Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Printed aerogels: chemistry, processing, and applications
Junzong
Feng
 ab,
Bao-Lian
Su
ab,
Bao-Lian
Su
 cd,
Hesheng
Xia
cd,
Hesheng
Xia
 e,
Shanyu
Zhao
e,
Shanyu
Zhao
 f,
Chao
Gao
g,
Lukai
Wang
f,
Chao
Gao
g,
Lukai
Wang
 b,
Osarenkhoe
Ogbeide
a,
Jian
Feng
b,
Osarenkhoe
Ogbeide
a,
Jian
Feng
 b and
Tawfique
Hasan
b and
Tawfique
Hasan
 *a
*a
aCambridge Graphene Centre, University of Cambridge, Cambridge CB3 0FA, UK. E-mail: th270@cam.ac.uk
bScience and Technology on Advanced Ceramic Fibers and Composites Laboratory, National University of Defense Technology, Changsha 410073, Hunan, P. R. China
cState Key Laboratory of Advanced Technology for Materials Synthesis and Processing & School of materials science and engineering, Wuhan University of Technology, Loushi Road 122, Wuhan 430070, P. R. China
dLaboratory of Inorganic Materials Chemistry, Department of Chemistry, University of Namur, 61 rue de Bruxelles, B-5000 Namur, Belgium
eState Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Chengdu 610065, P. R. China
fLaboratory for Building Energy Materials and Components, Swiss Federal Laboratories for Materials Science and Technology, Empa, CH-8600, Dübendorf, Switzerland
gMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, 38 Zheda Road, 310027, Hangzhou, P. R. China
First published on 1st February 2021
Abstract
As an extraordinarily lightweight and porous functional nanomaterial family, aerogels have attracted considerable interest in academia and industry in recent decades. Despite the application scopes, the modest mechanical durability of aerogels makes their processing and operation challenging, in particular, for situations demanding intricate physical structures. “Bottom-up” additive manufacturing technology has the potential to address this drawback. Indeed, since the first report of 3D printed aerogels in 2015, a new interdisciplinary research area combining aerogel and printing technology has emerged to push the boundaries of structure and performance, further broadening their application scope. This review summarizes the state-of-the-art of printed aerogels and presents a comprehensive view of their developments in the past 5 years, and highlights the key near- and mid-term challenges.
1. Introduction
Aerogels typify low-density bulk materials with nanostructured solid skeletons and open pores. Their skeletal and pore structure can be regulated at the nanoscale to achieve a number of extraordinary physical properties, including ultra-low density and thermal conductivity, ultra-high specific surface area and ultra-strong adsorption capacity. These properties make aerogels an ideal material family for a wide range of potential applications, including energy storage,1 catalyst supports,2 sensors,3 chemical adsorption,4 thermal insulation,5 biomedical,6 and shock absorption,7 thereby promoting the development of industries such as environmental protection,4 construction,8 chemical,9 aerospace,10 transportation,1 artificial intelligence,11 and medicine.6 Because of the controllability of the nanostructures, excellent physical properties, and potential application scopes, aerogels have attracted increasing interest from both the academic and industrial communities in the past two decades.Additive manufacturing, based on a variety of printing technologies, is considered as one of the pillars of the third industrial revolution.12,13 It is a “bottom-up” manufacturing process, as opposed to the traditional “top-down” subtractive strategy.14 Based on the artificial intelligence-aided design of patterns or three-dimensional (3D) models, it uses software and numerical control systems to stack metals, polymers, inorganics, and even medical-grade biomaterials, layer by layer, to create solid objects through a variety of technologies including inkjet, extrusion, sintering, melting, photonic curing, and spraying.15 It is not limited by mould manufacturing or processing technology, which simplifies process cycles and reduces material consumption. The technology also allows high manufacturing accuracy to create complex and fine patterns or structures. These greatly streamline the concept-to-design-to-manufacturing process, shortening the production cycle. As a powerful complement to traditional subtractive manufacturing methods, additive manufacturing therefore offers new possibilities in solving traditional manufacturing challenges.16,17
The first research combining printing technology and aerogel to obtain 3D printed aerogel scaffolds was reported in 2015.18 Since then, the field of printed aerogels has flourished and now represents an interdisciplinary research area intersecting aerogel and additive manufacturing technologies. There are two simple reasons behind this rapid development. First, the aerogel preparation method is always a liquid-to-solid sol–gel process. It can be easily adapted to ink formulation suitable for printing technologies based on liquid jetting or slurry extrusion. Second, application-specific shaping of aerogels is highly challenging as their low density and strength curb traditional post-machining options. The “bottom-up” printing technology family readily offers an alternative solution to create intricate shapes without complicated post-processing. In addition, current generation formulation and printing technologies can be tailored to design and manufacture macroporous structures. Indeed, printed aerogel scaffolds with designed macropores have been demonstrated to achieve better mass transfer performance than non-printed aerogels,19–21 which is critical to applications such as energy storage and tissue engineering.19–23
Based on the current landscape of aerogels using additive manufacturing technologies, the goal of this review is to provide a comprehensive overview of this rapidly growing research area, discussing the state-of-the-art science, technology and applications. In this review, we first briefly introduce aerogels and functional printing technology to the readers. Next, in line with the current understanding and standard practices in the field, we discuss the ink formulation and pertinent aerogel chemistry, printing considerations and processes, and various drying and post-treatment strategies. We then focus on the aerogel properties and their relevance to a wide range of application scopes. Finally, we provide a summary of research perspectives, critical hurdles and research opportunities. We hope that this review will help researchers in academia and industry to comprehensively understand the recent technical advancements and challenges in the field, and inspire new ideas and research directions.
2. Aerogels
The word ‘aerogel’ originates from ‘gel’, a 3D solid network with liquid filled pores, prepared by a sol–gel method. When the liquid in the pores of the gel is replaced by gas or air, the resulting light-weight nano-framework structure is called an aerogel.24 ‘Aerogel’ represents a broad description of the structure, and does not include restrictions on material compositions and preparation processes. The International Union of Pure and Applied Chemistry (IUPAC) defines a gel as a “non-fluid colloidal network or polymer network that is expanded throughout its whole volume by a fluid” and an aerogel as a “gel comprised of a microporous solid in which the dispersed phase is a gas. Microporous silica, microporous glass, and zeolites are common examples of aerogels”.25 But this definition is really too broad and may sometimes cause confusion because it is inappropriately defined as microporous (<2 nm), and without specific numerical restrictions to the porosity (the volume fraction of voids over a total volume). In their Review, Pierre A. C. et al. suggested that aerogels are dried gels with a very high relative pore volume (>90%).26 Ziegler C. et al. suggested that an aerogel is a solid with meso- (2–50 nm) and macropores (>50 nm) with diameters up to a few hundred nanometres and a porosity of more than 95% in which the dispersed phase is a gas.27 At present, the industrial community generally considers aerogels as “an open-celled, mesoporous, solid foam that is composed of a network of interconnected nanostructures and exhibits a porosity of no less than 50%”.28 However, with the rapid development of porous structures based on nanomaterials, there is also a recent trend of describing low-density bulk materials composed of uniformly dispersed one-dimensional (1D) nanofibres or two-dimensional (2D) nanosheets (such as graphene nanosheets or metal nanowires) as aerogels.29 These 1D and 2D material aerogels display highly porous networks with pore sizes in the range of a few hundred nanometres to a few micrometres, which is much larger than mesoporous structures. It can be seen that the above definitions focus on pore sizes (micro-, meso-, macro-pore) and porosities (e.g., 50, 90, 95%) but ignore the definition from the solid skeleton structure. Moreover, there is no uniform standard applied to these definitions, which may lead to overly strict or broad scope.Therefore, to clarify the scope of this review, we feel that it is necessary to briefly discuss the definition of aerogels, from both a structural and porosity standpoint. We recommend that materials that meet both the following statements can be called aerogels: (i) solids are uniformly dispersed in the whole structure and are connected through nanostructures (nanoparticles, nanofibres, or nanosheets with at least one dimension being <100 nanometres). (ii) The pores formed between the solids are open, filled with gas, and the porosity is no less than 50%. This definition covers all the examples discussed in this review.
With this above definition in mind, in this section, we introduce typical aerogel types, their various physical properties, and their traditional preparation processes and shaping methods.
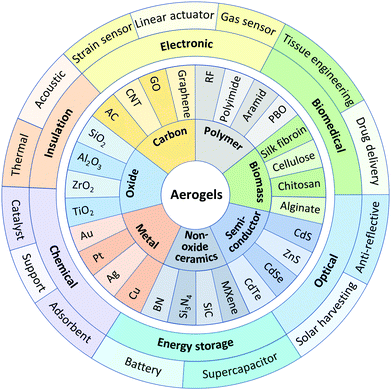
2.1 Types of aerogels and their properties
A wide range of materials can be used to fabricate aerogels. Examples of typical aerogels and their functional applications are shown in Fig. 1. Aerogels can be classified based on their chemical composition into oxides, carbon, polymers, biomass, metals, non-oxide ceramics, semiconductors, etc. Oxide aerogels such as silica (SiO2),30,31 alumina (Al2O3),32 zirconia (ZrO2),33 and titania (TiO2),34 are usually prepared by a sol–gel method using metal alkoxides, nitrates, or chlorides as raw precursors.35 Since oxides typically have high melting points and are not oxidized in ambient environments, their aerogels usually have good thermal stability. Carbon-based aerogels mainly include amorphous carbon (AC),36 as well as various carbon nanostructures such as carbon nanotubes (CNTs),37–39 graphene,40 and graphene oxide (GO).41 AC aerogels are generally formed by carbonization of organic polymer aerogels, while CNTs, graphene, and GO aerogels are often prepared via colloidal methods using these nanomaterials as the skeleton building blocks. In general, carbon-based aerogels are electrically conductive and highly resistant to acid or alkali corrosion. Organic polymer aerogels such as resorcinol-formaldehyde (RF),42,43 polyimide,44 aramid,45 and polybenzoxazine (PBO),46 are usually obtained via polymerization of monomers. Some organic polymer aerogels such as polyimide and aramid exhibit good flexibility. Polymer aerogels, however, generally have poor thermal stability. Exploiting this, certain polymer aerogels such as RF are used as precursors for carbon aerogels via pyrolysis in inert atmospheres. Biomass aerogels such as silk fibroin,47 nanocellulose,48 chitosan,49 and alginate50 are usually synthesized from biomass extracted from creatures and natural materials such as silkworm cocoons, wood and sea shells as raw materials and through cross-linking reactions. Biomass aerogels generally have very good biodegradability and biocompatibility. Metal aerogels such as gold (Au),51 platinum (Pt), silver (Ag), and copper (Cu) are usually made from their metal nitrates or chlorides via sol–gel chemical gelation with sodium borohydride as the reducing agent.52,53 These aerogels typically exhibit high catalytic activity and durability. Some of the metal aerogels with nanowire-structures exhibit good electrical conductivity and mechanical flexibility.54,55 Non-oxide ceramic aerogels include nitrides (e.g., boron nitride (BN),5,56 silicon nitride (Si3N4),57), and carbides (e.g., silicon carbide (SiC)58), and MXene59 (a 2D carbide and nitride family of transition metals (M), where X represents carbon or nitrogen) aerogels also belong to this group. Non-oxide ceramic aerogels are always prepared by an aerogel template method via a gas-solid phase reaction at high temperature,5,57,58 or by a colloidal dispersion method using the corresponding nanomaterials directly as the building blocks.59 Thanks to the excellent thermal stability of non-oxide ceramics, these aerogels can usually be used at higher temperatures than oxide aerogels. Semiconductor aerogels such as cadmium sulfide (CdS),60 zinc sulfide (ZnS),61 cadmium selenide (CdSe),62,63 and cadmium telluride (CdTe),64 are usually fabricated by a colloidal dispersion method, followed by gelation via destabilization or freeze casting. These aerogels retain the semiconducting and luminescent nature of the original materials, while their highly porous structure is beneficial for improving photocatalysis, photovoltaic and sensing performances. Depending on the composition and microstructure, different types of aerogels may exhibit different properties, specifically suited to target applications.2.2 Traditional preparation processes of aerogels
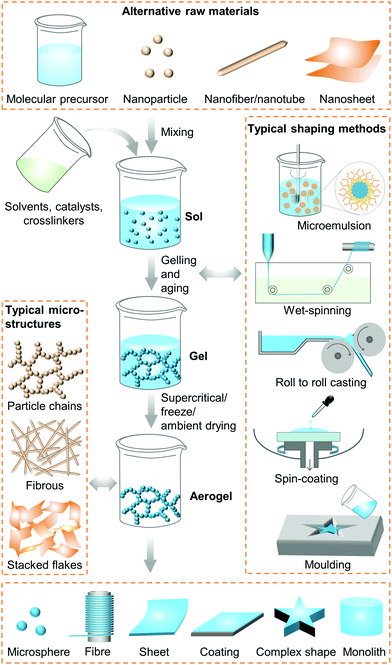
Based on the reaction environment where the nano-skeleton is created, the preparation methods of aerogels can be categorised into three major types. The first is a solid phase route that can be used to prepare perovskite oxide aerogels by solid-state gelation using carbon as the support.65 This method was first proposed in 2019. The second is the gas phase route with only a few reported examples, such as CNT aerogels by CVD66–68 or atomic layer deposition,69 and BN aerogels by template-assisted CVD.5,70 The third is the traditional and the most popular liquid phase route; Fig. 2. This involves creation and aging of sol-gels in solutions, followed by specific drying methods such as supercritical, freeze, or ambient drying. We note that for the case of nanoparticle-, nanofibre- or nanosheet-based aerogels, the primary sol step may not always be necessary.27,71 The most important and essential processes in the preparation of aerogels are the formation of a gel and its subsequent drying.The sol–gel method is generally used to prepare inorganic oxide,72–74 organic,6,75–77 and chalcogenide78,79 aerogels, and always uses organic alkoxides,73 metal chlorides,74,80 nitrates,81 small organic molecules,75,76 biopolymers,6 or prepolymerized molecules82 as the molecular precursor. Depending on the precursors used, the chemical reactions and gelation mechanisms vary.
The sol–gel chemistry of these aerogels has been comprehensively summarized in ref. 26 and 83. A simplified and generalised discussion on this process can be made for the most common SiO2 aerogels,84 where silicon alkoxides,85,86 sodium silicate,87 or prepolymerized siloxanes82 are used as molecular precursors. Among them, silicon alkoxides Si(OR)4 such as tetraethylorthosilicate (TEOS, Si(OCH2CH3)4) and tetramethylorthosilicate (TMOS, Si(OCH3)4) are the most widely used.85,86 This is because the hydrolysis and condensation rates for these materials are slow enough to be independently controlled, allowing for adjustment of the aerogel microstructure to suit different applications.88 The Si(OR)4 precursors are usually dissolved in solvents containing water and alcohol, and are hydrolyzed by acid (e.g., hydrochloric acid, nitric acid) or base (e.g., ammonium hydroxide, ammonium chloride) catalysts to form the molecular structure of Si–OH. The hydroxyl groups are then dehydrated and condensed to form a bridged Si–O–Si structure. This reaction process gradually forms nanoparticles in the solvent, with typical sizes in the range of a few nanometres to tens of nanometres. In this state, the suspension with nanoparticles dispersed in solution is called a sol. As the reaction progresses, the nanoparticles cross-link with each other to form a 3D networked structure with solvent in the pores, and the sol loses its fluidity and becomes a gel, like a viscoelastic solid. This transition from sol to gel is commonly called gelation. The sol–gel process is essentially a nucleation and growth process.84
The colloidal dispersion method skips the molecular step and directly uses nano-scale materials as the aerogel building blocks.27,71 A wide variety of starting materials have been investigated, including 0D oxide,89 semiconductor chalcogenide,61 and metal nanoparticles,62,90,91 1- and quasi-1-D metal nanowires,92 CNTs,37 and cellulose nanofibres,93 as well as 2D graphene sheets,94 and MXene.59 The dispersions of these nanomaterials can be broadly considered as colloids, either through steric or charge-stabilisation. Their specific gelation strategies have been summarized in detail in ref. 71. The 3D network structure from these materials is formed by physical or chemical cross-linking. The colloidal dispersion method is not limited to sol–gel chemical reactions, greatly expanding the types of aerogels that can be produced. During the preparation of the gel, the gelation time and density, particle size, pore size and other performance parameters of the final aerogels can also be controlled by adjusting a range of process parameters, such as the concentration of the starting nanomaterial, the type and concentration of catalysts, as well as processing temperature, making it a highly adaptable fabrication approach.84
Aerogels were first invented through supercritical drying in the 1930s.24 Since then, supercritical drying has remained the most popular drying method for aerogels. Indeed the majority of today's industrial production of aerogel thermal insulations still uses CO2 supercritical drying.95 After the 1990s, to reduce the cost and increase safety, extensive research investigations have been carried out to introduce ambient drying after solvent exchange, network strengthening, and surface modification.96–98 The biggest disadvantage of ambient drying is the large linear shrinkage of the aerogels, which can reach up to 15–25%.96 It may even be higher for the case of ultrahigh-porosity gels due to the weak strength of the gel skeleton.
The earliest freeze drying for aerogels appeared in 1989.99 However, when it was first introduced to dry oxide aerogels from alkoxide precursors, formation of cracks usually led to aerogel powders because of the volume expansion during the liquid-ice phase change.74 The use of freeze drying therefore remained very limited. However, since the advent of aerogels made from 2D materials such as graphene,100 freeze-drying has become the most commonly used drying method over the last 10 years.101 This is especially the case for aerogels with ultralow densities below 5 mg cm−3,52,54,102–109 since the flexibility of these 2D material skeletons can endure the expansion of liquid-ice phase change.
3. Functional printing
Among the existing manufacturing pathways, functional printing represents a group of technologies that can turn powders, liquids, or slurries into patterns or 3D shapes, even with complex geometries. Indeed, functional printing has brought about great innovations in the field of material processing technologies. In particular, workpieces or devices can be printed based on 3D printing, and can be constructed on demand, even with design modifications promptly and inexpensively.3.1 Printing technologies for additive manufacturing
Printing is an additive manufacturing process that exemplifies cost-effective production technologies suitable for either industrial scale-up or prototyping. This has propelled functional printing to the forefront of the materials manufacturing revolution. Compared to the traditional material removal-cutting and assembly processing mode, printing is a “bottom-up” manufacturing method by material accumulation, from scratch. It is significantly more adaptable and potentially less expensive compared to the traditional moulding method, and offers the production of structurally complex parts that were not previously possible.The traditional functional printing technologies are borrowed from graphics printing, and include inkjet, screen, and roll-to-roll gravure and flexographic printing.120,121 Among these, inkjet printing is a non-contact, mask-less, fast, and low-cost method with excellent design flexibility, and is the most popular choice for laboratory-scale or high-resolution additive manufacturing for functional devices.122,123 The drawbacks of inkjet printing are the throughput, strict ink formulation requirements and also, the concentration of functional materials in the resultant inks. These can be addressed by a wide range of modern 3D printing technologies, such as binder jetting, directed energy deposition, material extrusion and jetting, powder bed fusion, sheet lamination, and vat photopolymerisation.17,124–127 Although the resolution of 3D printing is often lower when compared to inkjet printing, the latter has seen rapid development over the past few years. Indeed, highly versatile printing strategies have already been demonstrated for metals,128–135 polymers,136–143 ceramics,144–148 inorganics,149,150 semiconductors,151 and biomaterials,152,153 as they can be directly or indirectly printed to form application-specific patterns or 3D shapes.
3.2 Products and applications enabled by functional printing
Fig. 3 shows a set of examples that can be fabricated by functional printing technologies. Combining with computer-aided design, they can be used to manufacture complex patterns,131,132 electrodes,149 hierarchical scaffolds,140,144 intricate load-bearing parts for heavy construction equipment like excavators,129 concrete walls,150 human implants,130,152 tissue frameworks,153 and even soft robots in a single print without assembly.139 Such versatility directly leads to products that can be applied in diverse areas such as sensors,138,151 energy storage,149,154 surgery,130,152 tissue engineering,153,155 electronics,135 mechanical parts,139,156 environmental remediation,140 automobiles and aerospace,133 and even in building construction.150,157 In the majority of cases, the printed products do not require any subsequent processing before use. Therefore, the design to manufacturing, and to subsequent application time is greatly reduced.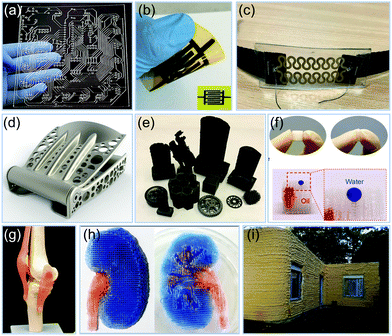 | ||
| Fig. 3 Examples of objects fabricated by functional printing technology. (a) Extrusion printed electronics interconnect pattern. Reproduced from ref. 135. Published by Springer Nature. (b) Screen printed microsupercapacitor. Reproduced with permission from ref. 154. Copyright 2019 WILEY-VCH. (c) Inkjet printed respiration rate sensor. Adapted from ref. 132. Published by MDPI. (d) Ti-6Al-4V seat buckle produced using direct metal laser sintering technology. Reproduced with permission from ref. 156. Copyright 2015 Elsevier Inc. (e) Polymer-derived ceramic parts fabricated via 3D printing. Reproduced with permission from ref. 148. Copyright 2018 WILEY-VCH. (f) Polydimethylsiloxane (PDMS) sponge for 3D water/oil separation by 3D printed template. Reproduced with permission from ref. 140. Copyright 2018 Elsevier B.V. (g) 3D printed menisci in a knee model. Adapted with permission from ref. 152. Copyright 2017 American Chemical Society. (h) 3D printed kidney structure composed of heterogeneous tissue parts. Reproduced from ref. 155. Published by Springer Nature. (i) 3D printed foam and concrete for construction. Adapted with permission from ref. 150. Copyright 2019 Elsevier B.V. | ||
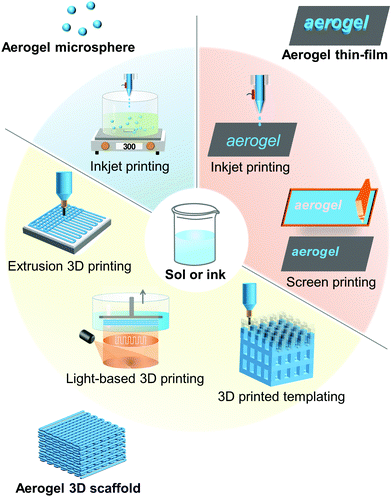
The required conditions for printing aerogels are relatively mild and usually do not involve very high temperatures. Therefore, the most commonly used printing technologies for aerogels are inkjet and screen printing for patterned thin-films, and photopolymerization, extrusion-based printing, and 3D printed templates for 3D scaffolds or complex shapes. In addition, inkjet printing can also be used to fabricate aerogel microspheres.
3.3 Benefits of printing compared to other aerogel shaping methods
Regardless of the fabrication techniques, aerogels are usually fragile due to their low solid content and nano-skeleton architecture. Compared to traditional aerogel shaping methods, the “bottom-up” additive manufacturing of functional printing methods for aerogels has some unique advantages. Table 1 lists a comparison between the reported aerogel printing techniques and the traditional aerogel shaping methods to create microspheres, fibres, supported and self-supported thin-films, complex geometries, and 3D scaffolds.| Shape of aerogel | Traditional shaping method | Printing method | Advantages via printing |
|---|---|---|---|
| Microsphere | Emulsion-gelation, Stöber, spraying, supercritical fluid | Inkjet printing with spinning | Simple control in diameter and good repeatability |
| Fibre | Spinning | Extrusion 3D printing | No requirements on fibre's flexibility, can directly form a device |
| Supported thin-film | Spin-, dip-coating, spray | Inkjet, screen printing | Easy access to patterns |
| Self-supported thin-film | Casting | 3D printing | Easy access to patterns |
| Complex part | Moulding | 3D printing | Without mould |
| 3D scaffold | — | Extrusion 3D printing or sacrificed 3D printed mould | Complex structure easily available, good controllability of macrostructure size |
Aerogel microspheres or microparticles can traditionally be prepared using several schemes, including emulsion-gelation,111,158 Stöber,159,160 spraying or electrospraying,110,161,162 and supercritical fluid163,164 methods. The diameters of the microspheres in these strategies are primarily controlled by either chemical reaction, applied voltage, or stirring and air flow rate. However, in practice, the batch-to-batch reproducibility in this approach is very challenging. There are various ways to limit these variations in the synthesis conditions. For example, if rotary stirring is used to synthesize aerogel particles, the size of the droplets deposited into the emulsion can be controlled through the precision nozzle of the inkjet printing, allowing synthesis of highly monodisperse microspheres.
On the other hand, traditional freeze- or wet-spinning technologies can be exploited to fabricate aerogel fibres.165 These methods can usually be used for flexible aerogels only, such as those made with graphene,112–114 GO,166 cellulose,167–170 and polyimide.165 Filaments can be fabricated using a 3D extrusion method, which is somewhat similar to the traditional spinning techniques. However, the added advantage here is that the filaments can be directly printed onto the substrate to form a device,171 without worrying about the mechanical robustness of the aerogel, or its transfer and integration on to the target substrate.
Substrate supported thin-films of aerogels can be prepared by the traditional spin coating,115–117 dip coating,172,173 and spray174,175 methods. However, these methods can hardly be used to create patterns without masks or stencils. Inkjet or screen printing can offer significant advantages here. First, they would allow creation of aerogels with specific patterns. Second, the thickness of the resultant coating could be better adjusted through ink formulation and/or printing parameters.
Self-supported aerogel thin-films can be conventionally prepared using roll-to-roll casting methods.118 The obtained aerogel film can then be additionally processed into the desired patterns, for example, by cutting. On the other hand, 3D printing offers the distinct advantage of direct patterning of self-supported thin-films, without the loss of any material.
Complex aerogel parts can be traditionally fabricated by pouring the sol into a mould,119 which, after gelation, is supercritically dried. The bulk aerogel may additionally require mechanical cutting or milling. This process, involving multiple steps, can only create structures with limited complexity. In contrast, 3D printing does not require a mould and can create significantly more complex structures. Additionally, due to the use of a computer aided approach, it is straightforward to modify the design of the aerogel shapes, significantly simplifying prototyping or production of specialised parts.
Similarly, 3D aerogel scaffolds cannot be realized through traditional shaping methods without using templates. On the other hand, from digital scaffold designs, 3D printing methods such as stereolithography (SLA),22 extrusion,176 or sacrificial 3D printed mould177 can be used to create 3D aerogel scaffolds with fine structures. As before, the adaptability in the design and adjustment of the channels, pore types, and porosity offers an added advantage. These can in turn be used to adjust the physicochemical properties of the scaffolds such as adsorption, mass transfer, and electrical conductivity for specific applications.
