymatic activities are related to the development of numerous diseases including Parkinson's disease, Alzheimer's disease and cancer. Identifying the location and expression levels of these enzymes in live cells are vital for the early-stage diagnoses and monitoring the efficient of therapies of diseases.35,36 In this section we will discuss three representative enzymes (steroid sulfatase, β-secretase and acetylcholinesterase) and their corresponding TP probes.Steroid sulfatase (STS) is primarily produced in the endoplasmic reticulum and responsible for the hydrolysis of aryl and alkyl steroid sulfate. The activity and reactions of STS is associated with physiological processes and pathological conditions including estrogen-related breast cancer. Inactive estrone sulfate can be hydrolyzed to estrone via STS desulfation, then bind to the estrogen receptor and stimulate the pathophysiology of breast cancer. In addition, STS expression is increased in breast tumors tissues when compared with normal tissues and has prognostic significance. The TP probe 63 was constructed based on a TP dye (4-hydroxy-1,8-naphthalimide) for the ratiometric detection of STS. With probe 63, a sulfate group and the methyl sulfonamide group serve as STS recognition site and an endoplasmic reticulum targeting group respectively (Scheme 26).37 A red-shifted emission was observed upon addition of STS due to enzymatic hydrolysis and release of the phenol form. Probe 63 was used to monitor the enhanced STS activity of breast tumor tissues over normal tissues using TP 3-dimensional imaging. Compared to single-photon imaging, TPEF imaging exhibited a penetration depth up to 120 μm using an excitation wavelength of 820 nm. Moreover STX64 (a STS inhibitor) was used to confirm the specific response between 63 and STS using TP ratiometric imaging.
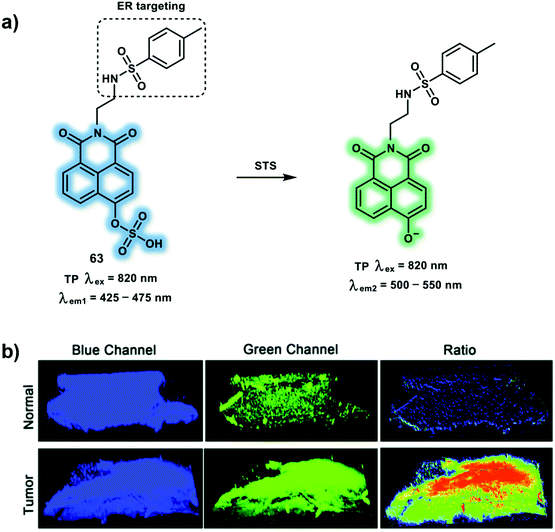 | ||
| Scheme 26 (a) TP probe 63 for the ratiometric detection of STS in endoplasmic reticulum. (b) The 3-dimensional TP fluorescence image of normal and tumor tissues with a magnification of 10×. Blue channel (425–475 nm) and green channel (500–550 nm); ratio images generated from the green channel and blue channel. Reproduced with permission from ref. 37. Copyright (2020) The Royal Society of Chemistry. | ||
β-Secretase (BACE1) is an important enzyme in the etiology of Alzheimer's disease. Production and accumulation of β-amyloid peptides (Aβ) is responsible for Alzheimer's disease pathogenesis. Aβ is generated by sequential cleavage from BACE1. In brief, BACE1 cleaves amyloid precursor protein at the β site, releasing a C-terminal fragment (CTFβ). The remaining CTFβ can then be cleaved by γ-secretase to yield Aβ. In addition, oxidative stress leads to enhanced BACE1 expression and activity in Alzheimer's disease. The TP probe 64 was developed based on FRET for the ratiometric detection of BACE1.38 With probe 64, the merocyanine derivative (mCyd) and Alexa Fluor 633 (AF633) are the energy donor and acceptor, respectively (Scheme 27). mCyd and AF633 are connected by a peptide (EVNL-DAEFRHDSGYK), which ensures that probe 64 can be cleaved with high selectively by BACE1 at its β site. The sensing mechanism was confirmed using mass spectrometry. Probe 64 exhibited a maximum emission at 651 nm upon TP excitation at 820 nm, which was ascribed to the emission profile of the fluorescent acceptor AF633. These observations were consistent with the design expectations for probe 64, that is prior to interaction with BACE1, FRET was observed. Subsequently, addition of BACE1 results in reduced AF633-based fluorescence emission at 651 nm and an increase in the emission at 578 nm. Probe 64 exhibits a linear fluorescence correlation with the concentration of BACE1 from 0.1 to 40.0 nM and TPEF emission ratio Igreen/Ired (green: 560–620 nm, red: 640–700 nm) in fresh cell lysates containing 0.05% DMSO, pH = 4.5. The detection limit was calculated to be 65.3 ± 0.1 pM. In addition probe 64 was able to visualize BACE1 changes using Axon 1125 (an inhibitor of BACE1) or superoxide in neurons by ratiometric fluorescence imaging. Moreover, compared to OP imaging (excitation at 552 nm), TP imaging (excitation at 820 nm) could detect BACE1 in the hippocampus region of AD mouse brain with deep penetration up to a thickness 300 μm. TP ratiometric imaging was then used to indicate higher concentrations of BACE1 in the S1BF and CA1 regions than CPu and LD regions in AD mouse brain. It has been reported that oxidative stress is likely to increase BACE1 gene promoter activity and cause BACE1 over transcription. Then, in order to gain more insight, a commercial ROS probe DCFH-DA was used to image oxidative stress where it was found that stress was higher in S1BF and CA1 over CPu and LD regions of Alzheimer's diseased brain slices. In addition, it was observed that both BACE1 and oxidative stress for each of those four regions in normal mouse brain slices were lower than in Alzheimer's disease mouse brain slices.