Fragile aerogels are very challenging to shape using traditional moulding and processing methods, especially in the micron scale. In the majority of cases, the functional printing methods can enable designing and shaping of aerogels on tens to hundreds of micron scale, addressing a critical shortcoming of the traditional sol–gel and mechanical processing methods. The controllability of the nanostructures through aerogel chemistry, and the macrostructural construction offered by various printing methods enables adaptability and precise control over aerogel designs from nanometre to centimetre scale. This significantly shortens, simplifies and improves the processes from basic chemicals or nanomaterials to useful device components, while reducing the production cost. In the next section, we discuss the processing and chemistry of printed aerogels in terms of ink preparation, drying, and post-treatment.
4. Printing technology for aerogels
Printed aerogels were first reported in 2015,18 realized by extrusion-based 3D printing of GO-based inks. Since then, printing of a wide variety of aerogels, including of graphene,178 SiO2,179 RF polymer,180 carbon,19 cellulose,181 metal,54 semiconductor,182 and g-C3N4183 have been reported. The technologies that have thus far been used to print aerogels include inkjet for microspheres,184 inkjet and screen for substrate-bound thin-films,182,185 and photopolymerization (light-based printing),22 extrusion186 and sacrificial template177 for 3D form factors. Simplified printing mechanisms of these technologies for aerogels are summarized in Fig. 4.The fabrication processes of the printed aerogels involve several steps: mixing of raw materials into highly consistent fluids with appropriate rheology/physical characteristics (i.e., formulation of functional ink), extrusion/printing, drying, and additional post curing processes such as pyrolysis or etching. Among them, the last two steps can be borrowed from conventional aerogel preparation processes, and therefore, are not particularly challenging to implement. Conversely, the first two steps, ink formulation and printing, face a range of technical difficulties and challenges. This is primarily due to the requirements of the specific printing technologies used, which can affect printing quality and feature resolution.
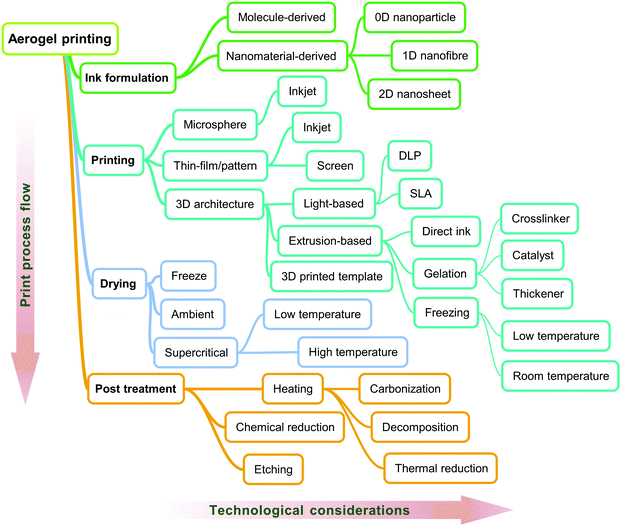
Based on the processes involved in printing of aerogels, the relevant technical characteristics and classifications are summarized in Fig. 5. In the following, we introduce them and related research progress in accordance with the sequence in Fig. 5.
4.1 Ink preparation processes and chemistry for printed aerogels
The word “ink” usually refers to a liquid or paste with suitable viscosity and rheological properties, specifically for inkjet, screen, certain types of 3D extrusion, or photopolymerization printers. Depending on the printing technique, a functional ink is usually composed of the active material, additives (e.g., binders and surfactants), and solvents. The matching of ink rheological properties specific to printing methods determines their printability.187 These rheological properties as well as the microstructure and final characteristics of the printed aerogels can be controlled by the process parameters in the ink preparation process, such as the ink composition, raw material morphology, and formulation. Based on different gelation principles in inks, we divide the discussion in this section into molecule-derived and nanomaterial-derived pathways. The chemical reaction processes follow the traditional sol–gel method which we will not repeat here. For this, we refer interested readers to ref. 188. In this review, we will mainly focus on the chemical reaction limitations caused by the printing requirements, as well as raw materials and reactions that may be specifically tailored for printing.Shear stresses in fluids may show a linear or nonlinear relationship with the increase in shear rates, that is, the viscosity may remain constant or vary with increasing shear rate. Based on this, fluids can be divided into Newtonian and non-Newtonian types.190Fig. 6a shows the variation of shear stress with shear rate for various fluids.191 As shown, the shear stress of a Newtonian fluid increases linearly with increasing shear rate (thus the viscosity remains constant, Fig. 6b).191 Newtonian fluids can therefore flow under a small external force. Liquids close to this ideal state, such as water, solvents, and mineral oil, are generally considered to be Newtonian fluids for practical calculations at standard conditions.
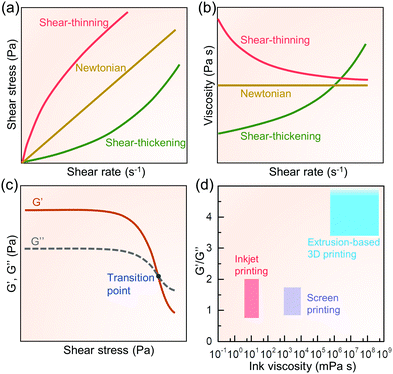 | ||
| Fig. 6 Newtonian and non-Newtonian fluids with (a) shear stress and (b) viscosity as a function of shear rate. (c) Typical storage (G′) and loss (G′′) modulus of shear-thinning non-Newtonian fluid as a function of shear stress. (d) Expected ink viscosity and G′/G′′ ranges suitable for different printing methods, data collected from ref. 120, 192, 201 and 203. | ||
The viscosity of non-Newtonian fluids is variable, and based on their decrease or increase with increasing shear rate, they can be classified into shear-thinning (pseudoplastic) and shear-thickening (dilatant) fluids (Fig. 6b).190 Shear-thinning behaviour of fluids is very important for the printing process. It ensures smooth flow of ink under a driving force during printing. Additionally, these fluids can maintain a certain level of self-support to retain the printed shape or pattern after the driving force is withdrawn. Polymer solutions, melts, and greases are generally shear-thinning fluids. In contrast to the shear-thinning fluids, the viscosity of the shear-thickening fluid increases with increasing shear rate. When at rest, it can gradually restore the state where it ‘flows’ easily. Aqueous solutions of potassium silicate and gum arabic are typical examples of shear-thickening fluids.
In addition to viscosity, viscoelasticity also plays an important role in the printability of inks.192 The storage modulus (G′) and loss modulus (G′′) characterize the ink's elasticity and viscous properties, respectively. The storage modulus, also known as elastic modulus, refers to the amount of elastic (reversible) energy stored by deformation and reflects the elasticity of a material. The loss modulus, also known as the viscous modulus, refers to the amount of energy lost due to viscous deformation (irreversible). The ratio of the plateau values of the storage modulus to the loss modulus (G′/G′′) reflects the viscoelasticity of the material. When the storage modulus is much greater than the loss modulus (G′ ≫ G′′), the material mainly undergoes elastic deformation, indicating that the substance is closer to a solid state. On the other hand, when G′ ≪ G′′, the material mainly undergoes viscous deformation, indicating that the substance is closer to a liquid state. When the storage and loss modulus are comparable, the material is in a semi-solid state. A gel is a typical semi-solid substance. The stress where the storage modulus begins to decrease with increasing shear stress, is called yield stress; Fig. 6c. This indicates the applied stress required to make the substance flow with irreversible plastic deformation.193 The point at G′ = G′′ is the transition point from solid-like to liquid-like state. For high viscosity printing such as screen and extrusion-based techniques, there needs to be a balance between G′ and G′′.192 A high-enough magnitude of G′ and G′′ (the value at the plateau region in Fig. 6c),194 and a suitable yield stress193,195 are important for effective printability of the ink in these cases.
In inkjet printing, viscosity and surface tension greatly affects the formation of ink droplets. The ejection behaviour of such droplets from printing nozzle can be characterized by Z, which is the inverse of the dimensionless Ohnesorge (Oh) number:196
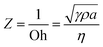 | (1) |
Among them, η, γ, and ρ are the viscosity, surface tension, and density of the ink, respectively, and a is the diameter of the nozzle. Through numerical simulation, it has been proposed that 1 < Z < 10 is considered suitable for inkjet printing for stable drop formation.197 However, although this proposed range facilitates reliable jetting, it is not very strict. For example, it has been shown through experiments that 1 < Z < 14 is sufficient to satisfy drop-on-demand (where individual droplets are ejected through the nozzles for each electrical actuating signal) inkjet printing.198–200 When Z < 1, the fluid is too viscous. This may cause the tail of the droplets to be elongated and drawn, or even failure of droplet formation. When Z > 14, the viscosity is so small that the droplets are easily broken, forming satellite droplets (secondary droplets), or sputtering after coming in contact with the substrate, reducing the print reliability and resolution. Compared to screen printing, the inks used for inkjet printing have a much lower viscosity, generally in the range of 4 to 30 mPa s,120,201 which is similar to that of water at 20 °C (1.0016 mPa s).202 The G′/G′′ for inkjet printing is typically in the range of 0.74 to 2 to allow for reliable printing (Fig. 6d).203
Screen printing uses a squeegee to scrape the ink from one end toward the other of the screen mesh, forcing the ink to penetrate through the pre-made holes to form a graphic printed on the substrate.192 A suitably high viscosity with shear thinning behaviour is needed to retain the transferred patterns on the substrate before the inks are ‘cured’. Too low viscosity may reduce the clarity of the printed graphics, while inks with too high viscosity with poor shear thinning property are difficult to transfer through the patterned openings in the screen mesh.204 The viscosity and the G′/G′′ of screen printing inks are generally in the range of 1 × 103 to 1 × 104 mPa s120,201 and 0.8 to 1.7 (Fig. 6d),192 respectively.
3D printable inks must have high viscosity to ensure that the printed structure is self-supporting, i.e., the extruded filaments do not collapse, or disperse after the applied shear force is withdrawn. In addition, as above, the inks must also have shear-thinning rheological properties. When the ink flows through the extrusion nozzle (usually with a diameter of tens to hundreds of microns) under pressure, the viscosity must be thinned to be low enough to prevent nozzle clogging and ensure continuous filament extrusion. Generally, the viscosities and the G′/G′′ of 3D printing inks are in the range of 6 × 105 to 5 × 108 mPa s and >3.4 (Fig. 6d),187,203 respectively. Besides high viscosity and high G′/G′′, high modulus in the plateau region and high yield stress are also required for extrusion-based 3D printing to prevent collapse of the printed object due to gravity and the pressure imposed by the extrusion nozzle.195
Light curing-based printing, whether it is a top-down system of SLA or a bottom-up projection system of digital light processing (DLP), requires the light-curing resins to quickly flow back into the gap formed after the workpiece is moved for the construction and light-curing of the subsequent layer. Hence, the requirement for the viscosities of the light curing resins is as low as possible to effectively and quickly backfill.142
| Composition of aerogel | Shape of product | Main raw material | Printing method | Drying method | Application | Ref. |
|---|---|---|---|---|---|---|
| SiO2 | Microsphere | TEOS, isopropyl alcohol | Inkjet | Ambient | — | 184 |
| SiO2 | 3D complex object | Dynasylan 40, SiO2 aerogel particle, PPGNH, 1-pentanol | 3D extrusion | Supercritical | Thermal insulation | 179 |
| SiO2–silk fibroin | 3D grid | Silkworm cocoons, TMOS, trimethoxysilane | 3D extrusion | Supercritical | Thermal insulation | 207 |
| SiO2–polymer | Letter pattern | TEOS, TMSM, ethanol–water | Photo-cure | Supercritical | — | 205 and 208 |
| SiO2–polymer | 3D complex structure | TEOS, APTMS, 2,4,6-trimethylbenzoyl-diphenyl-phosphineoxide, ethanol–water | 3D DLP | Ambient | Optical devices | 206 |
| Alginate | Microsphere | Alginic acid sodium salt, salbutamol sulphate, calcium chloride dihydrate | Inkjet | Supercritical | Drug delivery | 209 |
| RF | 3D microlattice | Resorcinol, formaldehyde, Na2CO3, water | 3D extrusion | Supercritical | Cell support | 180 |
| Carbon | 3D scaffold | Resorcinol, formaldehyde, hydroxypropyl methyl cellulose, fumed SiO2 | 3D extrusion | Supercritical | Electrode | 19 |
As discussed above, inkjet printing generally requires the ink to have low viscosity for reliable ejection of ink droplets from the nozzle. On the other hand, 3D extrusion-based printing requires the ink to have a relatively high viscosity to ensure that the printed filaments are self-supporting to retain their structure before curing.191
The traditional molecule-derived sols always have a low viscosity and can be directly used for inkjet printing without any special modification of the sol formulation. For example, SiO2 sol, prepared by a traditional two-step acid–base process, has directly been used for inkjet printing to prepare SiO2 aerogel microspheres.184 Similarly, alginate gel microspheres have been successfully demonstrated by inkjet printing aqueous solution of alginic acid sodium salt into calcium chloride (CaCl2) aqueous solution. The concentrations that are feasible for inkjet printing are 0.25–0.35 wt%. If it is >0.4 wt%, the viscosity is too high for printing as it results in high Reynolds and Ohnesorge number of the fluid, or even clogged nozzles.209
On the other hand, it is challenging to create 3D structures through printing from molecule-derived SiO2 or RF inks. This is because the inks themselves are not viscous enough to be self-supporting.180 Two strategies have been proposed to overcome this. The first is to add certain additives to increase the viscosity to retain the shape of the printed structure after 3D extrusion. In one method, SiO2 aerogel particles and poly(propylene glycol) bis(2-aminopropyl ether) (PPGNH) have been used to increase the viscosity of ethyl silicate-derived SiO2 sol.179 In another example, silk fibroin has been introduced into traditional SiO2 sol to increase the viscosity and create crosslinks between SiO2 and silk fibroin (Fig. 7a).207 Hydroxypropyl methyl cellulose and fumed SiO2 have also been added into traditional RF sols to achieve increased viscosity and the required shear-thinning behaviour.19
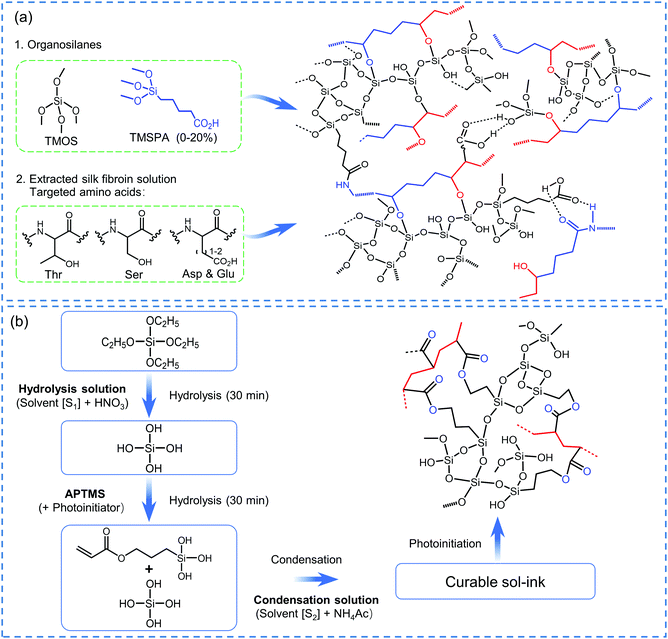 | ||
| Fig. 7 Schematic diagram of the SiO2-based ink preparation process and associated chemical reaction. (a) SiO2–silk fibroin sol for 3D extrusion printing. Adapted with permission from ref. 207. Copyright 2018 American Chemical Society. (b) SiO2-photocurable polymer sol for 3D digital light printing. Adapted with permission from ref. 206. Copyright 2018 American Chemical Society. | ||
The second strategy involves the introduction of a photocurable polymer (usually a chemical containing one or more acrylate groups),210 which cures under light and induces a rapid gelation. The gel can then be printed using a digital light processing printer.206 It is also necessary to ensure that the silicon source can be chemically connected with the photocurable polymer such that the final network structure contains a Si–O structure and not just the photo-cured resin. Common photocurable polymers that have been used for this are trimethoxysilylpropyl methacrylate (TMSM)205,208 and 3-acryloxypropyl trimethoxysilane (APTMS).206 An example preparation of the photocurable ink and the relevant chemical reaction is shown in Fig. 7b. Condensation can occur between Si–OH groups while the free-radical polymerization can take place between acrylate groups under the irradiation of light, resulting in a 3D cross-linked structure to form a gel containing SiO2.
There have only been a handful of demonstrations of printed aerogels from the traditional molecule-derived gel ink method. A large number of oxide (such as Al2O3, ZrO2) and organic (such as polyimide, melamine) aerogels that can be prepared by traditional sol–gel methods remain unexplored. This is primarily because the sol–gel processes for these aerogels are more challenging to control, for example, due to quick precipitation or gelation of sols, leaving little manoeuvrability for stable enough ink formulation for printing. However, the traditional molecule-derived sol–gel method is still the most effective way to tune the pore sizes in the nanoscale. Therefore, significant research opportunities in combining 3D printing and molecule-derived sol-gels exist.
0D Nanoparticle-based inks. Table 3 shows typical examples of 0D nanoparticle-based inks for printed aerogels. These materials are mostly used for inkjet and screen printing. The reported 0D structures include SiO2 aerogels,211 milled carbon aerogels,212,213 magnesium oxide (MgO)-TiO2 aerogels,185 and various oxide (zinc oxide (ZnO), cupric oxide, indium oxide, TiO2, and tin oxide)214 and semiconducting (CdSe/CdS) nanoparticles.182
| Composition of product | Shape of product | Main raw material | Printing method | Drying method | Application | Ref. |
|---|---|---|---|---|---|---|
| SiO2 | Pattern | SiO2 aerogel, DISPERBYK-2012, PVP-k30 binder, 1,5-pentadiol humectant, water | Inkjet | Ambient | Thermal insulation | 211 |
| PEDOT:PSS-carbon | Layer | PEDOT:PSS ink, activated carbon aerogel | Inkjet | Ambient | Actuators | 212 and 213 |
| MgO–TiO2 | Layer | MgO-TiO2 aerogel, EVA, p-xylene | Screen | Ambient | Solar cell | 185 |
| Metal oxides | Layer | Metal oxide nanoparticles, ethylene glycol, water | Inkjet | Ambient | Electronics | 214 |
| CdSe/CdS | Layer | Cadmium oxide, tri-noctylphosphine oxide, tri-noctylphosphine, H2O2 | Inkjet | Ambient or supercritical | Photo-electrochemical | 182 |
When aerogels are used for ink formulation, to ensure forming a homogeneous mixture, they are usually dispersed by ultrasonic agitation with the aid of surfactants or polymers in the ink solvent.185,211,214
In addition to the above examples, for SLA 3D printing, amorphous SiO2 nanopowders have also been demonstrated as the SiO2 source to synthesize porous SiO2 after thermal treatment at 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.215
°C.215
1D nanofibre-based inks. Table 4 shows selected examples of 1D nanofibre-based inks for printed aerogels. These are primarily based on CNTs,216 metal,54,217 and oxide217 nanowires, nanocellulose,218–222 and aramid fibres.223 The key printing methods used are 3D extrusion printing176,181 or SLA.22
| Composition of product | Shape of product | Main raw material | Printing method | Drying method | Application | Ref. |
|---|---|---|---|---|---|---|
| CNC | 3D complex structure | CNC, Kymene, water | 3D extrusion | Freeze | Tissue engineering | 181 |
| CNF | 3D complex structure | CNF, Kymene, water | 3D extrusion | Freeze | Tissue engineering | 176 |
| CNF/alginate | 3D construct | CNF, alginate, CaCl2, water | 3D extrusion | Freeze | Wound dressing | 225 |
| CNF/alginate | 3D grid | CNF, alginate, CaCl2, water | 3D extrusion | Ambient | Energy storage, humidity sensors | 226 |
| CNF | 3D complex structure | CNF, water, CaCl2 bath | 3D extrusion | Freeze | — | 227 |
| CNF | 3D scaffold | CNF, water | 3D extrusion | Freeze | Tissue engineering | 230 |
| Carbon-Li, Carbon-LiFePO4 | 3D anode/cathode | Wood pulp, LiFePO4 powder | 3D extrusion | Freeze | Lithium metal battery | 218 |
| Cellulose | 3D scaffold | Pulp, NMMO | 3D extrusion | Freeze | — | 219 |
| CNC | 3D complex structure | CNC, xyloglucan | 3D extrusion | Freeze | Thermal insulators | 220 |
| CNC/SA/gelatin | 3D scaffold | Cellulose, sodium alginate, gelatin | 3D extrusion | Freeze | Tissue regeneration | 221 |
| CNC/Au/PAM | 3D pattern | CNC, Au, AAm, MBAA, water | 3D extrusion | Supercritical | Plasmonic metamaterial | 229 |
| CNF | 3D scaffold | CNF, water | 3D extrusion | Freeze | TENG | 228 |
| Aramid | 3D grid | Aramid, KOH, DMSO | 3D extrusion | Supercritical | Thermoresponsive architecture | 223 |
| Ag | 3D architecture | Ag nanowire | Inkjet | Freeze | Electronic | 54 |
| Ag, MnO2, ZnO | 3D self-support architecture | AgNWs, GO, Ti3C2Tx, ZONWs, MONWs, CNT | 3D extrusion | Freeze | UV sensor, micro-supercapacitor | 217 |
| PEGDA/CNF | 3D scaffold | PEGDA, eosin Y, CNF | SLA | Freeze | Tissue engineering | 22 |
| CNT/GO/CNF | 3D all-in-one architecture | CNF, GO, CNT, water | 3D extrusion | Freeze | Evaporator | 216 |
Nanocelluloses are a family of nanomaterials derived from wood pulp.224 To date, cellulose nanofibrils (CNFs)22,176,216,218,222,225–228 and cellulose nanocrystals (CNCs)181,220–222,229 have been used to formulate inks for printed aerogels. CNF gels have high viscosity and shear-thinning properties as they are bonded together by strong hydrogen bonds.218,228 After mixing with water, the gels can be used directly as a 3D printable ink. After printing, placing the CNF gels in a CaCl2 bath can promote cross-linking and increase mechanical strength.227 CNCs are smaller in length compared to CNFs and as a result, have inferior self-supporting properties. Therefore, cross-linking additives are usually required for CNC inks to improve the printability and stability after printing.181 A common cross-linking additive for this is polyamide-epichlorohydrin (Kymene).176,181 Aramid nanofibre-based inks have been developed by using commercial Kevlar fibres as a raw material, dissolving in potassium hydroxide (KOH)-contained dimethyl sulfoxide (DMSO), and then forming a dispersion of aramid nanofibres.45,223 This ink with 2 wt% concentration exhibits enough viscosity and storage modulus for 3D extrusion printing to fabricate aramid aerogels.223
Other 1D structures that have been investigated for printing aerogels include CNTs,216 as well as Ag,54,217 MnO2,217 and ZnO nanowires.217 The challenge in printing these aerogels is that they usually have fewer chemically active sites compared to those in amorphous nanoparticles, making it often challenging to formulate inks with suitable properties. To address this, a small amount of 2D materials could be used as cross-linking agents to form bridges between nanowires and improve the overall viscosity and printability of the nanowire inks.217 Such cross-linking ability of GO or MXene with the nanowires has already been confirmed. Indeed, a small amount (0.67 vol%) of GO can greatly improve the printability of Ag, MnO2, and ZnO nanowire inks.217
2D nanosheet-based inks. One of the most exciting developments in the printed aerogels field was the demonstration of 3D printing of 2D GO inks and their annealing to form graphene aerogel scaffolds in 2015.18 Since then, the published literature reports related to the printing of 2D nanosheet-based aerogels have significantly outnumbered those on 0D- or 1D-nanomaterial-based aerogels. Table 5 shows selected examples of 2D nanosheet-based inks for printed aerogels. The 2D materials that have thus far been used include graphene,178,231,232 GO,18,41,109,233–238 and g-C3N4 nanosheets,183 the former two being the most common examples. The printing methods involved are primarily extrusion-based.171,186,231,233,239,240 In addition, drop-on-demand inkjet,109,238,241,242 projection micro-SLA (PmSLA) 3D printing,243,244 and 3D printed templates245,246 have also been used. The other constituents of the ink formulations for 2D nanosheets are solvents, and additives such as surfactants, crosslinkers, or thickeners, in which, water and organic solvents are primarily used. To increase the viscosity and printability, hydroxypropyl methylcellulose (HPMC),21,237 fumed SiO2 powder,18,20 RF sol,18 methyl cellulose,247 ascorbic acid,107 polyaniline,248 sodium alginate,249etc. have been used as cross-linkers, gelators, or thickeners. However, the surfactants or thickeners used in the inks usually need subsequent removal through post-treatment processes such as washing, acid etching or pyrolysis.
| Composition of product | Shape of product | Main raw material | Printing method | Drying method | Application | Ref. |
|---|---|---|---|---|---|---|
| PDMS/graphene | 3D sensor | GO, PDMS, PVA, THF | 3D extrusion | Freeze | Tactile sensor | 240 |
| Graphene | 3D microlattice | GO, fumed SiO2, resorcinol, formaldehyde | 3D extrusion | Supercritical | — | 18 |
| Graphene | 3D microlattice | Graphene, GO, fumed SiO2, RF | 3D extrusion | Supercritical | Supercapacitor | 20 |
| Graphene | 3D truss architecture | GO, water | Inkjet | Freeze | Strain sensor | 109 |
| Graphene | 3D monolith | GO, water | 3D extrusion | Freeze | — | 256 |
| Graphene | Pattern | GO, water | 3D extrusion | Freeze | Strain sensor | 171 |
| Graphene | 3D lattice | GO, EDA, water | Inkjet | Freeze | Sodium metal anode | 238 |
| Graphene | 3D pattern | Phenol, camphene, graphene, rGO, CNT | 3D extrusion | Sublimation | Supercapacitors | 178 |
| Graphene/Cu | 3D electrode | Cu, graphene, Pluronic F127 | 3D extrusion | Freeze | Supercapacitors | 231 |
| Graphene | Pattern | GO, water | 3D extrusion | Freeze | Wearable sensor | 235 |
| PPy-graphene | 3D microlattice | GO, Pluronic F127, PPy | 3D extrusion | Freeze | Supercapacitors | 234 |
| Graphene | 3D scaffold | GO, water | 3D extrusion | Freeze | Pressure sensor | 107 |
| Graphene-carbon | Micro-electrode | Carbon sphere, GO | 3D template | Freeze | Micro-supercapacitor | 246 |
| Graphene | 3D scaffold | Graphite | 3D extrusion | Freeze | — | 257 |
| Graphene-CNT | 3D lattice | GO, CaCl2, CNT | 3D extrusion | Freeze | Strain sensor | 258 |
| Graphene-polymer | 3D lattice | GO, CaCl2, polycaprolactone | 3D extrusion | Freeze | Fuse, micro-oscillator | 236 |
| Graphene | 3D electrode | GO, water | 3D extrusion | Freeze | Supercapacitor | 233 |
| Graphene | 3D web | Graphite, water | 3D extrusion | Freeze | Tactile sensor | 259 |
| MnO2/graphene | 3D scaffold | GO, HPMC | 3D extrusion | Freeze | Supercapacitor | 21 |
| MnO2/graphene | 3D electrode | GO, KMnO4, HCl | Inkjet | Freeze | Supercapacitor | 241 |
| ZnV2O6@Co3V2O8/graphene | 3D microlattice | V2O5, Co(Ac)2·4H2O, Zn(CH3COO)2·6H2O, NH4VO3, GO | 3D extrusion | Vacuum | Supercapacitor | 260 |
| Graphene | 3D scaffold | Graphene, EGB, DBP, PVB, ET | 3D extrusion | Ambient | Sensor | 232 |
| Graphene | 3D lattice | GO, EDA | 3D template | Freeze | Sensor | 243 |
| Graphene | 3D honeycomb | GO, water | 3D template | Freeze | Stretchable display | 245 |
| Graphene-LiFePO4, -Li4Ti5O12 | 3D electrode | GO, LiFePO4, Li4Ti5O12, Al2O3 | 3D extrusion | Freeze | Lithium-ion battery | 261 |
| Polyaniline/r-GO | 3D electrode | Graphite, polyaniline, NMP | 3D extrusion | — | Supercapacitor | 248 |
| Graphene | 3D lattice | LiClO4, propylene carbonate, GO | 3D extrusion | — | Supercapacitor | 262 |
| GO | 3D microlattice | GO, CaCl2 | 3D extrusion | Freeze | Supercapacitor | 41 |
| GO | 3D electrode | Graphite | 3D extrusion | Freeze | Microsupercapacitor | 263 |
| Graphene | 3D complex architecture | Graphite, Ag NPs, CNT, MoS2 | 3D extrusion | Freeze | Microsupercapacitor | 186 |
| Graphene | 3D truss architecture | GO, acrylic photopolymers, BisA-EDMA, PEGDA700 | 3D PmSLA | Supercritical or freeze | Energy storage | 244 |
| Graphene | 3D lattice | GO, HPMC, water | 3D extrusion | Freeze | Supercapacitor | 237 |
| Ni0.33Co0.66S2/graphene | 3D architecture | Sodium alginate, GO, Ni0.33Co0.66(OH)2·xH2O | 3D extrusion | Freeze | Energy storage | 249 |
| MoS2-Graphene | 3D electrode | Ammonium thiomolybdate, GO | Inkjet | Freeze | Sodium-ion battery | 242 |
| Graphene-CeZrLa oxide | 3D catalytic reactors | CeZrLa, CeZrLa/GO nanocomposites | 3D extrusion | Ambient | Nanocatalyst | 247 |
| g-C3N4-sodium alginate | 3D patterned membrane | g-C3N4, Pluronic F127, sodium alginate | 3D extrusion | Supercritical | Solar wastewater remediation | 183 |
| Graphene | 3D scaffold | Graphene, DNa, PEG, PEI, water | 3D extrusion | Ambient | Energy storage | 239 |
| r-GO | 3D framework | GO, Na3V2(PO4)3, water | 3D extrusion | Freeze | Sodium-ion battery | 264 |
It is apparent from Table 5 and the above discussion that the most commonly used 2D material for printed aerogels is graphene. Even though several other 2D materials such as hexagonal BN (h-BN),250 MXene,59,108 and molybdenum disulphide (MoS2) have recently been investigated,29,251,252 there exists a huge opportunity for fundamental research and application development for other 2D material printed aerogels.
4.2 Printing processes
Based on the shape and dimension of the intended macroscopic aerogel objects, printing methods can be grouped into three categories: microsphere, thin-film/pattern, and 3D architecture. This section discusses the ink requirements for these materials, which vary between the printing methods and the technologies used.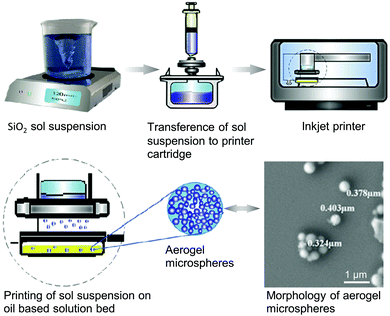 | ||
| Fig. 8 Inkjet printing process of SiO2 aerogel microspheres. Adapted with permission from ref. 184. Copyright 2015 Elsevier B.V. | ||
Compared to the previous emulsion-gelation111,158 or dropwise extrusion40,253–255 methods, inkjet printing of aerogel microspheres holds two advantages. First, it is a widely applicable and versatile technique, allowing precise control over microsphere dimensions. As long as the viscosity and the surface tension of the ink are kept to a suitably low range, inkjet printing can be used reliably. Although printings of only SiO2 and alginate aerogels have thus far been reported, we believe that other aerogel types can also be printed using this method in the near future. Second, due to the nature of the inkjet printing nozzle, the controllability of drop volume ensures small and highly repeatable microsphere diameter. This is not possible using the conventional dropwise extrusion approach.
Inkjet printing. The aerogel microspheres in the above section are very special application examples of the inkjet printing method. The prevalent application of graphics and functional inkjet lies in creating thin-film patterns of pigments or functional materials on a wide variety of substrates, including paper, glass, and silicon wafer.
When inkjet printing sols or inks in a membrane, the gelation process usually occurs in the ambient atmosphere, and is accompanied by a process of volatilization and shrinkage of the sols. Therefore, it is challenging to control the gelation rate of the inks and the structural shape and integrity of the printed aerogels. Thus far, printings of SiO2,211 carbon,213 and semiconductor182 aerogel films or patterns have been demonstrated from 0D nanoparticle-based inks. For example, using an ink formulation with activated carbon aerogel (ACA) nanoparticles, solvent, and conductive poly(3,4-ethylenedioxythiophene) (PEDOT) binders, layers of porous conductive composites have been directly printed on a membrane using a drop-on-demand inkjet printer.213 This particular printing process does not involve a chemical reaction. The use of binder allows for uniform patterning, with good control over the thickness per print repetition.
For sols or inks that are gelled by chemical reactions, it is challenging to keep a low enough and stable viscosity to achieve a suitable Z value for reliable drop on demand inkjet printing. To address this, a gelation approach via inkjet printing has been developed by simultaneously printing a semiconductor CdSe/CdS nanorod ink in one cartridge and a destabilization agent H2O2 in another,182 in a commercial colour inkjet printer; Fig. 9a. When printing, the two inks are mixed with a user-specified ratio, and an oxidation reaction of the surface ligands initiated to assemble the nanoparticle network to form gels. As in ref. 213, the thickness of the printed aerogel patterns can be increased with print repetitions; Fig. 9b. Very importantly, this gelation method can be extended to other aerogels from molecule-derived inks where the mixing of two components is carried out during printing. For example, the traditional two-step process of acid–base catalysed SiO2 gel can be tailored such that the pre-polymerized acid-catalyzed SiO2 sol precursor is loaded in one ink cartridge and the base catalyst in another. This approach can also address concerns on suitable ink viscosities and ink storage.
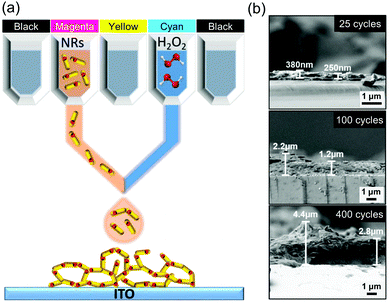 | ||
| Fig. 9 (a) Schematic of inkjet printing of semiconductor nanoparticle-based aerogels on conducting electrodes. (b) SEM images of the cross-section view of different printing cycles. Adapted with permission from ref. 182. Copyright 2019 WILEY-VCH. | ||
Screen printing. Screen printing can be used to create patterns on a wide variety of substrates on a large scale. The thickness of printed film can be controlled in the range of 10 to 100 μm,192 making it useful for printing solar cells,265 fuel cells,192 and electronic devices.191 Using screen printable inks of aerogel microparticles mixed with binders offers a particularly versatile route towards aerogel thin-film. For example, to improve the photovoltaic performance, TiO2 and MgO-doped TiO2 aerogel encapsulated with ethylene vinyl acetate (EVA) coatings can be screen-printed on silicon solar cells (Fig. 10).185 In this work, the aerogel particles with concentrations of 0.2–0.8 mg mL−1 are well distributed as micron-sized blocks within the 9 μm thick coating. A concentration of 15 wt% of EVA, a common binder for screen printable inks, provides the necessary viscosity and strength of the printed film.
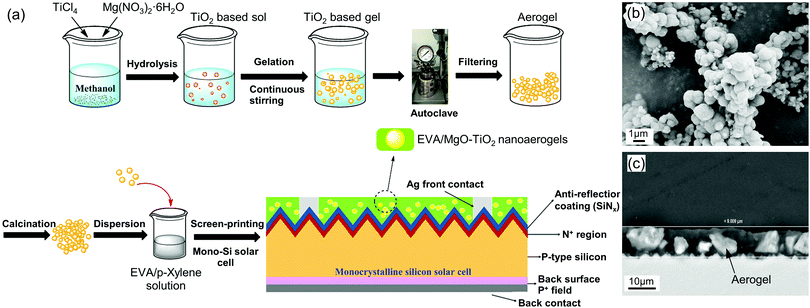 | ||
| Fig. 10 (a) Screen printing process for MgO-TiO2 aerogel particles onto the surface of a mono-Si solar cell. (b) SEM image of MgO-doped TiO2 aerogel. (c) Cross-section SEM image of a solar cell after coating with EVA/MgO-TiO2. Adapted with permission from ref. 185. Copyright 2018 John Wiley & Sons, Ltd. | ||
| Curing or gelation methods | Description | Advantages | Disadvantages |
|---|---|---|---|
| Light-based printing | Using photo-curable resin to cure layer by layer to form a 3D structure by ultraviolet or visible light in the presence of a photoinitiator. Printing aerogel accuracy: 10 μm.244 | Compatible with traditional molecule-derived sols. | Precursor sols must be compatible with photo-curable resins, initiators, etc. |
| Usually short curing time and relatively high efficiency. | The solution needs to be transparent to the light. | ||
| High printing accuracy. | |||
| Extrusion-based printing | Preparing the ink with appropriate viscosity firstly, and form the required 3D structure layer by layer by means of nozzle extrusion. Printing aerogel accuracy: 100–400 μm.19,260 | Various shapes printable. | Requirements for specific ink viscosity. |
| Suitable for non-molecular-based inks. | Need some curing measures. | ||
| Low equipment cost. | Diameter of nozzles always above 100 microns, print accuracy is not fine enough. | ||
| Inefficient for mass printing. | |||
| 3D printed templating | Using 3D printed resin as a template or mould. After injecting sol, the template is removed by dissolution or pyrolysis to form a 3D structure. Aerogel size accuracy: 200 μm.243 | Suitable for traditional molecule-derived sols. | Limited to the fluidity of sol or ink, fine structure is difficult to achieve. |
| Need to remove template. | |||
| No special requirements for sol control. | Limited to low viscosity sols or inks. |
Light-based printing. Based on photopolymerization, there are two main types of light-based 3D printing: SLA and DLP.210
SLA printing technology uses an ultraviolet laser as the light source. A lens system is used to control the scanning of the laser spot on the top surface of the liquid resin. When this layer is photopolymerized by the laser, the sample holding platform drops a certain distance (usually in the range of 25 to 100 μm).210 The cured layer therefore is immersed in liquid resin, and the surface of the liquid resin is scanned and photopolymerized again to create the subsequent layer. This process is repeated until a complete 3D structure is obtained.
DLP printing technology first uses the slicing software to cut the model into slices, and uses a projector to project the sliced image, usually from the bottom of a transparent container into the photocurable resin pool, so that photopolymerization occurs in a very thin resin layer. The sample holding platform then moves up by one layer's distance, and the projector projects the next sliced image and cures the layer. The cycle continues until a 3D printed object is created.
The differences between these two technologies are: The light source of SLA is a laser while for DLP it is a projector. Also, the resin curing of SLA is from spot to line, and then line to layer and eventually to object. On the other hand, for DLP, it is from layer to object.
For printing aerogels, light-based 3D printing methods always use a mixture of photo-curable resin and a pre-polymerized sol as precursors. In the presence of a photoinitiator, light with a specific wavelength and intensity is used to focus on the surface of the mixture, to complete the curing/drawing operation. The main challenge is the development of a photocurable resin that has a (i) short curing time to shorten the printing time, and (ii) low enough viscosity to ensure quick reflowing and filling so that the next layer can be printed.244
For traditional SLA or DLP printing of polymers, the inks are always composed of a photocurable resin (monomers and oligomers) and a photoinitiator. However, light-based 3D printing of molecule-derived oxide aerogels poses additional challenges. Taking SiO2 aerogel as an example, polymerization of the photocurable resin alone without that of photocurable SiO2 precursor can lead to discontinuous chemical structures or even print failure. It is therefore important to introduce a hybrid SiO2 precursor containing siloxane groups (Si–O–R) and acrylate groups, which can support both the hydrolysis-polycondensation reaction and photopolymerization reaction. Two such hybrid precursors, TMSM205,208 and APTMS,206 have been demonstrated for successful printing of SiO2 sols. The typical molecular structure and chemical reaction for APTMS when used as a hybrid precursor are shown in Fig. 7b.206 The light wavelengths that are used to initiate the photopolymerization are either in the visible green (λ = 532 nm),205,208 or UV (λ = 385 or 405 nm) region.244,266 In the first demonstration of printed aerogels by SLA in 2015, 3D letter-shaped aerogels were fabricated using a template with hollow letters as a mask (Fig. 11a–c).205 The light-induced instantaneous gelation spreads and completes within a few seconds in the entire illuminated area. It is suggested that the rapid gelation is due to the polymerization that releases heat and increases the local temperature, accelerating the gelation without any direct heating from the laser beam. In 2018, SiO2 aerogels with a 3D complex shape (Fig. 11d–f) were fabricated through light-based printing206 using a commercial DLP 3D printer. In this demonstration, each gel layer with a thickness of 200–400 μm can be completely cured within 2–5 s.
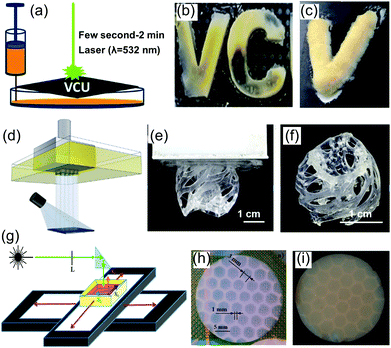 | ||
| Fig. 11 Schematic diagrams and photographs of light-based 3D printing processes for SiO2 aerogels. (a) Schematic diagram of a laser-based printing process with a mask, (b) wet-gel letters, and (c) dried aerogel letter “V”. (a-c) Adapted from ref. 205 with permission from the Royal Society of Chemistry. (d) Schematic diagram of a DLP printing process, (e) wet 3D gel after DLP printing, and (f) dried 3D aerogel structure after solvent removal. (d–f) Reproduced with permission from ref. 206. Copyright 2018 American Chemical Society. (g) Schematic diagram of the setup used to fabricate a honeycomb pattern of cross-linked aerogel, (h) digital photo of the alcogel, and (i) dried honeycomb aerogel composite. (g–i) Adapted with permission from ref. 266. Copyright 2018 American Chemical Society. | ||
In addition to the above, direct production of aerogel-polymer composites can also be realised using light-based printing. By adjusting the catalysts, the siloxane groups of the hybrid precursors can be hydrolysed-condensed first to form a gel. The gel can be then exposed to a laser in a hexagonal pattern to completely penetrate the entire gel, initiating photopolymerization reaction to form a polymer.266 After solvent exchange and supercritical drying, a SiO2 aerogel composite, composed of an in situ formed honeycomb wall of polymer (which is exposed to the laser) and hexagonal aerogel filler can be obtained (Fig. 11g–i).266
Besides molecule-derived SiO2 aerogels, PmSLA has also been used to print 3D GO and polyethylene glycol diacrylate (PEGDA)/CNF aerogels.22,244 For the GO aerogels,244 the authors used GO flakes with photocurable acrylates (bisphenol A ethoxylate dimethacrylate, and PEGDA) and photoinitiator. This reported PmSLA technique (Fig. 12) can achieve a resolution down to 10 μm to form more intricate structures than previously reported methods.244
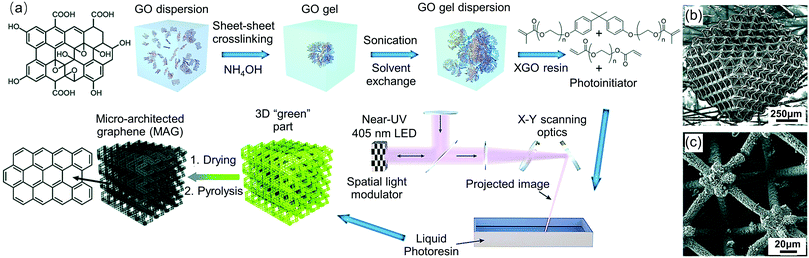 | ||
| Fig. 12 Fabrication of PmSLA printed graphene aerogels using photo-activated GO resin. (a) Schematic illustration of a synthesis process of micro-architectured graphene (MAG). (b) and (c) SEM images of crosslinked GO particle (XGO)-derived MAG. Adapted from ref. 244 with permission from the Royal Society of Chemistry. | ||
Extrusion-based printing. Extrusion-based 3D printing of aerogels can be achieved by automated movement of extrusion nozzles which have a typical inner diameter of several hundred microns. Extrusion can be enabled by pneumatic pressure via air-powered fluid dispensers, with the air pressure and the writing speed being the key parameters for successful 3D printing. For aerogel printing, extrusion-based 3D printing is the most versatile method. It can be widely used in both molecule-derived and nanomaterial-based inks, as long as the rheology of the inks is adjusted to a suitable range.
The resolution of the printed structures largely depends on the movement precision of the nozzle or the sample holding platform. For extrusion-based 3D printing, the main challenges are: (i) to achieve uniform dispersion of each component in the ink,186,233,239,249 (ii) to adjust the rheological properties of the ink not only with shear-thinning behaviour for reliable extrusion, but also with enough viscoelasticity to self-support and retain the shape immediately after printing,18,186,231,232,240,249 and (iii) to control/maintain the nanostructures of the gels through adjustment of chemical compositions on the basis of meeting the rheological requirements for printing.19
Based on the curing method, extrusion-based 3D printing can be divided into three categories: (i) Direct ink writing without curing or additive agent. For this, the ink consists of nano-materials and solvents only. No curing agent or additive is included. This ink usually has a high enough viscosity. (ii) Gelation or thickening with cross-linking or gelating agent which increases the ink viscosity, allowing it to self-support and maintain the printed shape after the deposition. (iii) Curing by freeze-casting. In this strategy, cooling is applied to solidify the printed slurry immediately after deposition, stopping the liquid flow to maintain the printed shape. Selected examples of the print parameters for extrusion-based 3D printed aerogels are summarized in Table 7. Below we discuss these abovementioned extrusion strategies in more detail.
| Curing or gelation methods | Main composition of the inks | Concentration mg mL−1 | Viscosity Pa s | Substrate | Printing environment | Nozzle diameters μm | Moving speed mm s−1 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Direct ink writing without curing or additives | GO + water | 5–25 | — | — | Air | 200 | 3.5 | 235 |
| GO + water | 17.5–50 | 2 × 102–2 × 103 | Glass | Air | 108–210 | 1–8 | 263 | |
| Graphene + water | 80–230 | 8 × 103 | — | Air | 200–600 | — | 257 | |
| GO + water | 15 | — | PET | Air | 200 | — | 259 | |
| GO + water | 20 | — | PET | Air | 200 | — | 171 | |
| Large GO + water | 20 | 1.4 × 104 | — | Air | 400 | 10 | 256 | |
| CNT/GO, GO/CNF + water | 180, 90 | 3 × 103 | — | Air | 305 | — | 216 | |
| CNF + water | 5–21 | 1 × 103–2 × 103 | Plastic | Air | 410–840 | 5–12 | 230 | |
| CNF + water, CaCl2 bath | 20 | 2.7 × 105 | Glass | Air | 300 | 5 | 227 | |
| Cross-linking or gelating agent or thickening | SiO2 sol, aerogel particle, PPGNH + 1-pentanol | — | 1 × 103–2 × 105 | Glass | Air | 100–1190 | 12–18.4 | 179 |
| SiO2-SF sol + water | 112, 126 | — | Silicone | Air | 838 | 10 | 207 | |
| RF, Na2CO3 + water | 164 | 6 × 104 | — | Air | 410 | 30–150 | 180 | |
| RF, cellulose, SiO2 + water | 349 | 2 × 104 | Glass | Isooctane | 100–400 | 3 | 19 | |
| CNC, Kymene, + water | 118–300 | — | — | Air | 200–500 | 2 | 181 | |
| Ag nanowire, GO + water | 187.5 | 8 × 104 | PET | Air | 110–500 | 4–10 | 217 | |
| GO, fumed SiO2 + water | 156.7 | 2.3 × 103 | Silicon | Isooctane | 250 | — | 18 | |
| Polyaniline, GO + NMP/water | 17.6–20.9 | 6 × 102–3 × 103 | Polystyrene | Air | 400–600 | — | 248 | |
| T-CNF, Kymene + water | 28.6 | 6 × 102 | — | Air | 800 | 10 | 176 | |
| CNF, alginate, CaCl2 + water | 10–14 | 5 × 104–3 × 105 | Glass | Air | 580 | 2 | 225 | |
| CNF, alginate, CaCl2, water | 19.5 | — | — | Air | 410 | — | 226 | |
| GO, EDA + water | 10 | — | — | Air | — | Inkjet | 238 | |
| GO, CaCl2 + water | 20.1 | 1 × 105 | Glass | Air | 100–400 | 4–10 | 41 | |
| GO, CaCl2 + water | 20 | — | Glass | Air | 250 | 4–6 | 236 | |
| CNT/GO, CaCl2 + water | 10 | 8 × 104 | Glass | Air | 250 | 4–6 | 258 | |
| GO, ascorbic acid + water | — | 7 × 103 | Glass | Air | 400 | 4–20 | 107 | |
| GO, GNP, SiO2, RF + water | 284.6 | 2.4 × 103 | Silicon | Isooctane | 250 | — | 20 | |
| GO, HPMC + water | 90 | — | Glass | Air | 400 | 3 | 21 | |
| GO, HPMC + water | 90 | — | Glass | Air | 400 | 5 | 237 | |
| GNP, EGB, DBP, PVB + ethanol | 200 | 1 × 103 | — | Air | 300–500 | 5–15 | 232 | |
| GO, HMPC, Pluronic F127, (NH4)2CO3 + water | 40 | 1 × 103 | — | Air | 250 | — | 234 | |
| CeZrLa/GO, methyl cellulose + water | — | — | — | Air | 400–800 | 33 | 247 | |
| GO, NCOH, SA + water | 50 | 4 × 104 | — | Air | — | 5 | 249 | |
| Graphene, Pluronic F127, Cu + water | 25–60 | — | Graphite | Air | 100–1000 | 6–12 | 231 | |
| Graphene, DNa, PEG, PEI + water | 366 | 5 × 103 | Al2O3 | Air | 400 | — | 239 | |
| CNF, Cellulose + water | 112.5 | 2 × 106–6 × 106 | PET | Air | 840 | — | 228 | |
| CNC, Au, AAm, MBAA + water | 254 | — | Glass | Air | 500 | 1.6 | 229 | |
| g-C3N4, Au, SA + water | 90 | 3 × 104 | Glass | Glycerol–CaCl2 | 50–80 | 3–6 | 183 | |
| Freezing | Graphene, rGO, CNT + Phenol or camphene | 2–100 | — | — | Air | 159 | — | 178 |
| Cellulose, NMMO + water | 50 | — | — | Air | 413 | — | 219 | |
| CNC, xyloglucan + water | 40 | 30–2000 | Cu plate | −3 °C to 1 °C | 838 | 3 | 220 | |
| Aramid, KOH + DMSO | 20 | 1 × 102–4 × 102 | Cold plate | −10 °C | 330 | 14 | 223 | |
| Ag nanowire, PVA + water | 10 | — | Cold plate | −30 °C | 120 | Inkjet | 54 | |
| GO + water | 10 | — | Cold plate | −25 °C | — | Inkjet | 109 | |
| GO + water | — | — | Cold plate | −25 °C | — | Inkjet | 241 | |
| ATM, GO + water | 46 | — | Nickel foam | −30 °C | — | Inkjet | 242 |
(i) For direct ink writing without curing or additive agent, it is possible to reach the desired rheological properties through direct mixing of nanomaterials with solvents. This is typically achieved by exploiting the hydrogen bonding interaction on the surface of the nanomaterials. This ink can be printed directly in the air at room temperature. The deposited material can support itself and retain the printed shape without additional curing measures (Fig. 13). Using a single or multiple nanomaterial, such as graphene,257,259 GO-,216,235,256,263 and CNF-based,227,230 and a mix of 1D nanofibre and 2D nanosheet materials, inks have been formulated without any additives. These inks have been demonstrated to be directly 3D-printable for aerogel fabrication.
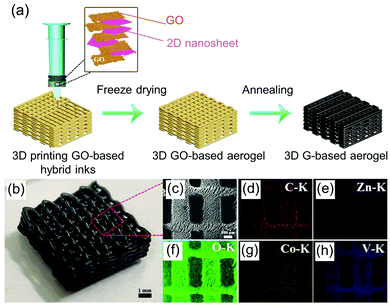 | ||
| Fig. 13 (a) Schematic illustration of 3D printing of a graphene-based aerogel electrode. (b) Photographs of as-prepared G/ZnV2O6@Co3V2O8 aerogel. (c) SEM image. (d–h) Corresponding EDS mapping images. Adapted with permission from ref. 260. Copyright 2019 WILEY-VCH. | ||
The printability of these inks largely depends on the solid content which governs their rheological properties. For GO aqueous inks from exfoliated layers, <5 mg mL−1 concentration is generally unsuitable for 3D extrusion printing due to low viscosity.18 Experimentally, the demonstrated concentration range of such dispersions to be printable is in the range of 5 to 50 mg mL−1.235,256,259,263 To avoid the use of a large amount of solvent or surfactants prevalent in the conventional liquid phase exfoliation method of 2D materials,267–269 and maintain the stability of high-concentration graphene inks against sedimentation, an alkaline solution with a pH of 14 was used.257 This increased the viscosity of the slurry by two orders of magnitude compared to that with a pH of 7. Using this strategy, the authors could maintain a slurry concentration of up to 50 mg mL−1 for a prolonged period.257
The obvious advantage of this additive-free ink is that it does not require any subsequent impurity removal process. However, the disadvantage that comes with this approach is that the viscosity of the ink is primarily determined by the concentration of the nanomaterials. This limits the manoeuvrability with material concentration and performance adjustment of the resultant structure, such as the density, mechanical strength, and electrical or thermal conductivity.