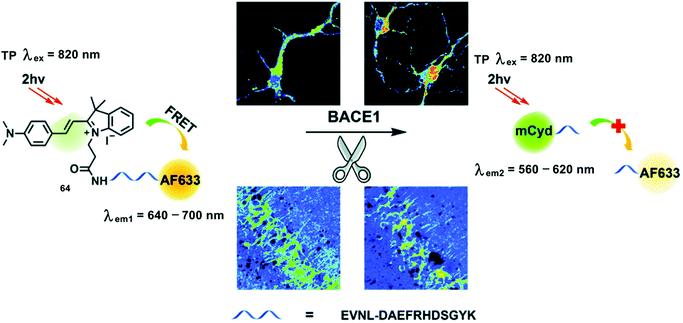 | ||
| Scheme 27 Illustration for the working principle of the designed TP ratiometric fluorescent probe 64 for the determination of BACE1 in neurons and mouse brain tissue slice. Reproduced with permission from ref. 38. Copyright (2020) The Royal Society of Chemistry. | ||
Depression is a common illness worldwide which can happen to people of all ages. When people are depressed, they feel persistently sad for weeks or months, rather than just a few days. Depression is responsible for worsening the effects of many chronic health conditions including arthritis, asthma, and diabetes. In a worse case scenario, depression can also lead to suicide. Oxidative stress in depression is a primary cause of neurotransmitter metabolism dysfunction in the brain. Acetylcholinesterase (AChE) is a key hydrolase that can catalyze the breakdown of acetylcholine (ACh). Therefore, abnormal concentrations of AChE correlate with the degradation of neurotransmission within the brain. Inspired by neostigmine (an AChE inhibitor), the novel TP probe 65 was developed to visualize the AChE activity during depression in mice brains (Scheme 28).39 The carbamate of neostigmine can bind covalently to a serine residue in the active site of AChE and then lead to carbamoylation of the active site serine in AChE. This specific binding induces the deactivation of AChE, which was employed as the recognition principle for AChE with probe 65. For probe 65, hemicyanine was chosen as a TP fluorophore and its inherent nitrogen cation (N+) facilitated binding with the anionic site of the hydrolytic center of AChE. Moreover, dimethyl carbamate is the specific recognition group towards AChE. The interaction between the carbamate and the hydroxyl group of the serine at the hydrolytic center of AChE results in carbamoylation of the serine. Probe 65 exhibits minimal fluorescence due to its pull-pull electron structure. When the protecting group of probe 65 was cleaved by AChE, the D–π–A-based hemicyanine is released resulting in an emission band centered at 560 nm using a TP excitation at 800 nm in PBS buffer solution. Probe 65 was then used to monitor AChE activity in PC12 cells using TPEF imaging. Probe 65 was then evaluated in mice brains due to its excellent performance in cellular imaging and excellent biocompatibility with cells. It is found that mice with depression-like behavior induced by chronic-restraint stress have elevated AChE activity in their brains. Finally, the relationship between O2˙− and AChE activity was evaluated using probe 65 in PC 12 cells using TP imaging. It was found that increased O2˙− resulted in an increase of AChE activity. However, a decrease in AChE activity was observed when caffeic acid ethyl ester (a O2˙− scavenger) was used to relieve the oxidative stress.
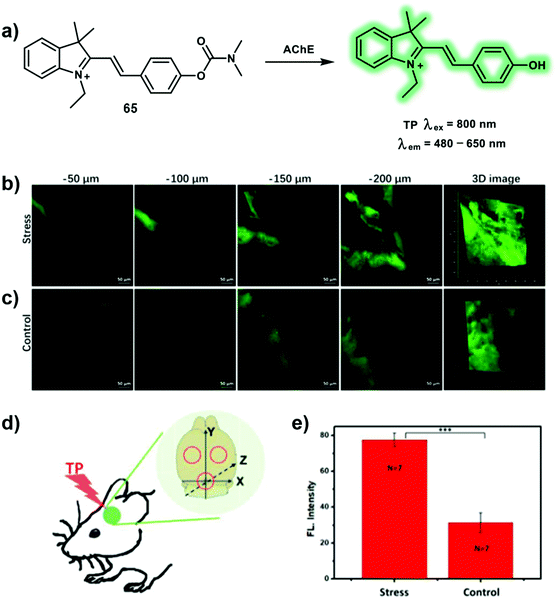 | ||
| Scheme 28 (a) TP probe 65 for the detection of AChE. (b and c) In situ TP fluorescence imaging of the brains of mice. (b) Stress: mice exposed to 14 consecutive days of chronic-restraint stress. (c) Control: mice not subjected to chronic-restraint stress. (d) Three different TP fluorescence imaging areas. (e) Relative fluorescence intensities of mice in b and c. The data are expressed as the mean ± SD; ***P < 0.001 for stress group compared with the control. Seven mice in each group. TP images, at a 20× magnification, of mice labeled with 0.15 mg kg−165via intraperitoneal injection. Fluorescence emission window: 480–650 nm. Scale bar = 50 μm. Reproduced with permission from ref. 39. Copyright (2019) American Chemical Society. | ||
5. Two-photon (TP) excitation-based fluorescent sensors for cellular microenvironment
pH, polarity and viscosity are important cellular microenvironment related parameters and play crucial roles in controlling signal transduction, transportation, interactions with biomacromolecules within live cells. The imbalance of these factors are hallmarks for the occurrence of various metabolic diseases causing cellular disfunction. In addition, the microenvironment is heterogeneous within cells, varying in specific settings such as the cytosol, or sub-organelles, or membrane. Hence, various probes have been developed to monitor microenvironmental distribution and fluxes. In this section, we will review some representative TP probes for cellular microenvironment (pH, polarity, and viscosity), again an emphasis will be placed on both the mechanism and applications.As an essential cellular microenvironmental factor, pH is strongly linked to various cellular behavior, such as cell proliferation and apoptosis, endocytosis processes and regulation of enzyme activity. pH values change from 4.0 to 8.0 in different cellular compartments, cytoplasmic pH is known to be near 7.2, while mitochondrial pH is roughly 8.0 and lysosomal pH is acidic (pH 4.0–5.5). Abnormal changes of pH can be considered as a hallmark of many diseases, including inflammation, Alzheimer's disease, and cancers. Probe 66 is a 2-naphthol-based ratiometric TP probe suitable for the detection of mitochondrial pH that uses benzochromene-2-one as the fluorophore and triphenylphosphonium salt as mitochondria targeting unit (Scheme 29).40 The fluorescence of probe 66 increases gradually at 604 nm with a concomitant decrease at 540 nm upon basification, a result of the enhanced ICT effect caused by formation of the stronger electron-donating phenolate. The measured pKa value of probe 66 is 7.95 ± 0.05, which is well-matched to mitochondrial pH (8.0). The maximum δ values of probe 66 are within the range of 20–70 GM at acidic pH (4.0) and alkaline pH (10.0). Probe 66 displays well-merged fluorescence with mCherry-mito-7 (a mitochondria-targeting protein) regardless of changes in mitochondrial membrane potential using TPM. The heterogeneity of mitochondrial pH (pHmito) using probe 66-labeled HeLa cells were evaluated using the fluorescence ratio (Ired/Igreen) under TP excitation at 820 nm. Additionally, pH values of mitochondria in the perinuclear position were higher than that in the periphery of cells. It was found that more acidic pHmito values were observed in Parkinson's disease model astrocytes when compared to wild-type astrocytes using pseudocolored ratiometric TPM images. Finally, probe 66 was used to evaluate the heterogeneous pHmito distribution in hippocampal tissues.