(ii) Ink viscosity can be better adjusted through gelation or thickening with other cross-linking or gelating agents. This adaptability allows the printing of a wider range of aerogel types, in particular, those that are difficult to extrude, such as molecule-derived SiO2179,207 and RF sol19 with low-viscosity, or 1D nanofibre CNCs181 and metal nanowires217 with a low number of reactive sites. Based on whether the additive chemically reacts with the original sol or ink, these additives can be further divided into three types: cross-linker, gelation catalyst, and thickener.
Chemical cross-linking agents form stable covalent bonds with the precursor or nanomaterials through chemical reactions, thereby increasing the viscosity of the sol or ink and the mechanical strength of the gel after printing. Generally, the formed chemical structure is retained in the final aerogel, and becomes a part of the aerogel's 3D network. Examples of cross-linking agents include silk fibroin biopolymers,207 RF,18 Kymene,176,181 acrylamide (AAm) and N,N′-methylenebis-(acrylamide) (MBAA) with post-printing UV-gelation.229 Examples of aerogels printed using these agents include SiO2,207 GO,18 CNC,181,229 and CNF.176
The gelation catalyst mainly changes the electric double layer balance of the system by catalysing the reaction or adjusting the pH value, so that the system undergoes cross-linking for gel curing. Although after this process most of the gelation catalysts generally remain in the pore solvent, a small amount still exists in the solid skeleton of the gel network. Subsequent processes such as washing or drying can remove it. For example, when printing GO-based inks, a trace amount (15 mmol L−1) of calcium chloride can be used as a gelation catalyst,41,236,258 increasing the storage modulus from 3500 Pa to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa and the yield stress from 40 Pa to 160 Pa, improving the printability and self-supporting property of the ink.41
000 Pa and the yield stress from 40 Pa to 160 Pa, improving the printability and self-supporting property of the ink.41
Thickeners, also known as viscosifiers, usually have high molecular weights (organic) or high surface area (inorganic), and can be used to increase the viscosity or storage modulus, and thus the printability of these inks. Generally, they do not chemically react with the gel network and only change the physical properties of the ink. A range of organic (such as hydroxypropyl methyl cellulose,19,21,237 PPGNH,179 and methyl cellulose247) or inorganic materials (including fumed SiO218–20 and nanoclay270) have been used as thickeners. In particular, fumed SiO2 has been used to prepare GO-18 and RF-based inks19 for extrusion 3D printing. Adding ∼10 wt% SiO2 to the inks could increase the storage modulus and yield stress by an order of magnitude,19 improving the extrusion printability of the inks. We note that the thickeners used in this example remain in the gel network skeleton. Subsequent steps such as high-temperature annealing or etching are required to remove them.
Recently, based on a similar method, a breakthrough on printing pure SiO2, polymer-reinforced SiO2, or SiO2–MnO2 aerogel objects was achieved.179 The authors demonstrated 3D extrusion of hybrid molecule-(ethyl silicate) and nanomaterial-derived (SiO2 aerogels) inks, coupled with an ammonia vapour post-treatment (Fig. 14),179 followed by solvent exchange and drying to solidify the printed structures. Here, SiO2 aerogel introduced to the ink formulation not only acts as a thickener in the ink, but also as the skeleton in the final printed structure.
 | ||
| Fig. 14 Extrusion-printed pure SiO2 or SiO2–MnO2 aerogels from inks with thickeners. (a) Schematic diagram of the 3D printing process, (b) 3D printed lotus flower of SiO2 gel using 38 layers. Photographs of the hydrogel (c) before solidification, (d) after ammonia-vapour-induced gelation and (e) after drying. Reproduced with permission from ref. 179. Copyright 2020 Springer Nature. | ||
(iii) For the case of freeze casting, the printing is carried out on a plate or environment at a temperature lower than the freezing point of the ink solvent. This ensures that the printed material is rapidly cooled and solidified immediately after deposition to retain the original shape. In addition to the conventional extrusion,178,219,220,223 3D drop-on-demand inkjet printing can also be combined with freeze-curing,54,109,241,242 where overhanging structures using a multi-nozzle system can be achieved. For this, water can be loaded in one of the nozzles to print “blank” ice as a temporary support.109 This strategy can also be divided into low-temperature- and room temperature freeze-curing.
For the case of low-temperature freeze-curing, water is used as the ink solvent (Fig. 15). The temperature of the cold plate is generally set from −30 °C to −25 °C when using 3D drop-on-demand inkjet printing.54,109,241,242 The newly deposited droplets make successive thin layers at the top of the previously printed layer, which usually melts and re-solidifies. Therefore, the different layers adhere well with each other without creating an interface. Using this method, graphene,109 MnO2-graphene,241 MoS2-graphene,242 and Ag nanowire54 3D aerogels have been printed on ice supports or Cu plates. DMSO has also been used as the solvent for aramid nanofibre-based inks. Since the freezing temperature of DMSO (18.5 °C) is higher than that of water, the cold plate can be set at −10 °C to ensure fast-enough casting.223 When 3D printing CNC aerogels by the extrusion method,220 the ambient temperature can be set between −3 °C and 1 °C. This temperature ensures that the top two printed layers do not freeze, ensuring good adhesion between all the printed layers.
 | ||
| Fig. 15 3D printing of GO-based gels and CNC gels cured by the low temperature freezing method. (a) 3D printing setup of GO-based gels. (b) 3D printed ice support. (c) 3D printed GO suspension. (d) Immersed printed ice structure into liquid nitrogen. (e) Freeze drying. (f) 3D ultralight graphene aerogel obtained by thermal reduction. (a–f) Reproduced with permission from ref. 109. Copyright 2016 WILEY-VCH. (g) Schematic illustration of the printing setup of CNC gels on a cold Cu plate, (h) photograph of the printing process, and (i) the final CNC aerogel. (g–i) Reproduced from ref. 220. Published by MDPI. | ||
A simple room temperature freeze-curing method was first reported to fabricate printed aerogels,178 using solvents that are solid at room temperature, have a high vapor pressure and are prone to sublimation at room temperature. Solvents such as phenol or camphene can be used, where they can be mixed with graphene above their melting temperature (for example, 50–60 °C), and be extruded in the air (Fig. 16). The ink solidifies as it cools to room temperature. Using a similar principle, cellulose/N-methylmorpholine-N-oxide (NMMO) ink at 70 °C could be 3D printed through extrusion, to obtain self-supported 3D cellulose gel.219
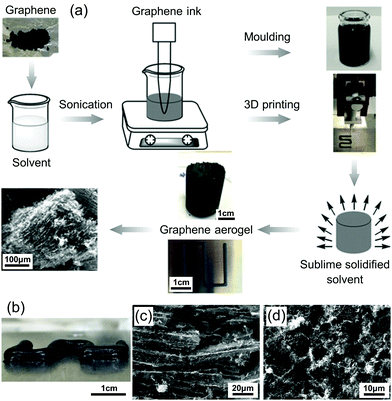 | ||
| Fig. 16 Printed graphene aerogels prepared by room temperature freeze gelation (RTFG). (a) Schematic illustration of the RTFG process. (b) Photograph of a three-layer thick graphene aerogel using phenol as a solvent. SEM images of graphene aerogels produced (c) in phenol and (d) in camphene. Reproduced from ref. 178. Published by WILEY-VCH. | ||
The advantage of a freeze-curing method is that because the viscosity is not taken into account, even low viscous Newtonian inks can be 3D printed. This allows printing of ultra-low concentration inks (for examples, <10 mg mL−1 or even as low as 2 mg mL−1)54,109,178 for ultra-low density 3D printed materials, with densities as low as 0.5 mg cm−3 for 3D graphene aerogel109 and 1.3 mg cm−3 for 3D Ag nanowire aerogel.54 On the other hand, because of low viscosity, retaining dimensional stability and size accuracy is challenging when using this method.
Aerogels from a 3D printed template. The 3D printed template method uses one of the polymer 3D printing technologies (e.g., SLA) to first print out a 3D polymer mould, leaving the desired aerogel volume empty with connected channels. Sols are then injected into the mould and after gelation, the mould is removed before or after drying, to finally obtain a 3D aerogel. There are two basic requirements for this method: the template should be removable without damaging the structure of the gel or aerogel; the viscosity of the sol is low enough for its complete filling into the tiny channels of the template.
The 3D printing templates that have been reported thus far include hollow polymer architectures by mask-image-projection-based SLA techniques,243 honeycomb pillar arrays by 3D printing using an acrylonitrile butadiene styrene ink,245 and hollow mould of high impact polystyrene in an inverse gyroid shape printed by a fused filament fabrication method.177 The sols or inks that have been demonstrated to fill the templates are traditional polyimide sol,177 GO/ethylenediamine (EDA) ink,243 and GO/L-ascorbic acid,245 which are all liquids with enough fluidity to ensure complete filling. After gelation and drying, the polymer templates could be removed either by demoulding,245 high temperature pyrolysis,243 or solvent dissolution,177 to obtain 3D aerogels177,243,245 with complex shapes (Fig. 17). Depending on the resolution of the 3D polymer template, the wall thickness of the GO-based aerogel can be as thin as 250 μm.245
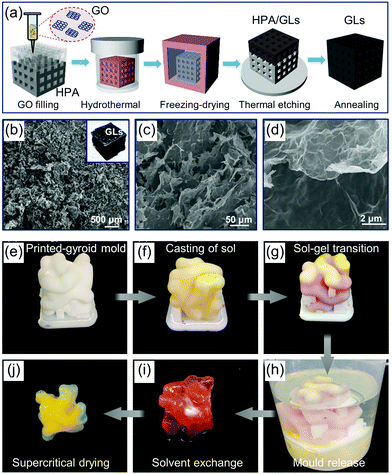 | ||
| Fig. 17 Fabrication process steps for (a–d) 3D graphene aerogel lattice and (e–j) polyimide aerogel by means of a 3D printed template. (a) Schematic diagram of using a 3D printed polymer mould to fabricate a graphene aerogel lattice through thermal etching. (b and c) SEM images of porous microstructures within the graphene aerogel lattice. (d) SEM image of the multilayer graphene cellular wall with typical wrinkled morphology. (a–d) Reproduced with permission from ref. 243. Copyright 2018 American Chemical Society. (e) Printed gyroid mould for the fabrication of polyimide aerogel, (f) cast sol in mould, (g) sol–gel transition, (h) mould removal process, (i) solvent exchange, and (j) supercritical drying. (e–j) Adapted with permission from ref. 177. Copyright 2019 American Chemical Society. | ||
The advantage of this method is that it can be applied to almost all the traditional sols used in the preparation of aerogels. This is unlike SLA which is only applicable to limited types of aerogels or extrusion printing with limitations in structure and narrow operating time window determined by the fluidity and cross-linking reaction.177 Furthermore, this 3D printed template method can produce more complex structures with desirable resolutions.
4.3 Drying methods
When fabricating a 3D printed aerogel, the next step after printing a static, well-defined gel is ‘drying’. This is a very important stage that determines whether a low-density nanoporous aerogel can be obtained. This is because conventional drying in ambient air usually exerts high capillary tension, which destroys the skeleton and induces large volume shrinkage, producing only cracked fragments or dense bulk material. This section discusses the phase change mechanism during drying, the rules for successful drying of aerogels, and three commonly used drying methods.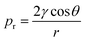 | (2) |
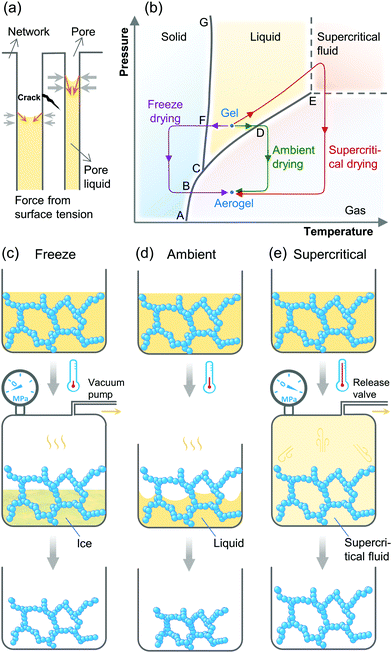 | ||
| Fig. 18 (a) Capillary forces inducing cracks or shrinkage in the pores of the gels. Adapted with permission from ref. 272. Copyright 1998 Wiley-VCH. (b) Typical phase diagram of solvent in the gel and the pressure–temperature changes in three routes for drying the gels to make aerogels. (c–e) Schematic illustrations of three drying routes (freeze, ambient, and supercritical). Adapted with permission from ref. 27. Copyright 2017 Wiley-VCH. | ||
If the drying process is conducted at atmospheric pressure with pore sizes in the nanometre range, it can be calculated from eqn (2) that the skeletons would experience oblique pulling stresses of ∼10 MPa (for a typical aquagel with hydrophilic skeleton and average diameter of 30 nm) as the liquids in the pores are evaporated. Such a large stress compresses the gel continuously during the drying process as the liquid evaporates, causing the gel to gradually shrink and become dense, thwarting the production of low-density aerogels. Additionally, when the pore radiuses on the two sides of a common wall are not equal, eqn (2) dictates that the two sides will be subjected to unequal stresses, causing the pore wall to break (Fig. 18a).188,272 In this case, only fragments can be obtained. Indeed, the drying process in the air already starts, to some extent, during printing in the ambient air which initiates the shrinkage process. This is why it is a common practice for 3D printing to be carried out directly in liquid baths.18–20,183
Therefore, the principles of successful drying to obtain aerogels are to: (i) minimize or (ii) avoid the capillary stress. The minimization route generally follows drying the gel at ambient pressure. This is why it is also called ambient drying. There are two ways to minimize the capillary stress. One is to change the liquid with a solvent with a lower surface tension, i.e., to reduce γ in eqn (2). The second is to modify the chemical state of the pore wall surface with hydrophobic chemical groups to increase the contact angle, i.e., to increase θ in eqn (2). Both measures can reduce the capillary stress pr, and thus minimize the shrinkage during drying. Furthermore, the capillary stress can be eliminated by avoiding the interfaces between liquid and gas, i.e., to prevent the phase transition from going through the curve connecting C–D–E in Fig. 18b. This can be realized by supercritical or freeze drying by forming a homogeneous supercritical fluid or by transforming the liquid–gas interfaces to solid–gas interfaces, respectively.
Freeze drying. Freeze drying involves freezing the solvent in the gel first, followed by its sublimation into the gaseous stage, under a vacuum environment, commonly realised by continuous operation of a vacuum pump. From the perspective of temperature, phase and vapor pressure changes, the liquid in the gel goes through the liquid–solid phase equilibrium curve connecting C–F–G by freezing to become a solid first, and then through the solid–gas phase equilibrium curve connecting A–B–C by sublimation under vacuum to become a gas (Fig. 18b and c). Though this method can bypass the liquid–gas phase equilibrium curve, avoiding the shrinkage of the gel caused by the capillary stress, it still needs to undergo two separate phase transitions (liquid–solid and solid–gas). The latter transition does not affect the gel's nanostructures. However, the liquid–solid transition process always results in a volume change because the densities of solid and liquid phases of the same solvent are typically different. In addition, this may also change the nanopore structure of the gel due to the liquid–solid transition process where solvent (such as water) crystals may grow much larger than the pore size of the gel.273 Therefore, when this drying method is used on conventional molecule-derived gels with mainly nanometre-size pores, obtaining aerogel monoliths could be challenging. Indeed, in such instances, only powders are usually obtained.83
The freeze-drying method is mostly used for drying nanomaterial-based gels, for example, of 0D metal nanoparticles,62 1D CNC,181,220,221 CNF,22,176,218,228 and Ag nanowires,54,217 2D graphene,231,257 and GO.241,242,244–246,254,264 Water is the most commonly used solvent. Tert-butanol, which has a freezing point (25.7 °C, Table 8) close to room temperature, can also be used as a solvent.274 To prevent the solvent crystals from growing too large, this process requires a fast cooling rate. This is usually achieved by immersion in liquid nitrogen.273 In addition, the room-temperature freeze-solidification method that uses high vapor pressure phenol (freezing temperature = 40.5 °C) or camphene (freezing temperature = 51.5 °C) as solvents178 allows sublimation under ambient conditions without the need for a vacuum unlike conventional freeze drying.
| Solvent | Freezing temperature (°C) | Critical temperature (°C) | Critical pressure (MPa) | Surface tensiona (mN m−1) |
|---|---|---|---|---|
| Data collected from ref. 272 and 276–283.a At room temperature. | ||||
| CO2 | −78 | 31 | 7.3 | 0.59 |
| H2O | 0 | 374 | 22.1 | 72.0 |
| Methanol | −97 | 239.4 | 8.1 | 22.3 |
| Ethanol | −114.3 | 243 | 6.3 | 21.9 |
| Acetone | −94.9 | 235 | 4.7 | 22.7 |
| Isopropanol | −88.5 | 235 | 4.7 | 21.2 |
| n-Hexane | −89.5 | 234.7 | 3.0 | 17.9 |
| n-Heptane | −90.5 | 267.3 | 2.7 | 19.7 |
| Tert-butanol | 25.7 | 233.2 | 4.0 | 20.1 |
Ambient drying. Ambient drying involves slow evaporation of the solvent in the gel pores at a heating temperature below the boiling point under ambient pressure (Fig. 18d). Water is the most commonly used solvent for inks to print gels, because of its very high surface tension (72.0 mN m−1, Table 8). However, the wet gel or printed structure dried under ambient conditions could exhibit a large linear shrinkage of ≥45%.217,230,275 Therefore, before drying, to minimize the shrinkage, the gels are always modified with water-repelling chemical groups to increase the contact angle first, then exchanged with lower surface tension solvents such as n-hexane284,285 or n-heptane (surface tensions of 17.9 and 19.7 mN m−1, respectively; Table 8).184 In addition, to control the volatilization rate of the solvent (such that the solvent can volatilize slowly, minimizing the shrinkage), the printed gel is usually placed in a container, leaving only a small hole to vent the vapour.206 Ambient drying has thus far been used to dry inkjet printed SiO2 microspheres184 and CdSe/CdS films,182 and 3D printed GO-based247 gels. These gels always have small dimensions which may help the diffusion of solvent from the inside of the structure to the surface. This additionally improves the uniformity of the solvent distribution in the overall structure such that it can be evaporated homogeneously, reducing the shrinkage to some extent.
Supercritical drying. Supercritical drying is the most commonly used method for traditional aerogel preparation. When the temperature and pressure of the liquid exceed the critical point (point E in Fig. 18b), the interface between the liquid phase and the gas phase disappears and becomes a homogeneous fluid. This is also termed as a ‘supercritical’ fluid, which fills the entire space, usually in an autoclave. In the absence of a liquid–gas interface, the high-pressure supercritical fluid can be slowly discharged through a pressure relief valve while maintaining the temperature above the critical point, producing an aerogel after releasing the pressure down to ambient and then cooling down to room temperature (Fig. 18e). This process essentially bypasses the liquid–gas phase equilibrium curve connecting C–D–E in Fig. 18b, through a liquid–supercritical–gas phase transition. The critical temperature and critical pressure data for common solvents are shown in Table 8. Based on the critical temperature of the solvent used, this process can be divided into low- and high-temperature supercritical drying.
Low-temperature supercritical drying uses CO2 as the drying medium which has a relatively low (31 °C) critical temperature (Table 8). The drying temperature of the autoclave is generally set at a mild temperature of 40–90 °C.209 The high-temperature supercritical drying strategy usually uses alcohol (such as ethanol and isopropanol) as the drying medium. In this case, the critical temperature is always above 230 °C (Table 8), while the drying temperature is set at 260–300 °C. The maximum pressures applied for these two drying methods are typically above 10 MPa.209 For the case of printed aerogels for supercritical drying, the most common route uses low-temperature supercritical drying of CO2. Examples of this strategy have been applied to SiO2-based,179,207 RF,19 aramid,223 alginate,209 CdSe/CdS,182 graphene,18,20,244 and g-C3N4-sodium alginate183 gels. There are also a few examples of SiO2–polymer aerogels prepared by high-temperature supercritical drying of ethanol.205,208 However, if the solvent in the printed gel is water, this process needs some modification. The critical temperature and pressure of water are difficult to achieve, and its solubility in supercritical CO2 fluid is small. Therefore, for both low- and high-temperature supercritical drying, the water in the printed gel needs to be replaced with an organic solvent, which is more CO2-soluble or has lower critical temperature and pressure, such as ethanol,209,223 methanol,207 and acetone.182
| Drying method | Advantages | Disadvantages | Preferable application |
|---|---|---|---|
| Freeze | Moderate cost of equipment | Phase change-induced volume change | Nanomaterial-based gels, specifically those with ultralow densities |
| Mild conditions | Pore size expansion effect | ||
| High energy consumption | |||
| Ambient | Simple process | Large shrinkage | Small size or thin gels |
| No pressure vessel required | Solvent-exchange requires a large amount of solvent | ||
| Continuous production | |||
| Supercritical | Small shrinkage | High equipment cost | Almost all kinds of gels |
| Best nanostructure preservation | High energy consumption | ||
| Hidden dangers with high temperature and pressure |
Based on the process characteristics, the degree of suitability of the drying method is also dependent on the gel types. For example, freeze drying is suitable for the drying of nanomaterial-derived gels,41,54,62 as the pores of these gels are usually larger. On the other hand, it is not suitable for molecule-derived gels with smaller pores. Likewise, ambient drying is suitable for gels with small dimensions such as inkjet printed microspheres.184 This is because the small dimension allows easy and complete replacement of the solvent and homogeneous evaporation. Finally, supercritical drying is the most versatile of all, and is suitable for almost all types of gels.
4.4 Post-treatment after drying
The step of post-treatment after the drying process is optional. This mainly involves sintering, chemical reduction, or a wide range of techniques for the removal of impurities. The basic requirement of post-treatments is not to damage the aerogel skeleton and pore structure. Therefore, the process is usually carried out in a controlled gas atmosphere. If a liquid phase is involved, a secondary solvent exchange and subsequent drying process are always required. This section discusses the three major post-treatment processes: heat treatment, chemical reduction, and etching.In the above section, we have comprehensively reviewed the technical aspects involved in the whole process of printing aerogels, including raw materials, ink formulation, printing methods, and drying and post-treatment strategies. Research progress in recent years has demonstrated many advantages of printed aerogels, including rich form factors (such as microspheres, films, patterns, and 3D architectures), on-demand shaping/fabrication, and the variety of materials (organic and inorganic aerogels such as nanocellulose, alginate, SiO2, graphene) that can be printed. In our opinion, these advantages will greatly promote the application of aerogels in conventional as well as new application areas, by overcoming previously unsurmountable obstacles.
5. Applications of printed aerogels
Traditional non-printed aerogels have already demonstrated huge potential for a wide range of applications. The use of printing technology can greatly promote these application opportunities, especially in the field of functional materials that require intricate structures and shaping. Indeed, printing technology offers unparalleled processability and designability on the micron scale, addressing a major weakness of traditional aerogels due to their brittleness and poor structural strength. In particular, printing enabled processability can be used to directly form and readily redesign complex aerogel structures. This makes aerogels significantly more versatile and designable for target applications. Based on the published literature on printed aerogels, we divide this section into six broad technology and application areas of thermal management, energy storage, electronics, chemical, biomedical, and light-harvesting.5.1 Printed aerogels for thermal management
Thermal insulation materials are widely used in aerospace, petroleum pipelines, industrial furnaces, chemical reactors, cold storage, and buildings. In terms of performance alone, aerogels are widely considered as one of the best thermal insulators because of their ultralow thermal conductivities. Indeed, the conventional SiO2 aerogel is also currently the most successfully commercialized aerogel product for thermal insulation. However, applications requiring thermal insulations usually demand various shapes, which must be assembled precisely and tightly to achieve effective insulation. The brittleness and difficulty in shaping aerogels therefore put them at a great disadvantage. Printing technologies can address the shaping of aerogel thermal insulations, making materials with fine and complex structures and ability to customize design and production, a reality.We note that the issue of mechanical reinforcement can be partially addressed using a support structure. For example, a honeycomb polymer reinforcement was in situ printed inside a SiO2 gel using photopolymerization (Fig. 11i).266 This allowed a well-integrated, high strength SiO2 aerogel, with a thermal conductivity of 0.0136 W m−1 K−1, between that of pure aerogel and pure polymer.266
Using inks containing SiO2 aerogel powder and an organic binder, SiO2 aerogel thin films with thicknesses of several microns were inkjet-printed for thermal insulations.211 The printed aerogels keep a relatively low thermal conductivity of 0.05 W m−1 K−1 in the range of 30 to 300 °C, though higher than that of pure aerogel powders (0.02 W m−1 K−1).
Extrusion-printed pure SiO2179 and SiO2–silk fibrion207 aerogels have also been investigated for thermal insulation. In particular, printed pure SiO2 aerogel (Fig. 19),179 demonstrates an ultralow thermal conductivity of 0.0159 W m−1 K−1, as low as most reported SiO2 aerogels prepared by traditional sol–gel processes.287 As pointed out earlier, extrusion printing allows the manufacturing of miniaturized and complex shapes of fragile aerogel objects, which are extremely challenging to make using traditional shaping methods (mould casting and subtractive manufacturing). Very importantly, the printed objects can be applied to small electronic components to ensure a low outer surface temperature (Fig. 19a–f), or to protect temperature-sensitive parts from a nearby heat source (Fig. 19g–j).
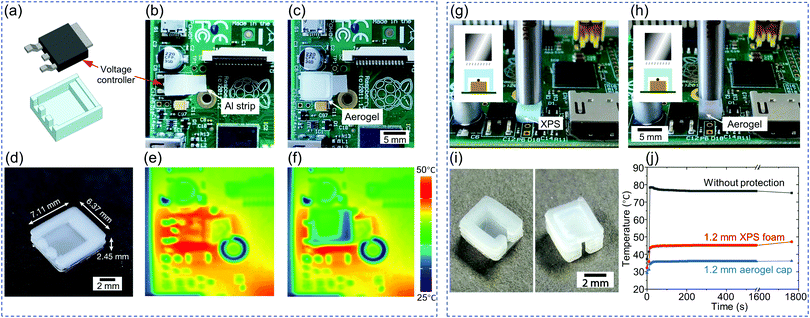 | ||
| Fig. 19 Performance of 3D-printed SiO2 aerogels for thermal insulation. (a) Sketch and (d) photograph of a printed aerogel. Photographs (b and c) and corresponding infrared images (e and f) of a circuit board, (b and e) without and (c and f) with the printed aerogel to cover the heat-generating component. Protection of a temperature-sensitive capacitor from a heat source with a (g) polystyrene foam cap (XPS) and (h) printed SiO2 aerogel cap. (i) 3D-printed aerogel cap. (j) Temperature evolution of the capacitor after contact with the cartridge heater. Adapted with permission from ref. 179. Copyright 2020 Springer Nature. | ||
5.2 Printed aerogels for energy storage
Due to high specific surface area, good mass transfer ability enabled by the open-pore structure and high electrical conductivity, carbon-based and metal aerogels have great application prospects in the field of energy storage. To fully realize the potential of aerogels in energy storage, two challenges must be overcome: (i) the formation of complex or even curved structures required by batteries or supercapacitors to minimize their volume; (ii) highly connected, direct and unobstructed hierarchical channels to ensure fast and effective mass transfer.186 3D printing can address both the above. Indeed, printed aerogels with excellent performance for supercapacitors, lithium batteries, sodium ion batteries, and hybrid energy storage have already been demonstrated, underscoring their huge potential in the field of energy storage.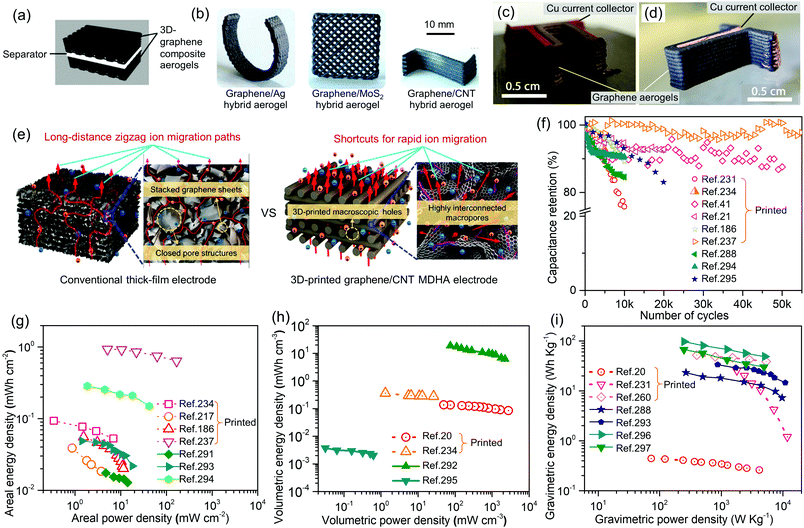 | ||
| Fig. 20 Printed aerogels for supercapacitors and electrodes. (a) Schematic illustration of the 3D-GCA symmetric supercapacitor. Reproduced with permission from ref. 20. Copyright 2016 American Chemical Society. (b) Photographs of the 3D-printed Graphene/Ag, Graphene/CNT, and Graphene/MoS2 mixed dimensional hybrid aerogels (MDHAs). Reproduced with permission from ref. 186. Copyright 2018 American Chemical Society. Photographs of a printed graphene aerogel supercapacitor composed of a (c) two-leg electrode and (d) one-leg electrode, both with Cu current collectors. (c and d) Reproduced with permission from ref. 231. Copyright 2017 American Chemical Society. (e) Schematic illustrations of insufficient ion migration for a conventional thick-film electrode and sufficient ion migration for the 3D-printed Graphene/CNT MDHA electrode. Reproduced with permission from ref. 186. Copyright 2018 American Chemical Society. Comparative survey from published literature: (f) capacitance retention as a function of cycle number, (g) areal energy density as a function of areal power density, (h) volumetric energy density as a function of volumetric power density, and (i) gravimetric energy density as a function of gravimetric power density. | ||
There have been detailed investigations to understand whether 3D printing technology can improve the rate capability of capacitors over non-printed bulk aerogels.19–21,237 The results show that when ink formulations of the two supercapacitors or electrodes are the same (i.e., the surface area, conductivity, and pore size of the aerogels are the same), 3D printed aerogel electrodes show better specific capacitances, 1.4–12.9 times those of the corresponding non-printed aerogels;19–21,237 see Table 10 for comparison. The capacitance retention values are also better than those of the capacitors based on non-printed aerogels (Table 10).
| Category | Electrode | Rate capability | Capacitance (at highest current density) | Thickness |
|---|---|---|---|---|
| 3D printed | DIW-CA19 | 69% (from 1 to 10 A g−1) | 86 F g−1 (10 A g−1) | — |
| GO-GNP-SiO2-220 | 88.7% (from 0.5 to 10 A g−1) | 63.6 F g−1 (10 A g−1) | 1000 μm | |
| 3D G/MnO221 | 56% (from 0.5 to 50 mA cm−2) | ca. 6.43 F cm−2 (50 mA cm−2) | 1000 μm | |
| SF-3D GA237 | 73.2% (from 5 to 100 mA cm−2) | ca. 1.16 F cm−2 (100 mA cm−2) | 2000 μm | |
| DIW-ACA19 | 79% (from 1 to 10 A g−1) | ca. 171 F g−1 (10 A g−1) | — | |
PG/MWCNT 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1178 1178 |
ca. 60.4% (from 1 to 100 A g−1) | ca. 100.5 F g−1 (100 A g−1) | — | |
| rCMG/Cu231 | ca. 75.9% (from 3 to 30 A g−1) | 13 F g−1 (30 A g−1) | — | |
| PPy-GA-0.1M234 | 90% (from 0.1 to 4 A g−1) | 395 F g−1 (1 A g−1) | 2000 μm | |
| 3DGC-141 | 85.9% (from 0.5 to 100 A g−1) | 183 F g−1 (100 A g−1) | 300 μm | |
| MnO2/GA241 | ca. 33% (from 1 to 10 A g−1) | 90 F g−1 (10 A g−1) | — | |
| G/ZnV2O6@Co3V2O8//G/VN260 | 78% (from 0.5 to 8 A g−1) | 116.8 F g−1 (8 A g−1) | — | |
| Graphene/MWCNT MDHA 2 mm186 | 71.4% (from 4 to 40 mA cm−2) | 456.69 mF cm−2 (40 mA cm−2) | 2000 μm | |
| Bulk (non-printed) | Bulk CA19 | 31% (from 1 to 10 A g−1) | ca. 17 F g−1 (10 A g−1) | — |
| Bulk GO-GNP-SiO2-220 | ca. 82% (from 0.5 to 10 A g−1) | ca. 47 F g−1 (10 A g−1) | 1000 μm | |
| Bulk G/MnO221 | 11% (0.5–50 mA cm−2) | ca. 0.50 F cm−2 (50 mA cm−2) | 1000 μm | |
| Bulk SF GA237 | 29.0% (from 5 to 100 mA cm−2) | ca. 0.40 F cm−2 (100 mA cm−2) | — | |
| pCA-KOH-700288 | 90.9% (from 1 to 10 A g−1) | 330 F g−1 (10 A g−1) | — | |
| CNF/RGO/MoOxNy292 | 62% (from 1 to 15 A g−1) | 421 F g−1 (15 A g−1) | — | |
| AAP(rGO-Co3O4//rGO)293 | 59.7% (from 2 to 30 mA cm−2) | 119.8 mF cm−2 (30 mA cm−2) | — | |
| FeOCl@CDCA294 | ∼58% (from 2 to 50 mA cm−2) | 74 F g−1 (50 mA cm−2) | — | |
| AASC(MnO2@CNTs@3DGA//PPy@CNTs@3DGA)-3.9295 | ∼84.6% (from 1 to 20 mA cm−2) | 950 mF cm−2 (1 mA cm−2) | — | |
| N-GA@V2O5 NWAs296 | ca. 50% (from 0.5 to 10 A g−1) | 360 F g−1 (10 A g−1) | — | |
| RGO/V2O5297 | 55.6% (from 1 to 8 A g−1) | 178 F g−1 (8 A g−1) | — | |
| Graphene hydrogel film-1 M H2SO4298 | 80% (from 1 to 20 A g−1) | ∼152 F g−1 (20 A g−1) | — | |
| GAMOF(Fe3+)299 | 74.4% (from 1 to 20 A g−1) | 353 F g−1 (20 A g−1) | — |
The ordered macropores formed between the printed gel filaments improve the penetration of the electrolyte and hence, ion diffusion, resulting in excellent rate capability.20 Compared with bulk aerogel electrodes with mainly sub-micron or smaller pores, which severely restricts ion diffusion, 3D printed aerogels can be designed to have faster ion diffusion kinetics (Fig. 20e).186 Additional performance enhancements can be achieved by printing finer aerogel features to further reduce the diffusion length.
From this perspective, deliberate design and printing of graphene aerogel microlattices with a gradient porous structure can further promote rapid diffusion of electrolyte ions and effective usage of the electrode volume.41,289,290 In addition, since extrusion-based 3D printing can increase the electrode thickness,19,21,217,260 which linearly increases the corresponding capacitance,21,237 this fabrication approach could also be used to tailor the overall performance metrics.
Fig. 20f–i show comparisons between printed and the state-of-the-art non-printed aerogel supercapacitors in terms of capacitance retention at long cycles, areal energy density, volumetric energy density, and gravimetric energy density. The performance parameters of the printed devices are comparable, and in the majority of the cases, better than their non-printed counterparts.288,291–299 In particular, we note that even after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1, two reports on supercapacitors fabricated by 3D printed graphene aerogels show a high capacitance retention of 90% (Fig. 20f),41,234 in comparison to rapid retention drops in the non-printed devices. This is very likely due to a combination of factors, including structural and electrochemical stability of the aerogel skeleton,41 and better diffusion kinetics. Therefore, the added advantage of creating complex structures and customised geometries from 3D printing offer significant device integration benefits over traditional bulk aerogels, which can only be formed by hand cutting.234
000 cycles at 10 A g−1, two reports on supercapacitors fabricated by 3D printed graphene aerogels show a high capacitance retention of 90% (Fig. 20f),41,234 in comparison to rapid retention drops in the non-printed devices. This is very likely due to a combination of factors, including structural and electrochemical stability of the aerogel skeleton,41 and better diffusion kinetics. Therefore, the added advantage of creating complex structures and customised geometries from 3D printing offer significant device integration benefits over traditional bulk aerogels, which can only be formed by hand cutting.234
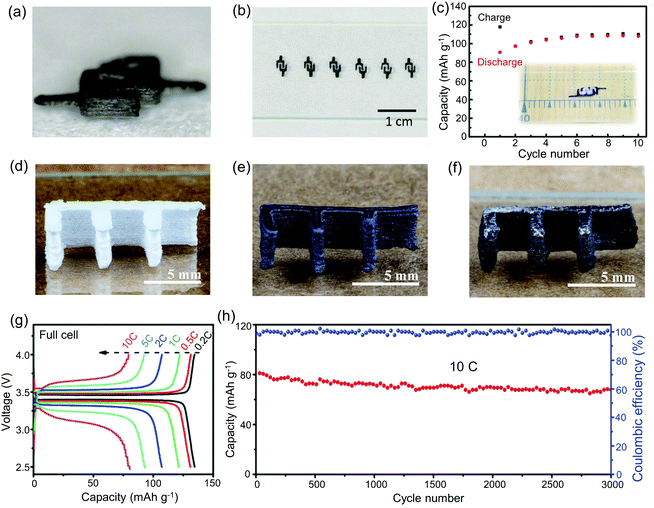 | ||
| Fig. 21 (a–c) Lithium-ion batteries and (d–h) lithium metal micro-batteries assembled by 3D printing of aerogels. (a) Digital image of a 3D-printed miniaturized electrode pair. (b) Digital image of the 3D-printed electrode arrays. (c) Cycling stability of the 3D-printed full cell. The inset is a digital image of the 3D-printed full cell consisting of LiFePO4/rGO, Li4Ti5O12/rGO, and polymer electrolyte. (a–c) Reproduced with permission from ref. 261. Copyright 2016 WILEY-VCH. Photographs of a (d) 3D printed CNF aerogel scaffold, (e) carbon scaffold, and (f) carbon/Li electrode with designed 3D structure for lithium metal micro-batteries. (g) Voltage profiles at various rates from 0.2 to 10C. (h) Long-term cycling performance at 10C for the full cell with carbon/LiFePO4 cathode and carbon/Li anode. (d-h) Reproduced with permission from ref. 218. Copyright 2019 WILEY-VCH. | ||
Rechargeable lithium metal batteries (LMBs) using lithium metal or lithium alloy as the anode material and non-aqueous electrolyte solution are considered as the next-generation Li-based rechargeable batteries. This is because of the highest theoretical energy density and lowest standard reduction potential of lithium metal among the candidate materials.300 However, the existing lithium metal electrodes are always planar, making it difficult to meet the requirements of miniaturization and customized form factors. Recently, CNF was used to manufacture LMBs via 3D extrusion printing.218 The 3D printed CNF (Fig. 21d) was first carbonised to obtain a carbon aerogel (Fig. 21e). Molten lithium was then injected to form a high aspect ratio carbon/Li anode (Fig. 21f). Importantly, the fully 3D printed LMBs show a high capacity of 80 mA h g−1 at a charge and discharge rate of 10C (Fig. 21g), with an 85% capacity retention after 3000 cycles (Fig. 21h). This stable cycling performance of the full cell is attributed to the macroporous structure of the 3D printed carbon aerogel scaffold. The authors suggest that the scaffold offers two benefits. First, it allows the cathode to have good electrolyte accessibility, which accelerates the charge transfer during cycling. Second, it helps to improve the anode ion accessibility and reduce the local current density of the lithium anode, effectively stabilizing Li and suppressing dendrite formation.218
Recently, highly porous aerogels of rGO238,264 and hybrid MoS2-graphene242 have been 3D printed to address this challenge. Using Na3V2(PO4)3 (NVP)- and GO-containing aqueous inks (Fig. 22),264 the first implementation of 3D printed SIBs was demonstrated. The printed continuous frameworks and hierarchically porous structure (Fig. 22b–d) can facilitate the rapid transport of Na+ and electrons. The capacity retention is over 90% after 900 cycles at 1C (Fig. 22f). In another example, an SIB with 3D inkjet printed hybrid MoS2-graphene aerogel electrodes was demonstrated.242 The results show that the 3D printed macroporous structure can sustain fast and stable high-capacity intercalation of large Na+ ions.
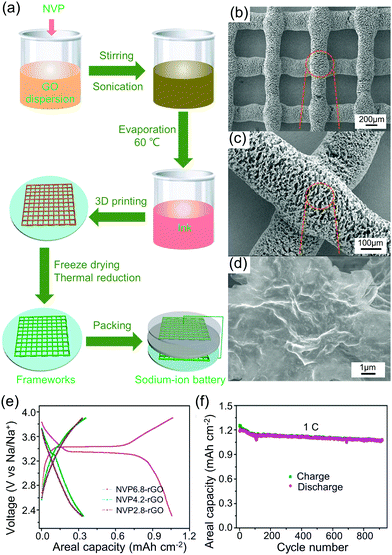 | ||
| Fig. 22 Sodium-ion batteries fabricated by 3D printed rGO aerogels. (a) Schematic illustration showing the fabrication process of 3D-printed hierarchical porous frameworks. (b–d) SEM images of 3D-printed rGO aerogel frameworks with different magnifications. (e) Charge and discharge profiles at 2C. (f) Long cycling performance of NVP6.8-rGO at 1C. Reproduced with permission from ref. 264. Copyright 2017 American Chemical Society. | ||
For sodium metal applications, inkjet printing of a 3D graphene lattice was demonstrated for sodium metal anodes.238 The 3D printed hierarchical and multiscale structure shows better stability (reversible and stable beyond 500 cycles) in coulombic efficiency than non-printed porous graphene papers (only stable to ∼170 cycles).
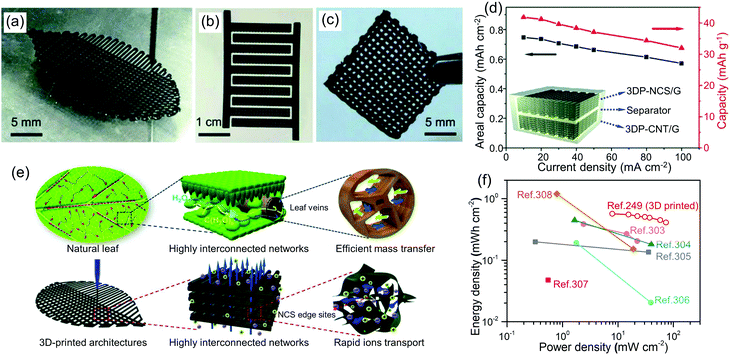 | ||
| Fig. 23 3D printed graphene-based hybrid energy devices. Photographs of the printed objects before drying (a and b) and aerogel (c). (d) Calculated specific capacitances of the device at different current densities. Inset: Schematic illustration of the hybrid energy storage device. (e) Schematic illustration of the mass transport process in a natural leaf and 3DP-NCS/G aerogel electrode. (a–e) Reproduced with permission from ref. 249. Copyright 2018 WILEY-VCH. (f) Ragone plots of areal energy densities versus power densities of the 3D printed graphene-based hybrid energy device compared with non-printed electrodes based on Ni–Co–S materials. | ||
The areal energy density of the printed aerogel device is higher than that of other Ni–Co–S based electrodes (Fig. 23f),303–308 partly because of its higher mass loading (17.86 mg cm−2)249 compared to other electrode materials (1.77–11.2 mg cm−2).303–305 Importantly, the capacitance of other electrodes with high mass loading drops significantly, due to the limited electron/ion transport capacity in the thick film electrodes.309 The advantage of 3D printing is that the architecture with interconnected network and highly open macropores created during the 3D printing process can improve both the electrical conductivity and serve as an effective mass transfer channel (Fig. 23e), shortening the travel distance for electrons/ions in the redox reaction, ensuring excellent electrochemical performance under high mass loading.249
5.3 Printed aerogels for electronics
With the rapid development of artificial intelligence, the demand for miniaturization of various electronic devices has accelerated, especially in the field of wearables. Among them, sensors and actuators, many of which can benefit from the unique properties of aerogels, represent an important family of devices. Requirements for miniaturization and flexibility for direct integration of aerogels on wearable substrates bring unprecedented challenges to their design and manufacture. As a broad technology platform, functional printing can address this need. Key applications in this category include strain or tactile sensors, mechanical linear actuators or electrical fuses, triboelectric nanogenerators (TENGs) and gas or chemical sensors.Graphene104,311,312 or fibrous carbon-based313 aerogels are highly flexible, and can endure high strain through expansion and compression processes, resulting in a large change in the contact area between the nanosheets or fibres, and consequently, the overall electrical resistance.311 The application of printing technology can further increase the deformation range of the flexible aerogels. For example, 3D printed porous GO-CNT aerogel mesh can reach 200% elongation, whereas traditional aerogels break at 25%.258
Materials for aerogel based 3D printed strain sensors demonstrated to date have mainly focused on graphene,18,109,171,235,259 and CNT-graphene hybrids.258 Tactile sensors from 3D printed PDMS microbeads/GO nanocomposite,240 or graphene235 aerogels have also been demonstrated. The densities of these printed aerogels are usually in the range of 0.5 to 25 mg cm−3,109,235,258 with typical electrical conductivity between 15.4 and 130 S m−1.109,235 We note that the miniaturization of these ultra-light sensors is directly related to the nozzle diameter of the extruders. The internal diameter of typical extrusion nozzles for current generation 3D printing is in the range of tens to hundreds of microns, with the best printing accuracy of 50 μm.258
Fig. 24 shows two examples of wearable strain235 and tactile259 sensors using 3D extrusion printed graphene aerogels. In the first example, four serpentine resistive aerogel sensors were integrated on to the finger joints on to gloves (Fig. 24a) to accurately monitor the complex and subtle changes in human finger joints (Fig. 24b and c).235 Inspired by the structure–activity relationship between the different microstructures of the spiral and radial lines in the spider web, the second example of an extrusion printed sensor (Fig. 24d–f) demonstrated a multi-resolution tactile platform to analyse and classify touch in different directions, distances and locations.259 The authors also designed a simulated graphene skin to manipulate the corresponding mechanical fingers with very small pressures (<3.83 × 10−3 Pa).259
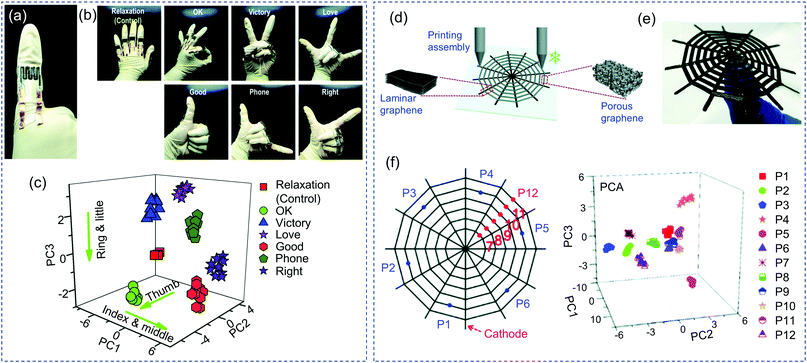 | ||
| Fig. 24 3D printed aerogel (a–c) strain and (d–f) tactile sensors. (a) Photograph showing the printed graphene aerogel sensor attached on the index finger joint. (b) Sensors placed on the finger joints, and different gestures with the deformation degree of the sensors, (c) 3D representation of the principal component analysis (PCA) result showing a clear clustering of the seven different gestures as variables in the trends of the joint movements. (a–c) Reproduced from ref. 235 with permission from the Royal Society of Chemistry. (d) Schematic illustration of print-manipulated graphene nanosheets assembled into different micro-structures in the spiral and radial threads. (e) Photograph of the multi-resolution graphene tactile sensor under pressure. (f) Electronic discrimination analysis of positions based on the multi-resolution graphene tactile sensor. (d–f) Reproduced from ref. 259 with permission from the Royal Society of Chemistry. | ||
The above examples imply that 3D printing technology has brought these aerogel-based resistive sensors a degree of design freedom that is difficult to achieve by traditional shaping methods. With this flexibility, complex connected structures in a millimetre-scale form-factor can be engineered to achieve a desired impedance range and pressure detection accuracy, promoting their development in customizable miniaturised sensors.314
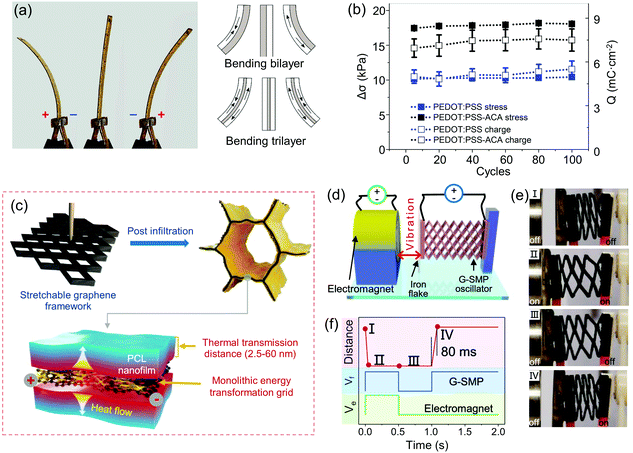 | ||
| Fig. 25 Printed aerogels as actuators and oscillators. (a) Photograph and schematic illustration of bending of an ionic electromechanically active polymer actuator. Adapted from ref. 315. Published by MDPI. (b) Durability of stress (filled) and charge density (empty) of PEDOT:PSS- and PEDOT:PSS-ACA-PVDF trilayer at linear actuation. Reproduced with permission from ref. 212. Copyright 2017 Elsevier B.V. (c) Schematic illustration of the fabrication process of a graphene/SMP nanocomposite aerogel that encompasses a monolithic graphene framework and polycaprolactone (PCL) nanofilm. (d) Schematic illustration of the high-speed G-SMP oscillator. (e) The original state (I) and three critical states (II, III, IV) during one oscillation cycle. (f) Impulse voltage on G-SMP (Vf), electromagnet (Ve), and the consequent distance change in a cycle. (c–f) Adapted with permission from ref. 236. Copyright 2019 American Chemical Society. | ||
Aerogels were first introduced in actuators in 2017, by inkjet printing a mixture of PEDOT-poly(styrenesulfonate) (PEDOT:PSS) conducting polymer and ACA on both sides of a PVDF membrane.212,213 Compared to the pure PEDOT: PSS variety, the actuator with PEDOT:PSS-ACA electrodes achieved a higher bending stress (Fig. 25b) due to the higher conductivity, ion diffusion rate, and specific capacitance. In this example, inkjet printing allowed the authors to control the shape and thickness of the polymer-aerogel hybrid films.
In another example, 3D extrusion printing technology was used to fabricate a micro oscillator based on graphene-shape memory polymer (G-SMP) nanocomposite aerogels (Fig. 25c–f).236 The stretchable 3D printed graphene aerogel framework, with high electrical (∼1100 S m−1) and thermal conductivity (∼3.6 W m−1 K−1), acts as an overall energy conversion grid for rapid heat generation and injection (Fig. 25c). This method increased the response speed of the oscillator to 50 milliseconds which is several orders of magnitude better than traditional bulk SMP composites, and is comparable to shape memory alloys. The G-SMP aerogel oscillator (Fig. 25d) can respond and oscillate with negligible hysteresis (80 ms) (Fig. 25e and f), because of its fast thermal energy generation and injection. The performance of the actuator is also very repeatable and stable, primarily due to the highly stretchable and intricate grid structure printed by 3D extrusion technology.
Based on 3D extrusion printing, two different types of TENGs have thus far been fabricated by graphene (Fig. 26a–c)107 and CNF (Fig. 26d–f)228 aerogels, respectively. In one example, a 3D printed graphene aerogel has been used as one of the electrodes of the TENG, the other being an Al foil. The Young's modulus of this printed aerogel is more than three times higher than that of the super-elastic bulk graphene materials (Fig. 26a). In this TENG structure, the two electrodes are separated by a fluorinated ethylene propylene (FEP) film (Fig. 26b). When the TENG is manually tapped, ten LEDs connected in series could be fully lit (Fig. 26c), indicating sufficient conversion efficiency.107
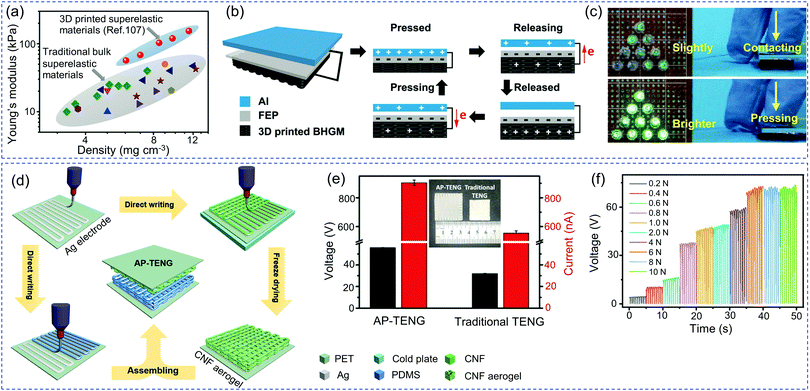 | ||
| Fig. 26 3D printed aerogels for TENGs. (a) Young's modulus of 3D-printed hierarchical graphene materials (BHGMs). (b) Schematic illustration of electricity generation in a nanogenerator in contact-separation mode. (c) Photographs of ten green LEDs powered by hand tapping at (top) small compression and (bottom) large compression. (a–c) Adapted with permission from ref. 107. Copyright 2019 WILEY-VCH. (d) Schematic illustration showing the fabrication process of an AP-TENG. (e) The output of a 3D hierarchical patterned AP-TENG and the mould-cast flat microporous TENG counterpart. (f) The output performance of the AP-TENG at different pressures. (d–f) Adapted with permission from ref. 228. Copyright 2019 Elsevier Ltd. | ||
In an all-printed TENG (AP-TENG) design,228 the two friction layers of PDMS and CNF aerogels, and the corresponding Ag electrodes were fabricated by 3D extrusion (Fig. 26d). The output voltage of this AP-TENG is 75% higher than that of a traditional TENG (Fig. 26e) and is stable under different pressures (Fig. 26f).