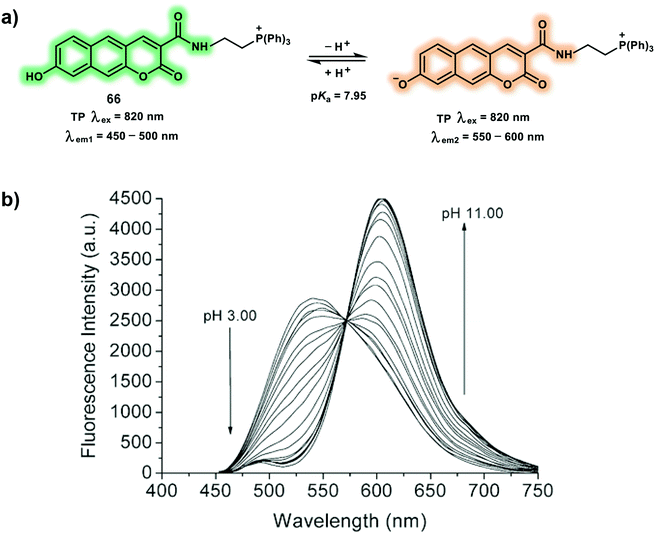 | ||
| Scheme 29 (a) TP probe 66 for the ratiometric detection of mitochondrial pH. (b) Change in fluorescence emission spectra of 66 with various pH in buffer solution (λex = 425 nm). Reproduced with permission from ref. 40. Copyright (2016) The Royal Society of Chemistry. | ||
Classical approaches for constructing polarity probes are mostly based on ICT systems, by linking an electron donor and an electron receptor with a conjugated spacer. Then when the surrounding solvent polarity changes, a marked blue/red shift in fluorescence or large variation in Φf will be observed. The TP probe 67 with a “D–π–A” structure facilitates the detection of lysosomal polarity in living systems (Scheme 30).41 A 1,8-naphthalimide was used as the TP fluorophore and electron acceptor, diphenylamine was introduced as electron donor, which was linked by phenyl group. Morpholine was used as a lysosome-targeting group to facilitate accumulation of the probe in lysosomes. When the polarity was varied from the non-polar solvent toluene to the polar solvent DMSO, the fluorescence spectra of probe 67 displays a red-shift in emission of up to 106 nm and a 43-fold fluorescence decrease with Φf from 0.308 to 0.009. Stronger fluorescence in lower polar lysosomes while weaker fluorescence in the higher polar media were observed for 67 which is caused by a positive solvatokinetic effect.42 Low lysosomal polarity monitored in inflammation, fatty liver and tumor mice using 67 in OP mode using excitation at 500 nm. The TP photophysical properties were evaluated and upon TP excitation, 67 displayed a maximum δ of 384 GM at 800 nm. Considering that the polarity may change continuously in zebrafish development processes, probe 67 was used to image the polarity variation in embryo and adult zebrafish models. A 1.8-fold fluorescence enhancement was observed for embryonic zebrafish at 500–550 nm, suggesting a low polarity environment for embryonic compared to adult zebrafish.
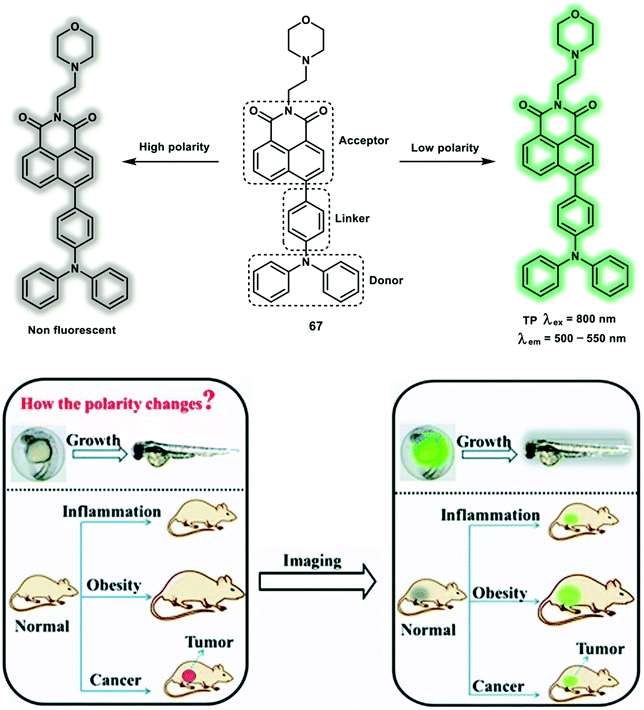 | ||
| Scheme 30 The design concept of the TP probe 67 for monitoring lysosomal polarity changes. Reproduced with permission from ref. 41. Copyright (2019) The Royal Society of Chemistry. | ||
As a primary cellular property of living cells, subcellular viscosity plays an important role in controlling diffusion and bimolecular reaction rates in diffusion-mediated cellular processes. Abnormal changes of cellular viscosity are associated with many disorders and diseases, such as Alzheimer's disease, diabetes and hypertension. Most of the viscosity-responsive probes are molecular rotors, which contain a rotatable conjugated moiety, as such changes in environmental viscosity alter the fluorescence intensity through rotation restriction. Probe 68 is a turn-on TP probe for monitoring mitochondrial viscosity with TP excitation and deep red emission (Scheme 31).43 The probe was designed based on the concept that the restriction of free rotation of hemicyanine facilitates stronger fluorescence emission, and is constructed using carbazole, hemicyanine (indole salt) as molecular rotors and mitochondria-targeting group. Notably, when evaluated in methanol–glycerol systems of low viscosity (1.4 cP), probe 68 was weakly fluorescent under 520 nm excitation, indicating that the fluorescence is quenched by hemicyanine rotation. However, in a high viscosity systems (956 cP), a 32-fold enhancement in emission intensity due to the inhibition of free rotation of the single bond between the carbazole moiety and indole salt was observed. The maximum δ of probe 68 was 121 GM at 740 nm. HeLa cells and zebrafish labeled with probe 68 after monensin or nystatin loading displayed enhanced fluorescence in both the OP mode and TP mode. The ability of probe 68 for tracking viscosity was verified in inflammation, fatty liver and tumor models, respectively, using OP excitation at 560 nm.
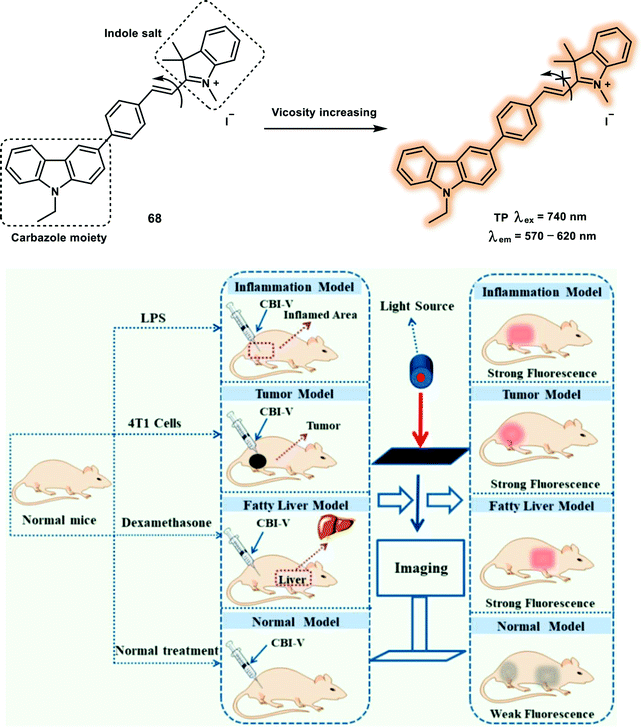 | ||
| Scheme 31 TP probe 68 for the detection of mitochondrial viscosity and its application for imaging in live mice. Reproduced with permission from ref. 43. Copyright (2019) American Chemical Society. | ||
6. Two-photon (TP) excitation-based fluorescent sensors for subcellular localization/protein oxidization
The development of TP probes that specifically localize in subcellular compartment is of great significance for elucidating cellular activities and processes. For instance, mitochondria produce ATP and supply energy through oxidative phosphorylation (OXPHOS) pathways in a chain of electron transport. While the Golgi complex is responsible for lipids and protein transportation from the endoplasmic reticulum. To detect subcellular organelles in living systems, TP excitation-based sensors that target specific organelle or biomacromolecule have been reported. This section will include some representative TP probes for subcellular localization and protein labelling.The most prominent role of the mitochondria is producing energy in the form of ATP. In addition, mitochondria are involved in controlling various cellular biological processes, such as the biosynthesis of amino acids and lipids, programmed cell death, cytosolic calcium ions (Ca2+) and ROS levels. Given mitochondria play a key role in the regulation of apoptotic pathways, as such many anticancer drugs have been developed to target the mitochondria in order to create tumor cells more susceptible to anticancer treatment. A dual-mode mitochondria-targeting probe 70 with aggregation-induced emission (AIE) and Raman output was designed for mitochondrial imaging of living cells (Scheme 32).44 Dual-mode live-cell imaging with probe 70 was conducted using a homemade fluorescence-stimulated Raman scattering microscope system. Probe 70 developed containing three units, α-cyanostilbene as the AIE skeleton, pyridinium salt as the targeting group for the mitochondria, and diphenylacetylene as Raman signal reporter with a Raman signal peak at 2225 cm−1. Dual-mode probe 70 is a hybrid of 69 (an AIE fluorophore) and diphenylacetylene unit. Probe 70 exhibits absorption at 380 nm and fluorescence output at around 500 nm with a large Stokes shift of 120 nm in DMSO. The δ of 70 was determined to be 14.2 GM under TP excitation at 800 nm. The AIE nature of 70 was evaluated in a mixed DMSO–water system. With water fraction increasing from 0% to 70%, the fluorescence intensity decreased due to enhanced twisted intramolecular charge transfer (TICT) processes caused by increased polarity. Fluorescence increase was observed as the water fraction increased from 70% to 99% which was attributed to AIE. The relative Raman intensity versus 5-ethynyl-2′-deoxyuridine at 2223 cm−1 was 6.0. Stimulated Raman scattering (SRS) exhibited a linear relationship with the concentration of probe 70 (ISRS = 0.1567c), in which c represents the concentration of the probe. In addition, probe 70 exhibited maximal TP excitation at 780 nm, and enhanced TPEF intensity upon increasing probe concentration. The TPEF fluorescence of probe 70 (1 mM) increased by approximately 70% with increasing water fraction from 0 to 40 vol% in DMSO–water mixtures. Finally, the TPEF and SRS signals of HeLa cells stained with probe 70 were collected, the merged images indicate colocalization of TPEF and the SRS signal and a positive correlation between TPEF intensity and SRS signal was determined using statistical analysis.