The above examples demonstrate that the combination of 3D printing technology and nanoporous aerogels could enhance the contact area, surface properties, and mechanical flexibility of TENGs, improving the mechanical resilience and triboelectric response.
The mechanism of resistive gas sensing is that the resistance of the active sensing layer changes when gas molecules come in contact with its surface.201 The main performance parameters of the gas sensor include sensitivity, selectivity, detection limit, response and recovery time, and stability.320,322 Because aerogels have high specific surface area and open-pore structures, they can provide a large number of abundant active sites to efficiently adsorb and detect gases. Aerogel-based gas sensors therefore usually have high sensitivity, and can be designed to have low detection limits.3
Printing technology can be used to design aerogel's macroporous structure and facilitate their direct integration on to the substrates and contacts. The former may promote enhanced gas diffusion and sensing compared to the bulk aerogels. Graphene aerogel lattices by a 3D printed template method have already been used to demonstrate sensors for volatile organic compound (VOC) detection (Fig. 27).243 The conductivity of the graphene aerogel decreases after it comes in contact with liquid reagents, and the light intensity of the LED lamp becomes dim (Fig. 27a–d). This sensor can be used to detect different types of chemical reagents (Fig. 27e) and different concentrations (Fig. 27f), showing excellent measurement repeatability (Fig. 27g).243
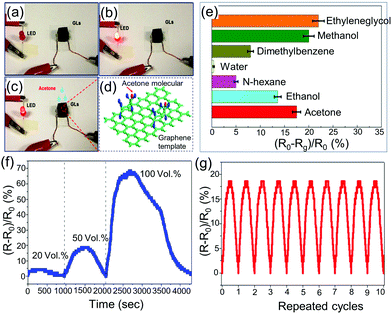 | ||
| Fig. 27 Printed aerogels for chemical sensor applications. (a) Photograph of a chemiresistive sensor with LED light detection. (b) The LED glows brightly in the initial state before chemical reagent is added. (c) LED light dimmed, indicating change in electrical resistance in response to chemical agent. (d) Schematic illustration of sensing of acetone through interaction with rGO. (e) Resistance changes of the chemiresistors for different chemical agents. (f) Resistance changes of the sensor under different volume% of acetone. (g) Cycling stability of acetone sensing over 10 times. Reproduced with permission from ref. 243. Copyright 2018 American Chemical Society. | ||
3D printing on aerogels allows engineering and fine tuning of the pores and skeleton structures on multiple scales. Therefore, the sensor sensitivity can be adjusted accordingly,243 while the mass transfer can be improved by careful design of hierarchical pores,270 increasing the adsorption and desorption rate, and improved detection efficiency.
 | ||
| Fig. 28 3D printed aerogels used as electrical conductors and UV sensors. (a) Stretchable light-emitting display constructed using GHC sandwich as conductors. (b) Photograph of GHC. (c) Circuit connection in the display electronic system. (d) Photographs of the stretchable light-emitting display. (e and f) Performance of the conductors under twisting and bending. (a–f) Adapted with permission from ref. 245. Copyright 2018 WILEY-VCH. (g) Schematic illustration of extrusion-based printed UV sensors. (h) As-prepared fibre-based UV sensor. (i) I–V curves of the fibre-based UV sensor in the dark and under UV (350 nm) illumination at a bias of 10.0 V. (j) Reversible switching of electrical current for the fibre-based UV sensor at 10.0 V biasing voltage in the dark state and under UV light irradiation. (g–j) Adapted from ref. 217 with permission from the Royal Society of Chemistry. | ||
3D printing technology has also been used to directly print Ag nanowire (AgNW) aerogel circular ring electrodes and ZnO nanowire (ZONW) aerogels as cylindrical photosensitive materials (Fig. 28g) to fabricate UV sensors directly onto microfibres (Fig. 28h). These UV sensors, which can be woven directly into fabrics at a precise location, with specific material combination through extrusion,217 offer stretchability or repeatable performance (Fig. 28j). Such manufacturing adaptability would be challenging to replicate using conventional approaches.
5.4 Printed aerogels for chemical applications
The intrinsic characteristics of aerogels such as large surface area, high porosity, and adjustable surface chemistry endow them with excellent supporting and adsorption capacity. Therefore, they are very attractive as potential catalyst supports or adsorbents in chemical synthesis or environmental protection.323 However, the mesoporous structure of aerogels exhibits relatively poor mass transfer performance. Therefore, when aerogel is used as a catalyst or adsorbent, it is generally applied in the form of particles, not in large monoliths. However, the drawback of poor mass transfer inside the aerogel particles, and low space utilization due to too large pores between the particles still exist. Since 3D printing offers design and regulation of aerogel macropores, it can be used to optimise mass transfer in materials, for example, through designs following Murray's law which mimics animal respiratory or plant vascular systems with hierarchical, area/volume preserving networks and precise pore diameter ratios,289 promoting their application in catalysis and adsorption.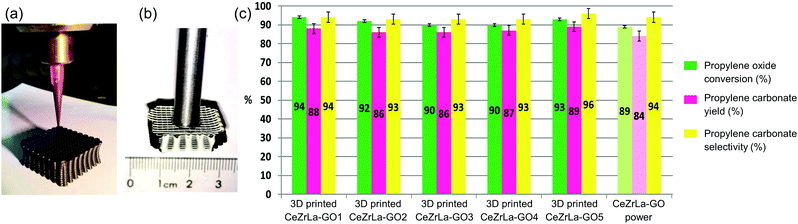 | ||
| Fig. 29 3D printed graphene aerogels loaded with CeZrLa for catalyst applications. (a) 3D printing process of GO-supported CeZrLa structures. (b) Typical GO-supported CeZrLa structure mounted onto an impeller shaft of a stirred batch reactor cell. (c) Experimental results (repeatability tests) for the green synthesis of propylene carbonate using the printed GO-supported CeZrLa catalyst (first five sets from left to right) in comparison to its powder counterpart. Adapted with permission from ref. 247. Copyright 2019 Elsevier Ltd. | ||
3D printing technology can promote microstructure design, and improve adsorption selectivity and capacity.140,328 Indeed, porous graphene lattices by 3D printed templates have been demonstrated as selective adsorbents for inorganic and organic solvents (Fig. 30),243 with adsorption capacities of organic solvents as high as >2000% (Fig. 30a). The nanoporous structure of the aerogels produces a strong adsorption by capillary tension, while the macropores created by printing provide a mass transfer channel to ensure that high-viscosity liquids like asphalt can be adsorbed and removed by Joule heating. Such designs also ensure the reusability and structural stability for multiple adsorption-combustion cycles for various combustible liquids (Fig. 30b).
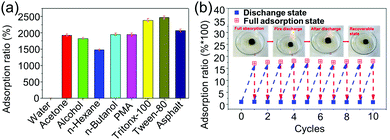 | ||
| Fig. 30 3D printed graphene aerogel lattices for adsorbent applications. (a) Adsorption ratios of different chemical solvents. (b) Cycling stability of adsorption capacity of alcohol with natural adsorption and fire discharge processes. Reproduced with permission from ref. 243. Copyright 2018 American Chemical Society. | ||
5.5 Printed aerogels for biomedical applications
The majority of aerogel applications in the fields related to biocompatibility include tissue engineering, drug delivery and biosensing. The high porosity of aerogels and their connectivity in open nanostructures are conducive to cell attachment, provide channels for nutrition and oxygen to cells, and additionally eliminate cell metabolism by-products, making aerogels a very attractive material for regenerative medicine.329For tissue regeneration, 3D printed aerogel scaffolds of a range of materials, including CNC,181 CNF,230 CNF/alginate,225 PEGDA/CNF,22 and gelatin/poly(lactic-co-glycolic acid) (PLGA)23 have been investigated. For example, 3D printed gelatin/PLGA23 has been evaluated for in vitro cartilage regeneration using scaffolds in conjunction with chondrocytes (Fig. 31).23 A large number of live cells (green) are observed in both the non-printed (Fig. 31a) and the 3D printed (Fig. 31g) samples, with cartilage-like tissue forming in both groups (Fig. 31b and h). Indeed, as seen in Fig. 31c and i, chondrocytes produced rich extracellular matrixes, covering both the scaffolds. However, the specimens from the non-printed group are irregularly shaped and form very thin tissues (Fig. 31d–f). In contrast, the specimens in the 3D printed group with controlled pore structure retain their original square shape and form thicker tissues (Fig. 31j–l). The authors propose that the improved cartilage regeneration in the 3D printed samples is primarily due to the controlled pore structure of the 3D printed scaffolds, which ensures uniform cell distribution and nutritional supply, forming a relatively uniform cartilage-like tissue. This printed aerogel scaffold has good biocompatibility and rich macroporous structure, all of which are beneficial for its application in tissue engineering.22,23
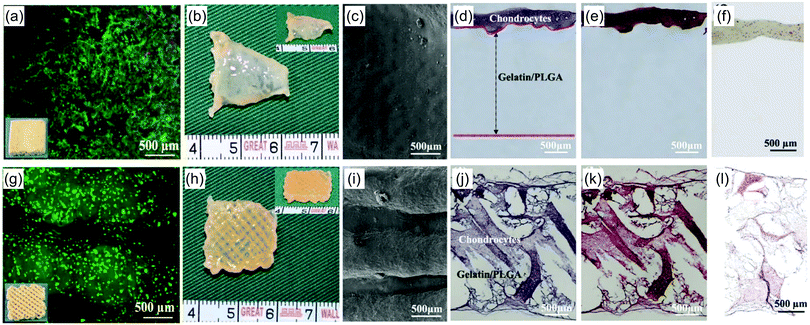 | ||
| Fig. 31 3D printed gelatin/PLGA for in vitro cartilage regeneration. Fluorescence micrographs of chondrocytes seeded on (a) non-printed freeze-shaped gelatin/PLGA scaffold and (g) 3D-printed scaffold for 24 h (live and dead cells are dyed green and red, respectively). Overall appearance of cell-scaffold constructs in (b) non-printed and (h) 3D-printed groups after six weeks of in vitro culturing. SEM images present the extracellular matrix deposition in the (c) non-printed and (i) 3D-printed groups after six weeks. Histological analysis of cell-scaffold constructs in (d–f) non-printed and (j–l) 3D-printed groups after six weeks of in vitro culturing. Reproduced with permission from ref. 23. Copyright 2019 Elsevier Ltd. | ||
A wide variety of drug carrier materials in an aerogel form have been investigated thus far, including SiO2,335 TiO2,336 chitosan,337 pectin,338 carrageenan,339 alginate,340 starch,341 and cellulose.342 These aerogels are traditionally shaped by one of the methods such as milling from monoliths, droplets through syringe into solution, and emulsion polymerization.338,343–345
Inkjet printing can produce fine ink droplets with diameters of tens of microns and as discussed before, can be used to fabricate ultrafine aerogel microspheres for drug delivery applications. Indeed, such a strategy to prepare alginate aerogel microspheres has been demonstrated for lung inhalation delivery of salbutamol sulphate (a drug used to treat bronchi or lung diseases); Fig. 32.209 Inkjet printing was used to obtain uniform and fine particle size (23.8 ± 4.5 μm, Fig. 32a and b) for high fluidity. Importantly, the salbutamol sulphate-loaded microsphere's fine particle fraction (indicating the flowability) is close to 50%, better than some commercial salbutamol sulphate formulations (26–28%).346,347 Additionally, fine nanopores in the aerogels ensure low continuous drug release for up to 10 hours (Fig. 32c). This method can be universally extended to other drug delivery approaches.
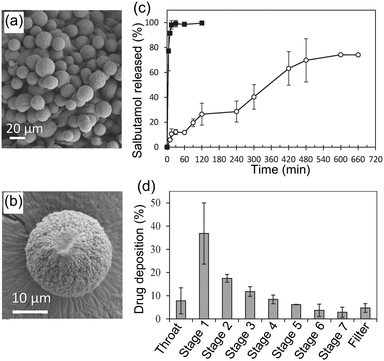 | ||
| Fig. 32 Inkjet-printed aerogels for drug delivery. (a and b) SEM images of alginate aerogel microspheres obtained by inkjet printing. (c) In vitro release profile of salbutamol sulphate from the aerogel microspheres (white circles) in phosphate buffer solution, compared to the dissolution profile of salbutamol sulphate (black squares) under the same operating conditions. (d) Drug deposition profile of salbutamol sulphate loaded in alginate aerogel microspheres with a next generation impactor. Adapted with permission from ref. 209. Copyright 2018 Elsevier B.V. | ||
5.6 Printed aerogels for light-harvesting applications
Solar energy can be harvested through conversion to heat, electricity, and energy-rich chemicals (fuel), based on mechanisms such as solar-thermal conversion, photovoltaic effect, photocatalysis, photosynthesis, and photoelectrochemistry.348,349 Thus far, non-printed aerogels have been used for a range of light-harvesting applications, including rGO-polyacrylamide (rGO-PAM),350 hollow-carbon-nanotubes,351 conjugated microporous polymer nanotubes,352 graphene,353 and MoS2-graphene254 for solar-steam generation, g-C3N4/GO for broad-spectrum photocatalysts,354 g-C3N4/graphene/NiFe hydroxide for solar-driven photoelectrochemical water oxidation,355 and SiO2–TiO2 for dye-sensitized solar cells.356 Printing technologies allow for fabrication and direct integration of these aerogels into light-harvesting devices, especially in fine and gradient structure configurations, and therefore, can greatly enhance device performance and overall manufacturability.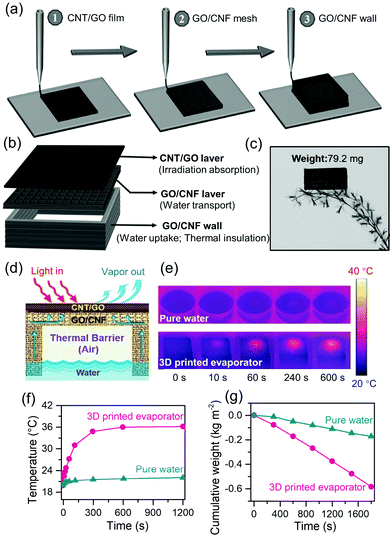 | ||
| Fig. 33 All-in-one 3D printed aerogel structure for solar steam generation. (a) Schematic diagram showing the 3D printing enabled fabrication process. (b) Three-layer structure of the 3D printed evaporator. (c) Photograph of light-weight evaporator. (d) Schematic illustration of solar steam generation. (e) Infrared images of pure water and the generator at various time points under 1 Sun illumination. (f) Curves showing surface temperature change of pure water and the generator as a function of irradiation time. (g) Cumulative weight loss of pure water and the generator through water evaporation over time. Reproduced with permission from ref. 216. Copyright 2017 WILEY-VCH. | ||
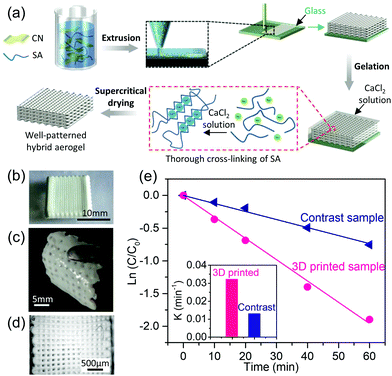 | ||
| Fig. 34 Photocatalysis application of printed Au/CN-SA aerogels. (a) Schematic illustration of the aerogel fabrication/printing process. Photographs of (b) a structure printed directly in air, (c) the printed structure after thorough cross-linking in a CaCl2 solution, and (d) supercritically dried aerogel. (e) Comparison of the photocatalytic methylene blue degradation rates (λ > 420 nm) of the 3D-printed structure and non-printed contrast aerogel sample. Adapted with permission from ref. 183. Copyright 2018 WILEY-VCH. | ||
6. Conclusions and perspectives
6.1 Research perspectives: challenges and future directions
In the past 5 years, significant breakthroughs have been achieved in combining aerogels and advanced printing technologies, leading to the realization of a variety of printed aerogels with a wide range of functionalities. Indeed, this has clearly brought a great improvement in aerogel properties and application-specific adaptability, considerably expanding their scope. However, this field is still in its infancy and great strides are needed before they can be widely accepted and exploited on an industrial scale as an additive manufacturing technology. In this section, we summarize the research achievements, and present our opinion on the key challenges that need to be overcome in the near- to mid-term from the perspectives of design and ink formulation, printing process, and application.(i) Design and synthesis of precursors: For printing oxide aerogels from molecule-derived formulation, the types of aerogel precursors that have been studied thus far are very few, and are limited to only SiO2-based precursors. New types of precursors and chemical structures need to be designed and synthesized for printing other materials and oxides such as polymers, Al2O3 and ZrO2.
For photocurable printed aerogels, the design and synthesis of new precursor materials containing both photocurable groups and hydrolysable polycondensation groups are required.
For the formulation of inks using the nanomaterial-derived route, another important research direction is the development of inexpensive material synthesis strategies such that the materials can be directly used for ink preparation without any purification and other processes.
(ii) Standardization of ink formulation: Generally, the lower the target aerogel density, the lower the concentration of corresponding materials in the ink, and consequently, the ink viscosity (and vice versa). To meet various printing requirements and wide target application performance (such as a wide density range), designing and synthesizing inks with appropriate rheological properties to fit different printing technologies has always been a difficult research challenge. For example, 3D extrusion requires inks not only with suitable viscosity but also with shear-thinning behaviour to ensure smooth extrusion and self-supported filaments after printing. This can be addressed by introducing different ink additives. Rapid gelation formulations through light excitation, gas reaction, and liquid bath are also promising means to address this issue. However, these approaches are mostly empirically derived and do not currently have a general understanding or standardized protocol. Such universal criterion for extrusion ink formulation can considerably simplify this step.
(iii) Design of hierarchically porous structures: For applications such as tissue engineering and catalysis, pore size gradient and hierarchical distribution is critical to reconcile and coordinate two competing properties of volume utilization rate and mass transfer.289,359 For 3D printing aerogel scaffolds, the pore structure of the printed aerogel consists of the nanopores of the aerogel itself and the voids between the printed filament structures (i.e., pores above the micrometer range). The design and optimization of these pore structures, including their pore size hierarchy and proportions are important determinants of the aerogel performance. In spite of this, very limited research in this area has been carried out thus far. For specific applications, conceiving the hierarchically ideal pore structure through theoretical calculations,289 then adjusting the print parameters, ink formulation, and gelation process to realize the designed pore structure and to verify mass transfer and related performance would be a game changer for the aerogel family of materials.
(iv) Strengthening of aerogel structure: Since aerogels are low-density, high-porosity materials, their mechanical strength is very low. For the case of layer by layer 3D printing, additional structural weakness may appear at the interface between the layers. The traditional method of reinforcement is to add fibres to form a composite material. In the field of printing, this may lead to new challenges in terms of ink rheology and printability. Therefore, understanding the effect of reinforcements, and the design of skeleton crosslinking through chemical reactions while maintaining the print performance, could form an important research direction. Adding short fibres or introducing special supramolecular interaction or dynamic covalent bond linking between the printed layers may address this, and improve the Z-direction strength. This could offer huge flexibility in ink formulation for printed aerogels.
(i) Printing of fine and intricate structures: Certain applications demand extremely strict size and resolution, and require the basic structural unit to be sufficiently small. For example, tissue engineering requires very high print dimensional accuracy to mimic the environment of the cell.360 In addition to the dimensional accuracy, the availability of printing complex structures to achieve the best mechanical strength is also an important prerequisite for structural design.361 Since 3D extrusion printing of aerogels requires the use of a slurry in a semi-gel state, controlling the dimensional accuracy of printing is very challenging. The size of the extrusion nozzle and the step resolution of the stages, also directly affects the print dimensions and accuracy. However, reducing the nozzle diameter may not always offer a solution as narrower nozzles are prone to clogging when nanomaterial derived inks are used. Although PmSLA 3D printing has been used to demonstrate print resolutions down to 10 microns, this has only been demonstrated on GO inks.244 Printing aerogels with high-resolution and intricate structures still remains as one of the key research challenges. To this end, research advances in two-photon polymerization could allow achieving print resolutions as fine as 100 nm.360
(ii) Exploitable aerogel materials: The majority of the printed aerogels have focused on nanomaterial-derived inks such as 1D CNF or CNC (Table 4) and 2D nanosheets (such as GO, Table 5). Formulation of molecule- (such as for Al2O3, ZrO2) or 0D nanoparticle- (such as metal, non-oxide ceramics) derived inks is less common. This is, in our opinion, primarily due to the challenges involved in ink formulation. For example, control of the cross-linking degree of Al2O3 or ZrO2 precursors, and therefore the viscosity, is more difficult than that of the precursors of SiO2. The time window of printability is also shorter. Similarly, the 0D nanoparticle-derived inks (such as metal and non-oxide ceramics) may struggle to form a solid structure after the binder is removed as the particles do not entangle like 1D nanofibres or 2D nanosheets. The investigation into these materials is critical to expand the application scope of aerogels. Indeed, the huge potential of these materials for high temperature catalysis or support (Al2O3, Pt, SiC, etc.), and electromagnetic shielding and energy devices (e.g., MXene) make them worth exploring.
(iii) Improving the print speed: The efficiencies of inkjet or screen printing for aerogel microspheres or films are somewhat acceptable. However, for 3D printing such as light- or extrusion-based techniques, the print speed and overall print efficiency are low for mass production. Light-based 3D printing usually has a very low print speed of only a few millimetres per hour in the vertical direction,142 and is limited by the rheology of the inks. The print-head movement speed in extrusion-based 3D printing is usually less than 20 mm s−1 (Table 7). These are unacceptable for industrial scale production. Therefore, relevant research in improving the efficiency of 3D printing of aerogels is very important towards their industrial exploitation. The development of new printing methods, such as multi-nozzle, multi-material printing may greatly increase the production efficiency.362 For light-based printing, computed axial lithography143 or DLP assisted by continuous liquid interface production142 could be used. However, considerable challenges in ink formulation with these photocurable resins and aerogel precursors remain, and require extensive investigation.
(i) Comprehensive testing of printed aerogels: Printed aerogels clearly exhibit advantages in both mechanism and performance parameters in a wide range of application areas. However, for printed aerogel technologies to be adopted in industrial production for commercial applications, in-depth research into long-term performance and stability is needed, in particular, the changes in microstructure under realistic operating conditions, performance degradation rates, and their causes. In addition, the toxicity, health effects, and degradation of printed aerogels are also subjects that need close investigation, especially for tissue engineering and drug delivery.
(ii) Standardization of design and processing: the use of printing technologies can greatly improve the flexibility of design and allow customization and quick realization of prototypes. However, the presence of multiple design software, materials, printing and drying methods, and application fields, without standardization, is a hindrance towards their practical use. To serve a wider range of applications, we believe that it is necessary to learn from the experience of industrialized large-scale production, and standardize the design software, ink, process, testing methods, product performance, and protective measures for handlers to achieve complete and comprehensive quality control, ensuring continuity, repeatability and unity of production.
The above research perspectives and directions involve multiple disciplines and require strengthening the relationship not only between different branches of academia (such as experts in software and hardware, chemistry, chemical engineering, materials, environment, bioengineering, medicine, and health and safety), but also between industry and academia. This would promote joint interdisciplinary research and accelerate the transfer of knowledge and expertise from the laboratory to the pilot scale, and then to large-scale industrial production to serve industrial progress, economic development, and environmental and societal wellbeing.
6.2 Concluding remarks
The world of aerogels is exciting and fascinating. Apart from the mature thermal insulation industry, research and applications of aerogels have thrived in the past decade. From thermal insulation to energy and biotechnology applications, the social and economic impact of these porous solids, in our opinion, will become increasingly apparent in the coming years. However, in combination with the rapidly progressing additive manufacturing technologies, printed aerogels have only just begun to appear in the past 5 years. Indeed, recent research efforts and attempts in this direction already indicate great potential in many fields of applications and new market opportunities by overcoming the limitations of current aerogel materials. This statement is especially relevant to processing. For example, the unique designs of macrostructures such as minimum-surface Schwarts, Gyroid, and Diamond structures could be efficiently realized by 3D printing to fine-tune and improve application-specific aerogel properties. Therefore, in spite of the future technological challenges discussed above, we are convinced that the adoption of printing technologies could herald a new generation of low-cost, customizable, and adaptable aerogels that could potentially create new functionalities and boost the existing application areas, in particular, in energy, sensing, and bio-medical fields.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. Z. Feng, J. Feng, and L. Wang acknowledge financial support from the National Natural Science Foundation of China (51712360). B. L. Su thanks the Program for Changjiang Scholars and Innovative Research Team in University (IRT_15R52) of the Ministry of Education, China, the National Natural Science Foundation of China (U1663225), the 111 Project (Grant No. B20002) from the Ministry of Science and Technology and the Ministry of Education, China. T. Hasan and O. Ogbeide acknowledges support from the Engineering and Physical Sciences Research Council (EP/L016087/1) and Alphasense Limited, United Kingdom.References
- N. Shaari and S. K. Kamarudin, Int. J. Energy Res., 2019, 43, 2447–2467 CrossRef.
- H. Maleki and N. Hüsing, Appl. Catal., B, 2018, 221, 530–555 CrossRef CAS.
- J. Yang, Y. Li, Y. Zheng, Y. Xu, Z. Zheng, X. Chen and W. Liu, Small, 2019, 15, 1902826 CrossRef CAS.
- H. Maleki, Chem. Eng. J., 2016, 300, 98–118 CrossRef CAS.
- X. Xu, Q. Zhang, M. Hao, Y. Hu, Z. Lin, L. Peng, T. Wang, X. Ren, C. Wang, Z. Zhao, C. Wan, H. Fei, L. Wang, J. Zhu, H. Sun, W. Chen, T. Du, B. Deng, G. J. Cheng, I. Shakir, C. Dames, T. S. Fisher, X. Zhang, H. Li, Y. Huang and X. Duan, Science, 2019, 363, 723–727 CrossRef CAS.
- S. Zhao, W. J. Malfait, N. Guerrero-Alburquerque, M. M. Koebel and G. Nyström, Angew. Chem., Int. Ed, 2018, 57, 7580–7608 CrossRef CAS.