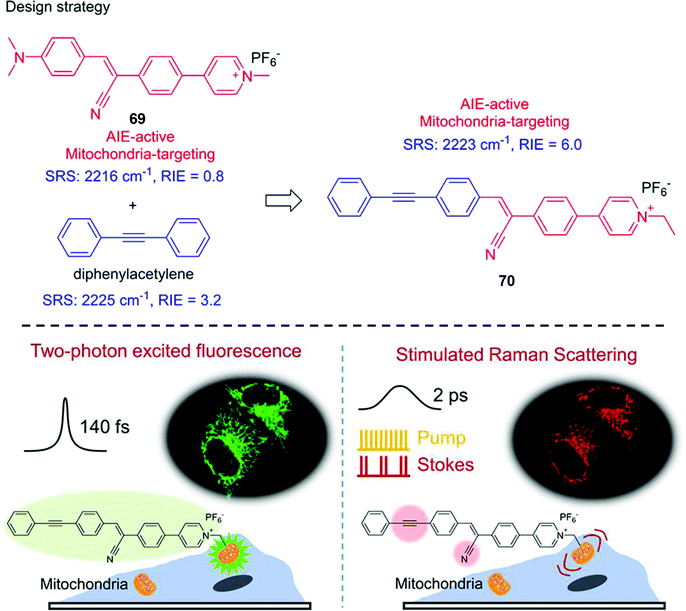 | ||
| Scheme 32 The design strategy and the application of an AIE-based probe 70 for the imaging of mitochondria using fluorescence and stimulated Raman scattering microscopy. Reproduced with permission from ref. 44. Copyright (2017) American Chemical Society. | ||
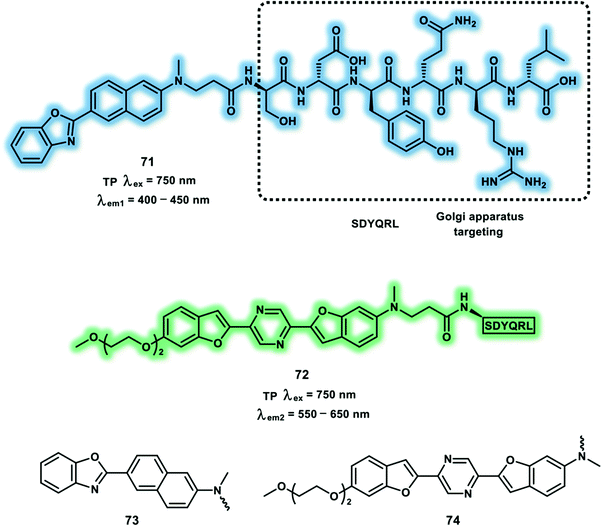
The Golgi complex is the vital processing and sorting station, which transports lipids and proteins synthesized in the endoplasmic reticulum and packages them into vesicles to transport to other organelles. Moreover, the Golgi complex is involved in glycosylation of proteins, transformation of the membrane and proteolysis. Blue- and yellow-emitting TP probes (71 and 72) for the Golgi apparatus were designed. These two probes use 6-(benzo[d]oxazol-2-yl)-2-naphthalylamine (73) and 2,5-bis(benzo[d]oxazol-2-yl)-pyrazine (74) derivatives as the fluorophores, and SDYQRL as the Golgi-apparatus-targeting unit (Fig. 2).45 Maximum δ × Φf for probes 71 and 72 were determined in EtOH and 1,4-dioxane/H2O (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) respectively with a value of 96 GM and 65 GM under 750 nm excitation. Probe 71 emits TPEF at 462 nm in HeLa cells, which was separated from the emission fluorescence of probe 72 at 560 nm. HeLa cells were labeled with TP probes 71 and 72 and exhibited superior Golgi apparatus staining compared to BODIPY TR ceramide, a OP commercial probe for the Golgi apparatus, revealing stronger interaction with TGN38 in the Golgi membrane. Then, probe 72 was used to monitor apoptosis in HeLa cells. Finally, both probe 71 and 72 were used to visualize the Golgi apparatus distribution in rat-hippocampal slices at depths of 120–270 μm using TPEF imaging.
1) respectively with a value of 96 GM and 65 GM under 750 nm excitation. Probe 71 emits TPEF at 462 nm in HeLa cells, which was separated from the emission fluorescence of probe 72 at 560 nm. HeLa cells were labeled with TP probes 71 and 72 and exhibited superior Golgi apparatus staining compared to BODIPY TR ceramide, a OP commercial probe for the Golgi apparatus, revealing stronger interaction with TGN38 in the Golgi membrane. Then, probe 72 was used to monitor apoptosis in HeLa cells. Finally, both probe 71 and 72 were used to visualize the Golgi apparatus distribution in rat-hippocampal slices at depths of 120–270 μm using TPEF imaging.
PDT is a photon-activated therapeutic modality that uses highly toxic ROS to kill cancer cells. Metal complexes have been widely used as photosensitizers for PDT, because the heavy-atom effect favors singlet-to-triplet intersystem crossing (ISC) and increases the excited-state lifetimes, leading to a boost in 1O2 and other ROS generation. Two efficient novel organoiridium photosensitizer complexes 75 and 76 were developed (Fig. 3).46 Both 75 and 76 were nontoxic to cancer cells in the dark, whereas an increased in cytotoxicity towards cancer cells a result of 1O2 generation was observed for OP or TP irradiation. The δ values of 75 and 76 were determined to be 115 GM and 70 GM, respectively, at 750 nm excitation. The generation of 1O2 by 75 and 76 with Φf of 0.73 and 0.81, respectively, under 465 nm irradiation, were evaluated using electron paramagnetic resonance spectroscopy. A549 spheroids incubated with 75 and 76 display a strong red phosphorescence at a depth up to 212 μm using TPM upon 750 nm excitation. The 1O2 generated using 76 was found to oxidize specific histidines in the 70 kilodalton heat shock protein (Hsp 70) and in aldose reductase (AR). While, the ROS generated upon irradiation increased the levels of enzymes involved in the glycolytic pathway.