- Y. Kebukawa, K. Okudaira, H. Yabuta, S. Hasegawa, M. Tabata, Y. Furukawa, M. Ito, A. Nakato, A. L. David Kilcoyne, K. Kobayashi, S. I. Yokobori, E. Imai, Y. Kawaguchi, H. Yano and A. Yamagishi, Geochem. J., 2019, 53, 53–67 CrossRef CAS.
- S. B. Riffat and G. Qiu, Int. J. Low-Carbon Technol., 2013, 8, 1–6 CrossRef CAS.
- I. Smirnova and P. Gurikov, Annu. Rev. Chem. Biomol. Eng., 2017, 8, 307–334 CrossRef.
- N. Bheekhun, A. R. A. Talib and M. R. Hassan, Adv. Mater. Sci. Eng., 2013, 406065 Search PubMed.
- K. Dong, X. Peng and Z. L. Wang, Adv. Mater., 2020, 32, 1902549 CrossRef CAS.
- J. D. Prince, J. Electron. Resour. Med. Libr., 2014, 11, 39–45 CrossRef.
- Manufacturing - The third industrial revolution | Leaders | The Economist, https://www.economist.com/leaders/2012/04/21/the-third-industrial-revolution, accessed 28 March 2020.
- A. Ambrosi and M. Pumera, Chem. Soc. Rev., 2016, 45, 2740–2755 RSC.
- B. Bhushan and M. Caspers, Microsyst. Technol., 2017, 23, 1117–1124 CrossRef.
- A. Zhakeyev, P. Wang, L. Zhang, W. Shu, H. Wang and J. Xuan, Adv. Sci., 2017, 4, 1700187 CrossRef.
- W. Gao, Y. Zhang, D. Ramanujan, K. Ramani, Y. Chen, C. B. Williams, C. C. L. Wang, Y. C. Shin, S. Zhang and P. D. Zavattieri, Comput. Des., 2015, 69, 65–89 Search PubMed.
- C. Zhu, T. Y. J. Han, E. B. Duoss, A. M. Golobic, J. D. Kuntz, C. M. Spadaccini and M. A. Worsley, Nat. Commun., 2015, 6, 6962 CrossRef CAS.
- S. Chandrasekaran, B. Yao, T. Liu, W. Xiao, Y. Song, F. Qian, C. Zhu, E. B. Duoss, C. M. Spadaccini, Y. Li and M. A. Worsley, Mater. Horiz., 2018, 5, 1166–1175 RSC.
- C. Zhu, T. Liu, F. Qian, T. Y. J. Han, E. B. Duoss, J. D. Kuntz, C. M. Spadaccini, M. A. Worsley and Y. Li, Nano Lett., 2016, 16, 3448–3456 CrossRef CAS.
- B. Yao, S. Chandrasekaran, J. Zhang, W. Xiao, F. Qian, C. Zhu, E. B. Duoss, C. M. Spadaccini, M. A. Worsley and Y. Li, Joule, 2019, 3, 459–470 CrossRef CAS.
- D. Sun, W. Liu, A. Tang, F. Guo and W. Xie, Soft Matter, 2019, 15, 8092–8101 RSC.
- W. Chen, Y. Xu, Y. Liu, Z. Wang, Y. Li, G. Jiang, X. Mo and G. Zhou, Mater. Des., 2019, 179, 107886 CrossRef CAS.
- S. S. Kistler, Nature, 1931, 127, 741 CrossRef CAS.
- J. Alemán, A. V. Chadwick, J. He, M. Hess, K. Horie, R. G. Jones, P. Kratochvíl, I. Meisel, I. Mita, G. Moad, S. Penczek and R. F. T. Stepto, Pure Appl. Chem., 2007, 79, 1801–1829 Search PubMed.
- A. C. Pierre and G. M. Pajonk, Chem. Rev., 2002, 102, 4243–4265 CrossRef CAS.
- C. Ziegler, A. Wolf, W. Liu, A. K. Herrmann, N. Gaponik and A. Eychmüller, Angew. Chem., Int. Ed, 2017, 56, 13200–13221 CrossRef CAS.
- “Aerogel.org” What is Aerogel? http://www.aerogel.org/?p=3, accessed 21 May 2020.
- S. M. Jung, H. Y. Jung, M. S. Dresselhaus, Y. J. Jung and J. Kong, Sci. Rep., 2012, 2, 849 CrossRef.
- D. Shin, J. Kim, C. Kim, K. Bae, S. Baek, G. Kang, Y. Urzhumov, D. R. Smith and K. Kim, Nat. Commun., 2017, 8, 16090 CrossRef CAS.
- L. Wang, J. Feng, Y. Jiang, S. Zhang, L. Li and J. Feng, J. Sol-Gel Sci. Technol., 2020, 94, 88–97 CrossRef CAS.
- F. Cao, L. Ren and X. Li, RSC Adv., 2015, 5, 18025–18028 RSC.
- G. O. Kiselev, A. P. Kiseleva, D. A. Ilatovskii, E. D. Koshevaya, D. A. Nazarovskaia, D. S. Gets, V. V. Vinogradov, P. V. Krivoshapkin and E. F. Krivoshapkina, Chem. Commun., 2019, 55, 8174–8177 RSC.
- Y. Kong, X. D. Shen and S. Cui, Mater. Lett., 2014, 117, 192–194 CrossRef CAS.
- A. E. Gash, T. M. Tillotson, J. H. Satcher Jr, L. W. Hrubesh and R. L. Simpson, J. Non. Cryst. Solids, 2001, 285, 22–28 CrossRef CAS.
- Y. J. Huang, Y. J. Lin, H. J. Chien, Y. F. Lin and K. C. Ho, Nanoscale, 2019, 11, 12507–12516 RSC.
- H. Bi, I. W. Chen, T. Lin and F. Huang, Adv. Mater., 2015, 27, 5943–5949 CrossRef CAS.
- M. B. Bryning, D. E. Milkie, M. F. Islam, L. A. Hough, J. M. Kikkawa and A. G. Yodh, Adv. Mater., 2007, 19, 661–664 CrossRef CAS.
- M. A. Worsley, S. O. Kucheyev, J. H. Satcher, A. V. Hamza and T. F. Baumann, Appl. Phys. Lett., 2009, 94, 073115 CrossRef.
- X. Zhao, W. Yao, W. Gao, H. Chen and C. Gao, Adv. Mater., 2017, 29, 1701482 CrossRef.
- Y. Jiang, Z. Xu, T. Huang, Y. Liu, F. Guo, J. Xi, W. Gao and C. Gao, Adv. Funct. Mater., 2018, 28, 1707024 CrossRef.
- R. W. Pekala, J. Mater. Sci., 1989, 24, 3221–3227 CrossRef CAS.
- M. Aghabararpour, M. Mohsenpour and S. Motahari, Mater. Res. Express, 2019, 6, 075059 CrossRef CAS.
- Z. Saadatnia, S. G. Mosanenzadeh, E. Esmailzadeh and H. E. Naguib, Sci. Rep., 2019, 9, 1370 CrossRef.
- J. Lyu, Z. Liu, X. Wu, G. Li, D. Fang and X. Zhang, ACS Nano, 2019, 13, 2236–2245 CrossRef.
- Y. Xiao, L. Li, S. Zhang, J. Feng, Y. Jiang and J. Feng, J. Mater. Sci., 2019, 54, 12951–12961 CrossRef CAS.
- P. Tseng, B. Napier, S. Zhao, A. N. Mitropoulos, M. B. Applegate, B. Marelli, D. L. Kaplan and F. G. Omenetto, Nat. Nanotechnol., 2017, 12, 474–480 CrossRef CAS.
- Z. Li, L. Zhong, T. Zhang, F. Qiu, X. Yue and D. Yang, ACS Sustainable Chem. Eng., 2019, 7, 9984–9994 CrossRef CAS.
- S. Takeshita, A. Sadeghpour, W. J. Malfait, A. Konishi, K. Otake and S. Yoda, Biomacromolecules, 2019, 20, 2051–2057 CrossRef CAS.
- R. Rodríguez-Dorado, C. López-Iglesias, C. García-González, G. Auriemma, R. Aquino and P. Del Gaudio, Molecules, 2019, 24, 1049 CrossRef.
- G. W. Nyce, J. R. Hayes, A. V. Hamza and J. H. Satcher, Chem. Mater., 2007, 19, 344–346 CrossRef CAS.
- F. Qian, A. Troksa, T. M. Fears, M. H. Nielsen, A. J. Nelson, T. F. Baumann, S. O. Kucheyev, T. Y.-J. J. Han and M. Bagge-Hansen, Nano Lett., 2020, 20, 131–135 CrossRef CAS.
- M. Georgi, B. Klemmed, A. Benad and A. Eychmüller, Mater. Chem. Front., 2019, 3, 1586–1592 RSC.
- P. Yan, E. Brown, Q. Su, J. Li, J. Wang, C. Xu, C. Zhou and D. Lin, Small, 2017, 13, 1701756 CrossRef.
- L. W. Yap, S. Gong, Y. Tang, Y. Zhu and W. Cheng, Sci. Bull., 2016, 61, 1624–1630 CrossRef CAS.
- M. Rousseas, A. P. Goldstein, W. Mickelson, M. A. Worsley, L. Woo and A. Zettl, ACS Nano, 2013, 7, 8540–8546 CrossRef CAS.
- L. Su, M. Li, H. Wang, M. Niu, D. Lu and Z. Cai, ACS Appl. Mater. Interfaces, 2019, 11, 15795–15803 CrossRef CAS.
- D. Lu, L. Su, H. Wang, M. Niu, L. Xu, M. Ma, H. Gao, Z. Cai and X. Fan, ACS Appl. Mater. Interfaces, 2019, 11, 45338–45344 CrossRef CAS.
- X. Zhang, R. Lv, A. Wang, W. Guo, X. Liu and J. Luo, Angew. Chem., Int. Ed., 2018, 57, 15028–15033 CrossRef CAS.
- L. Korala, J. R. Germain, E. Chen, I. R. Pala, D. Li and S. L. Brock, Inorg. Chem. Front., 2017, 4, 1451–1457 RSC.
- J. L. Mohanan, I. U. Arachchige and S. L. Brock, Science, 2005, 307, 397–400 CAS.
- A. Freytag, S. Sánchez-Paradinas, S. Naskar, N. Wendt, M. Colombo, G. Pugliese, J. Poppe, C. Demirci, I. Kretschmer, D. W. Bahnemann, P. Behrens and N. C. Bigall, Angew. Chem., Int. Ed, 2016, 55, 1200–1203 CrossRef CAS.
- V. Sayevich, B. Cai, A. Benad, D. Haubold, L. Sonntag, N. Gaponik, V. Lesnyak and A. Eychmüller, Angew. Chem., Int. Ed., 2016, 55, 6334–6338 CrossRef CAS.
- R. Wendt, B. Märker, A. Dubavik, A.-K. Herrmann, M. Wollgarten, Y. P. Rakovich, A. Eychmüller, K. Rademann and T. Hendel, J. Mater. Chem. C, 2017, 5, 10251–10259 RSC.
- B. Cai, K. Akkiraju, W. P. Mounfield, Z. Wang, X. Li, B. Huang, S. Yuan, D. Su, Y. Román-Leshkov and Y. Shao-Horn, Chem. Mater., 2019, 31, 9422–9429 CrossRef CAS.
- A. E. Aliev, J. Oh, M. E. Kozlov, A. A. Kuznetsov, S. Fang, A. F. Fonseca, R. Ovalle, M. D. Lima, M. H. Haque, Y. N. Gartstein, M. Zhang, A. A. Zakhidov and R. H. Baughman, Science, 2009, 323, 1575–1578 CrossRef CAS.
- H. Im, T. Kim, H. Song, J. Choi, J. S. Park, R. Ovalle-Robles, H. D. Yang, K. D. Kihm, R. H. Baughman, H. H. Lee, T. J. Kang and Y. H. Kim, Nat. Commun., 2016, 7, 10600 CrossRef CAS.
- Y. L. Li, I. A. Kinloch and A. H. Windle, Science, 2004, 304, 276–278 CrossRef CAS.
- J. T. Korhonen, P. Hiekkataipale, J. Malm, M. Karppinen, O. Ikkala and R. H. A. Ras, ACS Nano, 2011, 5, 1967–1974 CrossRef CAS.
- Y. Song, B. Li, S. Yang, G. Ding, C. Zhang and X. Xie, Sci. Rep., 2015, 5, 10337 CrossRef CAS.
- F. Matter, A. L. Luna and M. Niederberger, Nano Today, 2020, 30, 100827 CrossRef CAS.
- T. Linhares, M. T. Pessoa De Amorim and L. Durães, J. Mater. Chem. A, 2019, 7, 22768–22802 RSC.
- F. Peng, Y. Jiang, J. Feng, L. Li, H. Cai and J. Feng, J. Eur. Ceram. Soc., 2020, 40, 2480–2488 CrossRef CAS.
- Z. Zhao, D. Chen and X. Jiao, J. Phys. Chem. C, 2007, 111, 18738–18743 CrossRef CAS.
- Y. Nakanishi, Y. Hara, W. Sakuma, T. Saito, K. Nakanishi and K. Kanamori, ACS Appl. Nano Mater., 2020, 3, 49–54 CrossRef CAS.
- Y. Xiao, L. Li, S. Zhang, J. Feng, Y. Jiang and J. Feng, Macromol. Mater. Eng., 2019, 1900137 CrossRef.
- S. Zhang, J. Feng, J. Feng and Y. Jiang, Chem. Eng. J., 2017, 309, 700–707 CrossRef CAS.
- S. Bag, P. N. Trikalitis, P. J. Chupas, G. S. Armatas and M. G. Kanatzidis, Science, 2007, 317, 490–493 CrossRef CAS.
- K. S. Subrahmanyam, D. Sarma, C. D. Malliakas, K. Polychronopoulou, B. J. Riley, D. A. Pierce, J. Chun and M. G. Kanatzidis, Chem. Mater., 2015, 27, 2619–2626 CrossRef CAS.
- P. D. Brown, S. K. Gill and L. J. Hope-Weeks, J. Mater. Chem., 2011, 21, 4204–4208 RSC.
- B. J. Clapsaddle, B. Neumann, A. Wittstock, D. W. Sprehn, A. E. Gash, J. H. Satcher, R. L. Simpson and M. Bäumer, J. Sol-Gel Sci. Technol., 2012, 64, 381–389 CrossRef CAS.
- J. Mrowiec-Białoń, A. B. Jarzębski, L. Pajak, Z. Olejniczak and M. Gibas, Langmuir, 2004, 20, 10389–10393 CrossRef.
- D. Levy and M. Zayat, The Sol-Gel Handbook, 2015, vol. 2, pp. 519–574 Search PubMed.
- S. Karamikamkar, H. E. Naguib and C. B. Park, Adv. Colloid Interface Sci., 2020, 276, 102101 CrossRef CAS.
- S. D. Bhagat, H. Hirashima and A. Venkateswara Rao, J. Mater. Sci., 2007, 42, 3207–3214 CrossRef CAS.
- D. B. Mahadik, A. V. Rao, P. B. Wagh and S. C. Gupta, AIP Conference Proceedings, American Institute of PhysicsAIP, 2013, vol. 1536, pp. 553–554 Search PubMed.
- Z. Shao, F. Luo, X. Cheng and Y. Zhang, Mater. Chem. Phys., 2013, 141, 570–575 CrossRef CAS.
- F. Rechberger and M. Niederberger, Nanoscale Horiz., 2017, 2, 6–30 RSC.
- C. A. Saez Cabezas, G. K. Ong, R. B. Jadrich, B. A. Lindquist, A. Agrawal, T. M. Truskett and D. J. Milliron, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 8925–8930 CrossRef CAS.
- D. Wen, W. Liu, D. Haubold, C. Zhu, M. Oschatz, M. Holzschuh, A. Wolf, F. Simon, S. Kaskel and A. Eychmüller, ACS Nano, 2016, 10, 2559–2567 CrossRef CAS.
- R. Du, Y. Hu, R. Hübner, J. O. Joswig, X. Fan, K. Schneider and A. Eychmüller, Sci. Adv., 2019, 5, eaaw4590 CrossRef.
- L. Dong, W. Wang, J. Chen, X. Ding, B. Fang, X. Miao, Y. Liu, F. Yu, H. Xin and X. Wang, Biomater. Sci., 2018, 6, 2312–2315 RSC.
- H. Zhang, S. Lyu, X. Zhou, H. Gu, C. Ma, C. Wang, T. Ding, Q. Shao, H. Liu and Z. Guo, J. Colloid Interface Sci., 2019, 536, 245–251 CrossRef CAS.
- H. Yang, Z. Li, G. Sun, X. Jin, B. Lu, P. Zhang, T. Lin and L. Qu, Adv. Funct. Mater., 2019, 29, 1901917 CrossRef.
- G. Markevicius, R. Ladj, P. Niemeyer, T. Budtova and A. Rigacci, J. Mater. Sci., 2017, 52, 2210–2221 CrossRef CAS.
- P. R. Aravind, P. Shajesh, G. D. Soraru and K. G. K. Warrier, J. Sol-Gel Sci. Technol., 2010, 54, 105–117 CrossRef CAS.
- M. A. Einarsrud, S. Haereid and V. Wittwer, Sol. Energy Mater. Sol. Cells, 1993, 31, 341–347 CrossRef CAS.
- S. S. Prakash, C. J. Sankaran, A. J. Hurd and S. M. Rao, Nature, 1995, 374, 439–443 CrossRef CAS.
- D. Klvana, J. Chaouki, M. Repellin-Lacroix, G. Pajonk, M. Repellin-lacroix and G. Pajonk, J. Phys., Colloq., 1989, 50, 29–32 Search PubMed.
- X. Zhang, Z. Sui, B. Xu, S. Yue, Y. Luo, W. Zhan and B. Liu, J. Mater. Chem., 2011, 21, 6494–6497 RSC.
- M. J. Oh and P. J. Yoo, Korean J. Chem. Eng., 2020, 37, 189–208 CrossRef CAS.
- H. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS.
- Y. Si, X. Wang, C. Yan, L. Yang, J. Yu and B. Ding, Adv. Mater., 2016, 28, 9512–9518 CrossRef CAS.
- Z. Wang, R. Wei, J. Gu, H. Liu, C. Liu, C. Luo, J. Kong, Q. Shao, N. Wang, Z. Guo and X. Liu, Carbon, 2018, 139, 1126–1135 CrossRef CAS.
- W. Gao, N. Zhao, W. Yao, Z. Xu, H. Bai and C. Gao, RSC Adv., 2017, 7, 33600–33605 RSC.
- R. Bian, G. He, W. Zhi, S. Xiang, T. Wang and D. Cai, J. Mater. Chem. C, 2019, 7, 474–478 RSC.
- M. Peng, Z. Wen, L. Xie, J. Cheng, Z. Jia, D. Shi, H. Zeng, B. Zhao, Z. Liang, T. Li and L. Jiang, Adv. Mater., 2019, 31, 1902930 CrossRef.
- M. Han, X. Yin, K. Hantanasirisakul, X. Li, A. Iqbal, C. B. Hatter, B. Anasori, C. M. Koo, T. Torita, Y. Soda, L. Zhang, L. Cheng and Y. Gogotsi, Adv. Opt. Mater., 2019, 7, 1900267 CrossRef.
- Q. Zhang, F. Zhang, S. P. Medarametla, H. Li, C. Zhou and D. Lin, Small, 2016, 12, 1702–1708 CrossRef CAS.
- S. Liao, T. Zhai and H. Xia, J. Mater. Chem. A, 2016, 4, 1068–1077 RSC.
- P. Paraskevopoulou, D. Chriti, G. Raptopoulos and G. C. Anyfantis, Materials, 2019, 12, 1543 CrossRef CAS.
- Z. Xu, Y. Zhang, P. Li and C. Gao, ACS Nano, 2012, 6, 7103–7113 CrossRef CAS.
- Z. Xu, L. Peng, Y. Liu, Z. Liu, H. Sun, W. Gao and C. Gao, Chem. Mater., 2017, 29, 319–330 CrossRef CAS.
- G. Li, G. Hong, D. Dong, W. Song and X. Zhang, Adv. Mater., 2018, 30, 1801754 CrossRef.
- X. Y. Sun, W. B. Luo, J. Meng, X. Qing, W. Y. Fu, Y. Shuai and C. G. Wu, Sens. Actuators, A, 2015, 228, 69–74 CrossRef CAS.
- A. S. Gaikwad, S. A. Gupta and A. M. Mahajan, Mater. Res. Express, 2016, 3, 086403 CrossRef.
- X. Wang, Y. Zhang, J. Luo, D. Wang, H. Gao, J. Zhang, Y. Xing, Z. Yang, H. Cao and W. He, New J. Chem., 2018, 42, 6525–6531 RSC.
- M. A. B. Meador, E. J. Malow, R. Silva, S. Wright, D. Quade, S. L. Vivod, H. Guo, J. Guo and M. Cakmak, ACS Appl. Mater. Interfaces, 2012, 4, 536–544 CrossRef CAS.
- L. S. White, D. R. Echard, M. F. Bertino, X. Gao, S. Donthula, N. Leventis, N. Shukla, J. Kośny, S. Saeed and K. Saoud, Transl. Mater. Res., 2016, 3, 015002 CrossRef.
- G. Hu, J. Kang, L. W. T. Ng, X. Zhu, R. C. T. Howe, C. G. Jones, M. C. Hersam and T. Hasan, Chem. Soc. Rev., 2018, 47, 3265–3300 RSC.
- L. W. T. Ng, G. Hu, R. C. T. Howe, X. Zhu, Z. Yang, C. G. Jones, T. Hasan, L. W. T. Ng, G. Hu, R. C. T. Howe, X. Zhu, Z. Yang, C. G. Jones and T. Hasan, Printing of Graphene and Related 2D Materials, Springer International Publishing, 2018, pp. 103–134 Search PubMed.
- A. Moya, G. Gabriel, R. Villa and F. Javier del Campo, Curr. Opin. Electrochem., 2017, 3, 29–39 CrossRef CAS.
- A. Kamyshny and S. Magdassi, Chem. Soc. Rev., 2019, 48, 1712–1740 RSC.
- T. D. Ngo, A. Kashani, G. Imbalzano, K. T. Q. Nguyen and D. Hui, Composites, Part B, 2018, 143, 172–196 CrossRef CAS.
- P. Wu, J. Wang and X. Wang, Autom. Constr., 2016, 68, 21–31 CrossRef.
- 2012, ASTM International F2792-12a.
- A. J. Pinkerton, Opt. Laser Technol., 2016, 78, 25–32 CrossRef.
- T. DebRoy, T. Mukherjee, J. O. Milewski, J. W. Elmer, B. Ribic, J. J. Blecher and W. Zhang, Nat. Mater., 2019, 18, 1026–1032 CrossRef CAS.
- A. Nycz, M. W. Noakes, B. Richardson, A. Messing, B. Post, J. Paul, J. Flamm and L. Love, Solid Freeform Fabrication 2017, Proceedings of the 28th Annual International, 2017, pp. 24–33 Search PubMed.
- R. J. Mobbs, M. Coughlan, R. Thompson, C. E. Sutterlin and K. Phan, J. Neurosurg. Spine, 2017, 26, 513–518 Search PubMed.
- A. Al-Halhouli, L. Al-Ghussain, S. El Bouri, H. Liu and D. Zheng, Polymers, 2019, 11, 1518 CrossRef CAS.
- A. Al-Halhouli, L. Al-Ghussain, S. El Bouri, F. Habash, H. Liu and D. Zheng, Appl. Sci., 2020, 10, 480 CrossRef.
- T. Fiegl, M. Franke and C. Körner, Opt. Laser Technol., 2019, 111, 51–57 CrossRef CAS.
- J. H. Martin, B. D. Yahata, J. M. Hundley, J. A. Mayer, T. A. Schaedler and T. M. Pollock, Nature, 2017, 549, 365–369 CrossRef CAS.
- Y. Zheng, Z. Z. He, J. Yang and J. Liu, Sci. Rep., 2014, 4, 4588 CrossRef.
- S. Sun, X. Gan, Z. Wang, D. Fu, W. Pu and H. Xia, Addit. Manuf., 2020, 33, 101176 CAS.
- C. Zhang, X. Lu, G. Fei, Z. Wang, H. Xia and Y. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 44774–44782 CrossRef CAS.
- D. Lv, W. Chen, W. Shen, M. Peng, X. Zhang, R. Wang, L. Xu, W. Xu, W. Song and R. Tan, Sens. Actuators, B, 2019, 298, 126890 CrossRef CAS.
- T. Chen, O. R. Bilal, K. Shea and C. Daraio, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5698–5702 CrossRef CAS.
- J. H. Shin, J. H. Heo, S. Jeon, J. H. Park, S. Kim and H. W. Kang, J. Hazard. Mater., 2019, 365, 494–501 CrossRef CAS.
- D. A. Walker, J. L. Hedrick and C. A. Mirkin, Science, 2019, 366, 360–364 CrossRef CAS.
- J. R. Tumbleston, D. Shirvanyants, N. Ermoshkin, R. Janusziewicz, A. R. Johnson, D. Kelly, K. Chen, R. Pinschmidt, J. P. Rolland, A. Ermoshkin, E. T. Samulski and J. M. DeSimone, Science, 2015, 347, 1349–1352 CrossRef CAS.
- B. E. Kelly, I. Bhattacharya, H. Heidari, M. Shusteff, C. M. Spadaccini and H. K. Taylor, Science, 2019, 363, 1075–1079 CrossRef CAS.
- H. Chen, X. Wang, F. Xue, Y. Huang, K. Zhou and D. Zhang, J. Eur. Ceram. Soc., 2018, 38, 5294–5300 CrossRef CAS.
- H. Zhang, Y. Yang, B. Liu and Z. Huang, Ceram. Int., 2019, 45, 10800–10804 CrossRef CAS.
- Y. de Hazan and D. Penner, J. Eur. Ceram. Soc., 2017, 37, 5205–5212 CrossRef CAS.
- K. Terrani, B. Jolly and M. Trammell, J. Am. Ceram. Soc., 2020, 103, 1575–1581 CrossRef CAS.
- S. A. Brinckmann, N. Patra, J. Yao, T. H. Ware, C. P. Frick and R. S. Fertig, Adv. Eng. Mater., 2018, 20, 1800593 CrossRef.
- S. H. Park, M. Kaur, D. Yun and W. S. Kim, Langmuir, 2018, 34, 10897–10904 CrossRef CAS.
- B. Furet, P. Poullain and S. Garnier, Addit. Manuf., 2019, 28, 58–64 CAS.
- M. Rieu, M. Camara, G. Tournier, J. P. Viricelle, C. Pijolat, N. F. de Rooij and D. Briand, Sens. Actuators, B, 2016, 236, 1091–1097 CrossRef CAS.