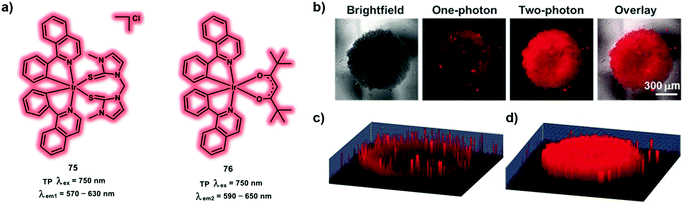 | ||
| Fig. 3 (a) Structures of the complexes 75 and 76. (b–d) Phosphorescence imaging of A549 spheroids. Spheroids were incubated with 76 (10 μM) for 2 h. (b) Comparison of brightfield, one-photon (λex = 458 nm), and two-photon (λex = 750 nm) excitation, λem = 620 ± 30 nm. OP (c) and TP (d) 3D Z-stack images were taken every 16.3 μm from the top to bottom of the spheroids. Images were taken under a 10× objective. Scale bar: 300 μm. Reproduced with permission from ref. 46. Copyright (2017) The Authors (P. Zhang, C. K. C. Chiu, H. Huang, Y. P. Y. Lam, A. Habtemariam, T. Malcomson, M. J. Paterson, G. J. Clarkson, P. B. O'Connor, H. Chao and P. J. Sadler). Published by Wiley-VCH Verlag GmbH & Co. KGaA. | ||
7. Dual-responsive two-photon (TP) excitation-based fluorescent sensors
Significant effort has been devoted to the development of single response fluorescent probes for specific analytes. However, a specific disease is usually associated with a variety of biological markers. For example, ROS exhibit crosstalk in redox homeostasis. With increased ONOO− and H2O2 in drug-induced liver injury (DILI) having been reported. Similarly, ROS and Aβ are associated with the progression Alzheimer's disease.15 While hypoxia can lead to a decrease of cellular GSH and upregulation of nitroreductase. Compared to single-responsive probes for one analyte, dual-responsive probes can provide different information by facilitating measurement of two analytes within the same biological system. Many dual-responsive OP probes have been developed, while dual-responsive probes with TPEF are relatively rare. Therefore, the development of dual-responsive TP probes is appropriate when exploring biologically-related effects associated with multiple analytes involved in many diseases and disorders.47 This section will include some representative sensors designed to detect more than one analyte using TP probes and their applications.DILI has attracted significant attention, however the biological role and changes in fluxes of HOCl during DILI remain unknown. N-Acetyl cysteine (NAC) displays some protective effects against DILI. While, H2S, a downstream product of NAC, may be involved in DILI. Hence, developing a dual-responsive probe for H2S and HOCl could help reveal the functions of H2S and HOCl in DILI. To recognize and image H2S and HOCl simultaneously, a TP probe 77 was constructed by linking 7-amino coumarin and rhodamine B using a piperazine (Scheme 33).48 Thiolactone and azido were exploited as HOCl and H2S reactive group, respectively. Upon addition of HOCl (0–100 μM), a gradual fluorescence increase at 580 nm was observed for 545 nm excitation. The separate addition of H2S (0–100 μM), resulted in an increase of the emission at 445 nm upon 360 nm excitation which was ascribed to D–π–A structure formation of the coumarin derivative. With probe 77 in hand, endogenously generated H2S and HOCl were imaged in NAC-induced and LPS/PMA-stimulated RAW264.7 cells, respectively, under TP excitation at 750 nm. A significant green fluorescence enhancement with increased HOCl levels during DILI after combined administration of fluoxetine and duloxetine was observed, which indicates that HOCl could be an efficient indicator for DILI. In addition, NAC-pretreated cells displayed an increased blue fluorescence and decreased yellow fluorescence by TPM imaging, indicating an enhanced level of H2S and suppressed concentration of HOCl. Dual-color fluorescence images of Kunming mice liver tissue confirmed the capability of probe 77 for visualizing H2S and HOCl fluctuations during DILI.
N-Methyl-D-aspartic acid (NMDA) receptor over-activation is strongly involved in depression. Zinc cation (Zn2+) and hydrogen ion (H+) serve as binding partners of NMDA receptor and regulate the activity. Therefore, monitoring simultaneous changes of Zn2+ and H+ levels are meaningful for understanding depression-related signal pathways. A dual-color fluorescent probe 78 was developed for monitoring of Zn2+ and H+ fluctuations in mice brains with depression-like behavior (Scheme 34).49 2,2′-Dipicolylamine (DPA) was used as the recognition site for Zn2+ and naphthalene fluorescein was used as the H+ reporter. Fluorescence of the coumarin quenched by DPA, upon binding to Zn2+, was restored via a blocked PeT process. Probe 78 displays a linear relationship between the fluorescence emission intensity at 460 nm and the concentration of Zn2+ (0–10 μM). When the pH changed from 6.5 to 9, the emission intensity of probe 78 at 680 nm gradually increased linearly. While the well-separated fluorescence emission wavelength facilitated the simultaneous detection of Zn2+ and H+. Probe 78 was used to image Zn2+ and H+ fluctuations in the brains of mice with depression. The mice with depression-like behavior exhibited decreased Zn2+-related TP blue fluorescence (excitation at 800 nm) and H+-related red fluorescence, indicating a reduction in Zn2+ and pH levels in mice with depression-like behavior.
Disruption of mitochondrial dynamics including fusion, fission and movement, mitophagy are involved in many diseases, such as Huntington's, Alzheimer's, and Parkinson's diseases. To uncover the O2˙− and pH flux changes within mitochondrial dysfunction of living cells, a TP probe 79 was developed for the simultaneous monitoring of O2˙− and pH (Scheme 35).50 With probe 79 a caffeoyl and fluorescein were used as responsive units towards O2˙− and pH, respectively, and the cationic triphenylphosphonium was used as a mitochondria-targeting unit. Under TP excitation at 800 nm, the probe displays distinguishable blue and green fluorescence signals allowing for the simultaneous detection of O2˙− and pH. In the presence of O2˙−, a turn-on blue fluorescence signal at 450 nm was observed under excitation at 800 nm or 400 nm. In addition, when the pH increased from 5.0 to 9.0, a similar increase of green fluorescence at 520 nm was observed upon 488 nm excitation. Drp1 deficiency induced hyperfused mitochondrial accumulations can lead to apoptosis of cancer cells. Drp1-knocked down 4T1 cells displayed enhanced blue and green fluorescence upon excitation at 405 nm and 488 nm, respectively. Moreover, elevated O2˙− and pH levels in mdivi-1 (an inhibitor of Drp1)-pretreated tumor-bearing mice was visualized using TPEF imaging.
The mitochondrion is an effective target for developing PDT agents, since it produces energy for cells and regulates apoptosis. Recently, a mitochondria-targeting agent 83 was developed for PDT in cancer cells, which is co-activatable by GSH and H2O2 (Scheme 36).5180 is resistant to nucleophilic attack by GSH, while the boronic ester can be cleaved by H2O2, followed by a 1,6-benzyl elimination to release unprotected thiol-containing intermediate 81 which is converted into the photosensitizer 82. More importantly, 82 exhibits strong photosensitizer ability for the generation of both 1O2/O2˙−. The δ of 82 was determined to be 41 ± 4 GM at 800 nm in methanol. The triphenylphosphine ligand of probe 83 was used to target mitochondria. Significant turn-on fluorescence emission was observed when both GSH and H2O2 were present, but just a high concentration of GSH (ca. 10 mM) in mitochondria did not induce a response by 83 prior to oxidation by H2O2. Moreover, HeLa cells treated with H2O2 and 83 were killed upon irradiation using a TP laser (800 nm), and were imaged using co-staining with calcein-AM and propidium iodide.
 | ||
Scheme 36 (a) Schematic presentation for the GSH/H2O2 mediated activation of 80. (b) Synthetic routes of probe 83. (c) Fluorescence response of 80 (10 μM) to GSH (1 mM) and H2O2 (1 mM) in 20% acetonitrile –PBS buffer (pH 7.4) at 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, λex = 405 nm. Inset: Fluorescence enhancement (I/I0) over time. Reproduced with permission from ref. 51. Copyright (2020) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. °C, λex = 405 nm. Inset: Fluorescence enhancement (I/I0) over time. Reproduced with permission from ref. 51. Copyright (2020) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Conclusions and outlook
This review describes research carried out within numerous research groups over the past five years towards the development of TP probes and the use of two photons in disease therapy. The probes developed should provide a better understanding of the various biologically-related species and their functions within physiology and pathology. We describe the development of dual-responsive sensors and theranostic systems based on TPEF, which we believe will pave a way in providing a deeper understanding of the complex role that different species play in disease-specific processes.When starting the process of designing an efficient TP probe, it is vital not to forget important lessons derived from the development of successful OP probes. As such it is important to remember the most important fluorescent mechanisms used for the development of OP probes including ESIPT, FRET, PeT and ICT, still remain to be explored in the area of TP probes. Importantly, most current TP fluorophores have relatively short emission wavelengths though they can be excited using NIR wavelengths. As such we encourage researchers to start developing TP fluorophores with NIR emissions which are needed in order to minimize emission light scattering and achieve deeper penetration during imaging. In addition, TP fluorophores with large δ values should be designed and developed to reduce photodamage of samples due to the decreased need for high-power pulsed lasers. In addition to turn-on TP probes it is vital to develop ratiometric TP probes which can facilitate quantitative measurements in biomedical applications due to internal calibration. In particular, we believe that FRET based systems could provide a potential strategy for the design of ratiometric TP probes. Then, in order to gain insight into the complex nature of biological systems, the development of dual/multiple-responsive probes and multiple-color fluorophores is essential to develop TP systems capable of accurate and early disease diagnosis. Last but not least, factors including water solubility, Φf, photostability cytotoxicity and cell permeability currently well known in the design of OP probes should be considered when developing efficient TP probes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. W. wishes to thank China Scholarship Council and The University of Bath for supporting his PhD in the UK. T. D. J. would like to thank the EPSRC and The University of Bath for funding. T. D. J. wishes to thank the Royal Society for a Wolfson Research Merit Award and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University for support (2020ZD01). J. L., P. L. and B. T. thank the support by the National Natural Science Foundation of China (22074083, 91753111, and 21927811), the Key Research and Development Program of Shandong Province (2018YFJH0502), National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2017ZX09301030004).References
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS.
- H. M. Kim and B. R. Cho, Chem. Rev., 2015, 115, 5014–5055 CrossRef CAS.
- W. Denk, J. H. Strickler and W. W. Webb, Science, 1990, 248, 73–76 CrossRef CAS.
- M. Göppert-Mayer, Ann. Phys., 1931, 401, 273–294 CrossRef.
- W. Kaiser and C. G. B. Garrett, Phys. Rev. Lett., 1961, 7, 229–231 CrossRef CAS.
- Y. Fu and N. S. Finney, RSC Adv., 2018, 8, 29051–29061 RSC.
- B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3–40 CrossRef CAS.
- L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X.-P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Chem. Soc. Rev., 2020, 49, 5110–5139 RSC.
- A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Chem. Soc. Rev., 2018, 47, 8842–8880 RSC.
- S. Xu, H.-W. Liu, L. Chen, J. Yuan, Y. Liu, L. Teng, S.-Y. Huan, L. Yuan, X.-B. Zhang and W. Tan, J. Am. Chem. Soc., 2020, 142, 2129–2133 CrossRef CAS.
- H.-J. Lee, C.-W. Cho, H. Seo, S. Singha, Y. W. Jun, K.-H. Lee, Y. Jung, K.-T. Kim, S. Park, S. C. Bae and K. H. Ahn, Chem. Commun., 2016, 52, 124–127 RSC.
- W. Li, B. Fang, M. Jin and Y. Tian, Anal. Chem., 2017, 89, 2553–2560 CrossRef CAS.
- L. Zhou, Q. Wang, X.-B. Zhang and W. Tan, Anal. Chem., 2015, 87, 4503–4507 CrossRef CAS.
- L. Wu, A. C. Sedgwick, X. Sun, S. D. Bull, X.-P. He and T. D. James, Acc. Chem. Res., 2019, 52, 2582–2597 CrossRef CAS.
- L. C. Murfin, M. Weber, S. J. Park, W. T. Kim, C. M. Lopez-Alled, C. L. McMullin, F. Pradaux-Caggiano, C. L. Lyall, G. Kociok-Köhn, J. Wenk, S. D. Bull, J. Yoon, H. M. Kim, T. D. James and S. E. Lewis, J. Am. Chem. Soc., 2019, 141, 19389–19396 CrossRef CAS.
- D. Cheng, Y. Pan, L. Wang, Z. Zeng, L. Yuan, X. Zhang and Y.-T. Chang, J. Am. Chem. Soc., 2017, 139, 285–292 CrossRef CAS.
- X. Li, R.-R. Tao, L.-J. Hong, J. Cheng, Q. Jiang, Y.-M. Lu, M.-H. Liao, W.-F. Ye, N.-N. Lu, F. Han, Y.-Z. Hu and Y.-H. Hu, J. Am. Chem. Soc., 2015, 137, 12296–12303 CrossRef CAS.
- L. Yuan, L. Wang, B. K. Agrawalla, S.-J. Park, H. Zhu, B. Sivaraman, J. Peng, Q.-H. Xu and Y.-T. Chang, J. Am. Chem. Soc., 2015, 137, 5930–5938 CrossRef CAS.
- Z. Mao, M. Ye, W. Hu, X. Ye, Y. Wang, H. Zhang, C. Li and Z. Liu, Chem. Sci., 2018, 9, 6035–6040 RSC.
- Y. L. Pak, S. J. Park, D. Wu, B. Cheon, H. M. Kim, J. Bouffard and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 1567–1571 CrossRef CAS.
- Q. Xu, C. H. Heo, G. Kim, H. W. Lee, H. M. Kim and J. Yoon, Angew. Chem., Int. Ed., 2015, 54, 4890–4894 CrossRef CAS.
- U. Tamima, M. Santra, C. W. Song, Y. J. Reo and K. H. Ahn, Anal. Chem., 2019, 91, 10779–10785 CrossRef CAS.
- L. Chen, M. K. Cho, D. Wu, H. M. Kim and J. Yoon, Anal. Chem., 2019, 91, 14691–14696 CrossRef CAS.
- H. Li, Q. Yao, F. Xu, N. Xu, X. Ma, J. Fan, S. Long, J. Du, J. Wang and X. Peng, Anal. Chem., 2018, 90, 4641–4648 CrossRef CAS.
- Z. Mao, W. Feng, Z. Li, L. Zeng, W. Lv and Z. Liu, Chem. Sci., 2016, 7, 5230–5235 RSC.
- Y. Li, Y. Shu, M. Liang, X. Xie, X. Jiao, X. Wang and B. Tang, Angew. Chem., Int. Ed., 2018, 57, 12415–12419 CrossRef CAS.
- B. Dong, X. Song, X. Kong, C. Wang, Y. Tang, Y. Liu and W. Lin, Adv. Mater., 2016, 28, 8755–8759 CrossRef CAS.
- H. Zhang, R. Liu, J. Liu, L. Li, P. Wang, S. Q. Yao, Z. Xu and H. Sun, Chem. Sci., 2016, 7, 256–260 RSC.
- Y. Zhang, X. Wang, X. Bai, P. Li, D. Su, W. Zhang, W. Zhang and B. Tang, Anal. Chem., 2019, 91, 8591–8594 CrossRef CAS.
- K. Liu, X. Kong, Y. Ma and W. Lin, Angew. Chem., Int. Ed., 2017, 56, 13489–13492 CrossRef CAS.
- C. de la Torre, A. Toscani, C. Marín-Hernández, J. A. Robson, M. C. Terencio, A. J. P. White, M. J. Alcaraz, J. D. E. T. Wilton-Ely, R. Martínez-Máñez and F. Sancenón, J. Am. Chem. Soc., 2017, 139, 18484–18487 CrossRef.
- Y. W. Jun, T. Wang, S. Hwang, D. Kim, D. Ma, K. H. Kim, S. Kim, J. Jung and K. H. Ahn, Angew. Chem., Int. Ed., 2018, 57, 10142–10147 CrossRef CAS.
- X. Wang, P. Li, Q. Ding, C. Wu, W. Zhang and B. Tang, Angew. Chem., Int. Ed., 2019, 58, 4674–4678 CrossRef CAS.
- H.-W. Liu, L. Chen, C. Xu, Z. Li, H. Zhang, X.-B. Zhang and W. Tan, Chem. Soc. Rev., 2018, 47, 7140–7180 RSC.
- J. Zhang, X. Chai, X.-P. He, H.-J. Kim, J. Yoon and H. Tian, Chem. Soc. Rev., 2019, 48, 683–722 RSC.
- W. Li, S. Yin, X. Gong, W. Xu, R. Yang, Y. Wan, L. Yuan and X. Zhang, Chem. Commun., 2020, 56, 1349–1352 RSC.
- L. Ge, Z. Liu and Y. Tian, Chem. Sci., 2020, 11, 2215–2224 RSC.
- X. Wang, P. Li, Q. Ding, C. Wu, W. Zhang and B. Tang, J. Am. Chem. Soc., 2019, 141, 2061–2068 CrossRef CAS.
- A. R. Sarkar, C. H. Heo, L. Xu, H. W. Lee, H. Y. Si, J. W. Byun and H. M. Kim, Chem. Sci., 2016, 7, 766–773 RSC.
- J. Yin, M. Peng and W. Lin, Chem. Commun., 2019, 55, 11063–11066 RSC.
- P. Wang and S. Wu, J. Photochem. Photobiol., A, 1995, 86, 109–113 CrossRef CAS.
- J. Yin, M. Peng and W. Lin, Anal. Chem., 2019, 91, 8415–8421 CrossRef CAS.
- X. Li, M. Jiang, J. W. Y. Lam, B. Z. Tang and J. Y. Qu, J. Am. Chem. Soc., 2017, 139, 17022–17030 CrossRef CAS.
- J.-W. Choi, S. T. Hong, M. S. Kim, K. C. Paik, M. S. Han and B. R. Cho, Anal. Chem., 2019, 91, 6669–6674 CrossRef CAS.
- P. Zhang, C. K. C. Chiu, H. Huang, Y. P. Y. Lam, A. Habtemariam, T. Malcomson, M. J. Paterson, G. J. Clarkson, P. B. O'Connor, H. Chao and P. J. Sadler, Angew. Chem., Int. Ed., 2017, 56, 14898–14902 CrossRef CAS.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228–2248 RSC.
- X. Jiao, Y. Xiao, Y. Li, M. Liang, X. Xie, X. Wang and B. Tang, Anal. Chem., 2018, 90, 7510–7516 CrossRef CAS.
- X. Wang, X. Bai, D. Su, Y. Zhang, P. Li, S. Lu, Y. Gong, W. Zhang and B. Tang, Anal. Chem., 2020, 92, 4101–4107 CrossRef CAS.
- W. Zhang, X. Wang, P. Li, H. Xiao, W. Zhang, H. Wang and B. Tang, Anal. Chem., 2017, 89, 6840–6845 CrossRef CAS.
- J. Sun, K. Du, J. Diao, X. Cai, F. Feng and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 12122–12128 CrossRef CAS.
Footnote |
| † Equal contribution. |
| This journal is © The Royal Society of Chemistry 2021 |