- F. Yang, V. Tadepalli and B. J. Wiley, ACS Biomater. Sci. Eng., 2017, 3, 863–869 CrossRef CAS.
- H.-W. Kang, S. J. Lee, I. K. Ko, C. Kengla, J. J. Yoo and A. Atala, Nat. Biotechnol., 2016, 34, 312–319 CrossRef CAS.
- L. Yu, Z. Fan, Y. Shao, Z. Tian, J. Sun and Z. Liu, Adv. Energy Mater., 2019, 9, 1901839 CrossRef.
- J. W. Jung, J. S. Lee and D. W. Cho, Sci. Rep., 2016, 6, 21685 CrossRef CAS.
- B. Dutta and F. H. Sam Froes, Titanium Powder Metallurgy: Science, Technology and Applications, Elsevier Inc., 2015, pp. 447–468 Search PubMed.
- C. Gosselin, R. Duballet, P. Roux, N. Gaudillière, J. Dirrenberger and P. Morel, Mater. Des., 2016, 100, 102–109 CrossRef.
- C. A. García-González, J. J. Uy, M. Alnaief and I. Smirnova, Carbohydr. Polym., 2012, 88, 1378–1386 CrossRef.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- H. Cai, Y. Jiang, L. Li, J. Feng and J. Feng, J. Sol-Gel Sci. Technol., 2019, 91, 44–53 CrossRef CAS.
- A. G. Howard and N. H. Khdary, Mater. Lett., 2007, 61, 1951–1954 CrossRef CAS.
- C. Jiménez-Saelices, B. Seantier, B. Cathala and Y. Grohens, Carbohydr. Polym., 2017, 157, 105–113 CrossRef.
- E. Başaran, T. Alp Arıcı, A. Özcan, Ö. Gök and A. S. Özcan, J. Sol-Gel Sci. Technol., 2019, 89, 458–472 CrossRef.
- C. A. García-González and I. Smirnova, J. Supercrit. Fluids, 2013, 79, 152–158 CrossRef.
- Y. Wang, Y. Cui, Z. Shao, W. Gao, W. Fan, T. Liu and H. Bai, Chem. Eng. J., 2020, 390, 124623 CrossRef CAS.
- Z. Wang, H. Yang, Y. Li and X. Zheng, ACS Appl. Mater. Interfaces, 2020, 12, 15726–15736 CrossRef CAS.
- I. Karadagli, B. Schulz, M. Schestakow, B. Milow, T. Gries and L. Ratke, J. Supercrit. Fluids, 2015, 106, 105–114 CrossRef CAS.
- J. Mroszczok, B. Schulz, K. Wilsch, G. Frenzer, S. Kasper and G. Seide, Mater. Today Proc., 2017, 4, S244–S248 CrossRef.
- B. Schulz, T. Meinert, D. Bierbüsse, M. Busen, N. Körtzinger, M. Stankowski and G. Seide, Chem. Ing. Tech., 2016, 88, 1501–1507 CrossRef CAS.
- J. Zhou and Y. Lo Hsieh, Nano Energy, 2020, 68, 104305 CrossRef CAS.
- T. Xue, H. Yang, B. Shen, F. Li, M. Su, X. Hu, W. Liu and Y. Song, J. Mater. Chem. C, 2019, 7, 6317–6322 RSC.
- B. Huo, D. Jiang, X. Cao, H. Liang, Z. Liu, C. Li and J. Liu, Carbon, 2019, 142, 13–19 CrossRef CAS.
- V. D. Phadtare, V. G. Parale, T. Kim, K. Y. Lee, S. Pathak and H. H. Park, J. Alloys Compd., 2020, 823, 153847 CrossRef CAS.
- L. Liu, Z. Cai, S. Lin and X. Hu, ACS Appl. Nano Mater., 2018, 1, 4910–4917 CrossRef CAS.
- J. Li, Y. Zhang, K. Ma, X. De Pan, C. X. Li, G. J. Yang and C. J. Li, J. Therm. Spray Technol., 2018, 27, 471–482 CrossRef CAS.
- V. C. F. Li, A. Mulyadi, C. K. Dunn, Y. Deng and H. J. Qi, ACS Sustainable Chem. Eng., 2018, 6, 2011–2022 CrossRef CAS.
- N. Teo, P. Joo, E. J. Amis and S. C. Jana, ACS Appl. Polym. Mater., 2019, 1, 1749–1756 CrossRef CAS.
- Y. Lin, F. Liu, G. Casano, R. Bhavsar, I. A. Kinloch and B. Derby, Adv. Mater., 2016, 28, 7993–8000 CrossRef CAS.
- S. Zhao, G. Siqueira, S. Drdova, D. Norris, C. Ubert, A. Bonnin, S. Galmarini, M. Ganobjak, Z. Pan, S. Brunner, G. Nyström, J. Wang, M. M. Koebel and W. J. Malfait, Nature, 2020, 584, 387–392 CrossRef CAS.
- Y. Ge, T. Zhang, B. Zhou, H. Wang, Z. Zhang, J. Shen and A. Du, J. Mater. Res., 2018, 33, 2052–2061 CrossRef CAS.
- V. C. F. Li, C. K. Dunn, Z. Zhang, Y. Deng and H. J. Qi, Sci. Rep., 2017, 7, 8018 CrossRef.
- F. Lübkemann, J. F. Miethe, F. Steinbach, P. Rusch, A. Schlosser, D. Zámbó, T. Heinemeyer, D. Natke, D. Zok, D. Dorfs and N. C. Bigall, Small, 2019, 15, 1902186 CrossRef.
- P. He, X. Tang, L. Chen, P. Xie, L. He, H. Zhou, D. Zhang and T. Fan, Adv. Funct. Mater., 2018, 28, 1801121 CrossRef.
- B. A. García-Torres, A. Aguilar-Elguezabal, M. Román-Aguirre and L. Álvarez-Contreras, Mater. Chem. Phys., 2016, 172, 32–38 CrossRef.
- F. Meng, Z. Dehouche, A. Nutasarin and G. R. Fern, Int. J. Energy Res., 2018, 42, 3915–3927 CrossRef CAS.
- X. Tang, H. Zhou, Z. Cai, D. Cheng, P. He, P. Xie, D. Zhang and T. Fan, ACS Nano, 2018, 12, 3502–3511 CrossRef CAS.
- L. Wang, S. Chen, T. Shu and X. Hu, ChemSusChem, 2020, 13, 1330–1353 CrossRef CAS.
- H. Maleki, L. Durães, C. A. García-González, P. del Gaudio, A. Portugal and M. Mahmoudi, Adv. Colloid Interface Sci., 2016, 236, 1–27 CrossRef CAS.
- S. M. S. Murshed and P. Estellé, Renewable Sustainable Energy Rev., 2017, 76, 1134–1152 CrossRef CAS.
- R. P. Chhabra, Rheology of Complex Fluids, SpringerNew York, 2010, pp. 3–34 Search PubMed.
- M. Zeng and Y. Zhang, J. Mater. Chem. A, 2019, 7, 23301–23336 RSC.
- M. R. Somalu, A. Muchtar, W. R. W. Daud and N. P. Brandon, Renewable Sustainable Energy Rev., 2017, 75, 426–439 CrossRef CAS.
- H. W. Lin, C. P. Chang, W. H. Hwu and M. Der Ger, J. Mater. Process. Technol., 2008, 197, 284–291 CrossRef CAS.
- R. Durairaj, A. Seman and N. N. Ekere, in ESTC 2006 - 1st Electronics Systemintegration Technology Conference, Institute of Electrical and Electronics Engineers Inc., 2006, pp. 347–353.
- M. Wei, F. Zhang, W. Wang, P. Alexandridis, C. Zhou and G. Wu, J. Power Sources, 2017, 354, 134–147 CrossRef CAS.
- B. Derby, Annu. Rev. Mater. Res., 2010, 40, 395–414 CrossRef CAS.
- B. Derby, N. Reis, K. A. M. Seerden, P. S. Grant and J. R. G. Evans, Materials Research Society Symposium - Proceedings, 2000, vol. 625, pp. 195–201 Search PubMed.
- F. Torrisi, T. Hasan, W. Wu, Z. Sun, A. Lombardo, T. S. Kulmala, G. W. Hsieh, S. Jung, F. Bonaccorso, P. J. Paul, D. Chu and A. C. Ferrari, ACS Nano, 2012, 6, 2992–3006 CrossRef CAS.
- G. Hu, L. Yang, Z. Yang, Y. Wang, X. Jin, J. Dai, Q. Wu, S. Liu, X. Zhu, X. Wang, T. C. Wu, R. C. T. Howe, T. Albrow-Owen, L. W. T. Ng, Q. Yang, L. G. Occhipinti, R. I. Woodward, E. J. R. Kelleher, Z. Sun, X. Huang, M. Zhang, C. D. Bain and T. Hasan, Sci. Adv., 2020, 6, eaba5029 CrossRef CAS.
- G. Hu, T. Albrow-Owen, X. Jin, A. Ali, Y. Hu, R. C. T. Howe, K. Shehzad, Z. Yang, X. Zhu, R. I. Woodward, T. C. Wu, H. Jussila, J. Bin Wu, P. Peng, P. H. Tan, Z. Sun, E. J. R. Kelleher, M. Zhang, Y. Xu and T. Hasan, Nat. Commun., 2017, 8, 278 CrossRef.
- J. Dai, O. Ogbeide, N. Macadam, Q. Sun, W. Yu, Y. Li, B. L. Su, T. Hasan, X. Huang and W. Huang, Chem. Soc. Rev., 2020, 49, 1756–1789 RSC.
- J. Rumble, CRC handbook of chemistry and physics, CRC Press, 100th edn, 2019 Search PubMed.
- S. Naficy, R. Jalili, S. H. Aboutalebi, R. A. Gorkin, K. Konstantinov, P. C. Innis, G. M. Spinks, P. Poulin and G. G. Wallace, Mater. Horiz., 2014, 1, 326–331 RSC.
- L. W. T. Ng, X. Zhu, G. Hu, N. Macadam, D. Um, T. Wu, F. Le Moal, C. Jones and T. Hasan, Adv. Funct. Mater., 2019, 29, 1807933 CrossRef.
- S. Saeed, R. M. Al-Sobaihi, M. F. Bertino, L. S. White and K. M. Saoud, J. Mater. Chem. A, 2015, 3, 17606–17611 RSC.
- I. Cooperstein, E. Shukrun, O. Press, A. Kamyshny and S. Magdassi, ACS Appl. Mater. Interfaces, 2018, 10, 18879–18885 CrossRef CAS.
- H. Maleki, S. Montes, N. Hayati-Roodbari, F. Putz and N. Huesing, ACS Appl. Mater. Interfaces, 2018, 10, 22718–22730 CrossRef CAS.
- K. M. Saoud, S. Saeed, M. F. Bertino and L. S. White, J. Porous Mater., 2018, 25, 511–520 CrossRef CAS.
- C. López-Iglesias, A. M. Casielles, A. Altay, R. Bettini, C. Alvarez-Lorenzo and C. A. García-González, Chem. Eng. J., 2019, 357, 559–566 CrossRef.
- F. P. W. Melchels, J. Feijen and D. W. Grijpma, Biomaterials, 2010, 31, 6121–6130 CrossRef CAS.
- J. Koo, J. W. Kim, M. Kim, S. Yoon and J. H. Shim, Int. J. Precis. Eng. Manuf. - Green Technol., 2020 DOI:10.1007/s40684-020-00189-4.
- I. Põldsalu, M. Harjo, T. Tamm, M. Uibu, A. L. Peikolainen and R. Kiefer, Sens. Actuators, B, 2017, 250, 44–51 CrossRef.
- I. Põldsalu, U. Johanson, T. Tamm, A. Punning, F. Greco, A. L. Peikolainen, R. Kiefer and A. Aabloo, Synth. Met., 2018, 246, 122–127 CrossRef.
- J. S. Gebauer, V. Mackert, S. Ognjanović and M. Winterer, J. Colloid Interface Sci., 2018, 526, 400–409 CrossRef CAS.
- F. Kotz, K. Arnold, W. Bauer, D. Schild, N. Keller, K. Sachsenheimer, T. M. Nargang, C. Richter, D. Helmer and B. E. Rapp, Nature, 2017, 544, 337–339 CrossRef CAS.
- Y. Li, T. Gao, Z. Yang, C. Chen, W. Luo, J. Song, E. Hitz, C. Jia, Y. Zhou, B. Liu, B. Yang and L. Hu, Adv. Mater., 2017, 29, 1700981 CrossRef.
- S. Liu, X. Shi, X. Li, Y. Sun, J. Zhu, Q. Pei, J. Liang and Y. Chen, Nanoscale, 2018, 10, 20096–20107 RSC.
- D. Cao, Y. Xing, K. Tantratian, X. Wang, Y. Ma, A. Mukhopadhyay, Z. Cheng, Q. Zhang, Y. Jiao, L. Chen and H. Zhu, Adv. Mater., 2019, 31, 1807313 CrossRef.
- L. Li, Y. Zhu and J. Yang, Mater. Lett., 2018, 210, 136–138 CrossRef CAS.
- D. Kam, M. Chasnitsky, C. Nowogrodski, I. Braslavsky, T. Abitbol, S. Magdassi and O. Shoseyov, Colloids Interfaces, 2019, 3, 46 CrossRef CAS.
- S. Sultan and A. P. Mathew, Nanoscale, 2018, 10, 4421–4431 RSC.
- J. Virtanen, M. Janka and S. Tuukkanen, IFMBE Proc. 65, 2017, 65, 1029–1032 Search PubMed.
- Q. Cheng, Y. Liu, J. Lyu, Q. Lu, X. Zhang and W. Song, J. Mater. Chem. A, 2020, 8, 14243–14253 RSC.
- H. Zhu, W. Luo, P. N. Ciesielski, Z. Fang, J. Y. Zhu, G. Henriksson, M. E. Himmel and L. Hu, Chem. Rev., 2016, 116, 9305–9374 CrossRef CAS.
- E. Espinosa, D. Filgueira, A. Rodríguez and G. Chinga-Carrasco, Bioengineering, 2019, 6, 65 CrossRef CAS.
- H. Françon, Z. Wang, A. Marais, K. Mystek, A. Piper, H. Granberg, A. Malti, P. Gatenholm, P. A. Larsson and L. Wågberg, Adv. Funct. Mater., 2020, 30, 1909383 CrossRef.
- K. M. O. Håkansson, I. C. Henriksson, C. de la Peña Vázquez, V. Kuzmenko, K. Markstedt, P. Enoksson and P. Gatenholm, Adv. Mater. Technol., 2016, 1, 1600096 CrossRef.
- C. Qian, L. Li, M. Gao, H. Yang, Z. Cai, B. Chen, Z. Xiang, Z. Zhang and Y. Song, Nano Energy, 2019, 63, 103885 CrossRef CAS.
- A. J. Hess, A. J. Funk, Q. Liu, J. A. De La Cruz, G. H. Sheetah, B. Fleury and I. I. Smalyukh, ACS Omega, 2019, 4, 20558–20563 CrossRef CAS.
- R. Ajdary, S. Huan, N. Zanjanizadeh Ezazi, W. Xiang, R. Grande, H. A. Santos and O. J. Rojas, Biomacromolecules, 2019, 20, 2770–2778 CrossRef CAS.
- V. G. Rocha, E. García-Tuñón, C. Botas, F. Markoulidis, E. Feilden, E. D’Elia, N. Ni, M. Shaffer and E. Saiz, ACS Appl. Mater. Interfaces, 2017, 9, 37136–37145 CrossRef CAS.
- K. Huang, J. Yang, S. Dong, Q. Feng, X. Zhang, Y. Ding and J. Hu, Carbon, 2018, 130, 1–10 CrossRef.
- S. Ling, W. Kang, S. Tao and C. Zhang, Nano Mater. Sci., 2019, 1, 142–148 CrossRef.
- Z. Qi, J. Ye, W. Chen, J. Biener, E. B. Duoss, C. M. Spadaccini, M. A. Worsley and C. Zhu, Adv. Mater. Technol., 2018, 3, 1800053 CrossRef.
- B. An, Y. Ma, W. Li, M. Su, F. Li and Y. Song, Chem. Commun., 2016, 52, 10948–10951 RSC.
- F. Guo, X. Zheng, C. Liang, Y. Jiang, Z. Xu, Z. Jiao, Y. Liu, H. T. Wang, H. Sun, L. Ma, W. Gao, A. Greiner, S. Agarwal and C. Gao, ACS Nano, 2019, 13, 5549–5558 CrossRef CAS.
- B. Yao, S. Chandrasekaran, H. Zhang, A. Ma, J. Kang, L. Zhang, X. Lu, F. Qian, C. Zhu, E. B. Duoss, C. M. Spadaccini, M. A. Worsley and Y. Li, Adv. Mater., 2020, 32, 1906652 CrossRef CAS.
- Y. Yu, Z. Wang, Z. Hou, W. Ta, W. Wang, X. Zhao, Q. Li, Y. Zhao, Q. Zhang and Z. Quan, ACS Appl. Energy Mater., 2019, 2, 3869–3877 CrossRef CAS.
- G. De La Osa, D. Pérez-Coll, P. Miranzo, M. I. Osendi and M. Belmonte, Chem. Mater., 2016, 28, 6321–6328 CrossRef CAS.
- G. Shi, S. E. Lowe, A. J. T. Teo, T. K. Dinh, S. H. Tan, J. Qin, Y. Zhang, Y. L. Zhong and H. Zhao, Appl. Mater. Today, 2019, 16, 482–492 CrossRef.
- C. Ma, R. Wang, H. Tetik, S. Gao, M. Wu, Z. Tang, D. Lin, D. Ding and W. Wu, Nano Energy, 2019, 66, 104124 CrossRef CAS.
- E. Brown, P. Yan, H. Tekik, A. Elangovan, J. Wang, D. Lin and J. Li, Mater. Des., 2019, 170, 107689 CrossRef CAS.
- Q. Zhang, F. Zhang, X. Xu, C. Zhou and D. Lin, ACS Nano, 2018, 12, 1096–1106 CrossRef CAS.
- R. M. Hensleigh, H. Cui, J. S. Oakdale, J. C. Ye, P. G. Campbell, E. B. Duoss, C. M. Spadaccini, X. Zheng and M. A. Worsley, Mater. Horiz., 2018, 5, 1035–1041 RSC.
- Z. Wang, X. Liu, X. Shen, N. M. Han, Y. Wu, Q. Zheng, J. Jia, N. Wang and J. K. Kim, Adv. Funct. Mater., 2018, 28, 1707043 CrossRef.
- Y. Chen, M. Guo, L. He, W. Yang, L. Xu, J. Meng, X. Tian, X. Ma, Q. Yu, K. Yang, X. Hong and L. Mai, J. Mater., 2019, 5, 303–312 Search PubMed.
- V. Middelkoop, T. Slater, M. Florea, F. Neaţu, S. Danaci, V. Onyenkeadi, K. Boonen, B. Saha, I. A. Baragau and S. Kellici, J. Clean. Prod., 2019, 214, 606–614 CrossRef CAS.
- Z. Wang, Q. Zhang, S. Long, Y. Luo, P. Yu, Z. Tan, J. Bai, B. Qu, Y. Yang, J. Shi, H. Zhou, Z. Y. Xiao, W. Hong and H. Bai, ACS Appl. Mater. Interfaces, 2018, 10, 10437–10444 CrossRef CAS.
- X. Tang, C. Zhu, D. Cheng, H. Zhou, X. Liu, P. Xie, Q. Zhao, D. Zhang and T. Fan, Adv. Funct. Mater., 2018, 28, 1805057 CrossRef.
- J. Wang, D. Liu, Q. Li, C. Chen, Z. Chen, P. Song, J. Hao, Y. Li, S. Fakhrhoseini, M. Naebe, X. Wang and W. Lei, ACS Nano, 2019, 13, 7860–7870 CrossRef CAS.
- M. A. Worsley, S. J. Shin, M. D. Merrill, J. Lenhardt, A. J. Nelson, L. Y. Woo, A. E. Gash, T. F. Baumann and C. A. Orme, ACS Nano, 2015, 9, 4698–4705 CrossRef CAS.
- H. Long, L. Chan, A. Harley-Trochimczyk, L. E. Luna, Z. Tang, T. Shi, A. Zettl, C. Carraro, M. A. Worsley and R. Maboudian, Adv. Mater. Interfaces, 2017, 4, 1700217 CrossRef.
- W. Yao, R. Mao, W. Gao, W. Chen, Z. Xu and C. Gao, Carbon, 2020, 158, 418–425 CrossRef CAS.
- X. Feng, J. Zhao, D. Sun, L. Shanmugam, J. K. Kim and J. Yang, J. Mater. Chem. A, 2019, 7, 4400–4407 RSC.
- H. Bian, J. Wei, Y. Zhou, J. Liu, Z. Shao, B. Wang and Z. Li, Propellants, Explos., Pyrotech., 2019, 44, 1613–1620 CrossRef CAS.
- J. Ma, P. Wang, L. Dong, Y. Ruan and H. Lu, J. Colloid Interface Sci., 2019, 534, 12–19 CrossRef CAS.
- L. Dong, Z. Chen, X. Zhao, J. Ma, S. Lin, M. Li, Y. Bao, L. Chu, K. Leng, H. Lu and K. P. Loh, Nat. Commun., 2018, 9, 76 CrossRef.
- F. Guo, Y. Jiang, Z. Xu, Y. Xiao, B. Fang, Y. Liu, W. Gao, P. Zhao, H. Wang and C. Gao, Nat. Commun., 2018, 9, 881 CrossRef.
- L. Liu, Y. Huang, F. Li, Y. Ma, W. Li, M. Su, X. Qian, W. Ren, K. Tang and Y. Song, Chem. Commun., 2018, 54, 4810–4813 RSC.
- J. Zhao, Y. Zhang, X. Zhao, R. Wang, J. Xie, C. Yang, J. Wang, Q. Zhang, L. Li, C. Lu and Y. Yao, Adv. Funct. Mater., 2019, 29, 1900809 CrossRef.
- K. Fu, Y. Wang, C. Yan, Y. Yao, Y. Chen, J. Dai, S. Lacey, Y. Wang, J. Wan, T. Li, Z. Wang, Y. Xu and L. Hu, Adv. Mater., 2016, 28, 2587–2594 CrossRef CAS.
- T. Liu, C. Zhu, T. Kou, M. A. Worsley, F. Qian, C. Condes, E. B. Duoss, C. M. Spadaccini and Y. Li, ChemNanoMat, 2016, 2, 635–641 CrossRef CAS.
- X. Yun, B. Lu, Z. Xiong, B. Jia, B. Tang, H. Mao, T. Zhang and X. Wang, RSC Adv., 2019, 9, 29384–29395 RSC.
- J. Ding, K. Shen, Z. Du, B. Li and S. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 41871–41877 CrossRef CAS.
- F. Brunetti, A. Operamolla, S. Castro-Hermosa, G. Lucarelli, V. Manca, G. M. Farinola and T. M. Brown, Adv. Funct. Mater., 2019, 29, 1806798 CrossRef.
- L. S. White, T. Selden, M. F. Bertino, C. Cartin, J. Angello, M. Schwan, B. Milow and L. Ratke, Ind. Eng. Chem. Res., 2018, 57, 1197–1206 CrossRef CAS.
- F. Bonaccorso, A. Lombardo, T. Hasan, Z. Sun, L. Colombo and A. C. Ferrari, Mater. Today, 2012, 15, 564–589 CrossRef CAS.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun’ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS.
- J. N. Coleman, M. Lotya, A. O’Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H. Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS.
- P. Wei, H. Leng, Q. Chen, R. C. Advincula and E. B. Pentzer, ACS Appl. Polym. Mater., 2019, 1, 885–892 CrossRef CAS.
- J. L. Gurav, I. K. Jung, H. H. Park, E. S. Kang and D. Y. Nadargi, J. Nanomater., 2010, 409310 Search PubMed.
- N. Hüsing and U. Schubert, Angew. Chem., Int. Ed., 1998, 37, 22–45 CrossRef.
- W. Liao, H. B. Zhao, Z. Liu, S. Xu and Y. Z. Wang, Composites, Part B, 2019, 173, 107036 CrossRef CAS.
- L. Ren, S. Cui, F. Cao and Q. Guo, Angew. Chem., Int. Ed., 2014, 53, 10147–10149 CrossRef CAS.
- J. J. Moyano, A. Gómez-Gómez, D. Pérez-Coll, M. Belmonte, P. Miranzo and M. I. Osendi, Carbon, 2019, 151, 94–102 CrossRef CAS.
- C. L. Yaws, H.-C. Yang and X. Pan, Chem. Eng., 1991, 98, 140–149 CAS.
- G. Körösi and E. S. Kováts, J. Chem. Eng. Data, 1981, 26, 323–332 CrossRef.
- G. W. Kauffman and P. C. Jurs, J. Chem. Inf. Comput. Sci., 2001, 41, 408–418 CrossRef CAS.
- D. R. Lide, CRC Handbook of Chemistry and Physics, 85th Edn, CRC Press, 2004 Search PubMed.
- R. Sui and P. Charpentier, Chem. Rev., 2012, 112, 3057–3082 CrossRef CAS.
- D. W. Matson and R. D. Smith, J. Am. Ceram. Soc., 1989, 72, 871–881 CrossRef CAS.
- A. H. N. Mousa, J. Chem. Thermodyn., 1977, 9, 1063–1065 CrossRef CAS.
- L. M. Radzhabova, G. V. Stepanov and I. M. Abdulagatov, Fluid Phase Equilib., 2011, 309, 128–144 CrossRef CAS.
- Y. Jiang, J. Feng and J. Feng, J. Sol-Gel Sci. Technol., 2017, 83, 64–71 CrossRef CAS.
- D. B. Mahadik, Y. K. Lee, C. S. Park, H. Y. Chung, M. H. Hong, H. N. R. Jung, W. Han and H. H. Park, Solid State Sci., 2015, 50, 1–8 CrossRef CAS.
- C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- P. C. Thapliyal and K. Singh, J. Mater., 2014, 127049 Search PubMed.
- X. Wei, S. Wan and S. Gao, Nano Energy, 2016, 28, 206–215 CrossRef CAS.
- X. Zheng, G. Shen, C. Wang, Y. Li, D. Dunphy, T. Hasan, C. J. Brinker and B. L. Su, Nat. Commun., 2017, 8, 14921 CrossRef CAS.
- L. Wu, Y. Li, Z. Fu and B.-L. Su, Natl. Sci. Rev., 2020, 7, 1667–1701 CrossRef.
- L. Fan, Y. Zhang, Z. Guo, B. Sun, D. Tian, Y. Feng, N. Zhang and K. Sun, Chem. – Eur. J., 2020, 26, 9314–9318 CrossRef CAS.
- Q. Zheng, A. Kvit, Z. Cai, Z. Ma and S. Gong, J. Mater. Chem. A, 2017, 5, 12528–12541 RSC.
- D. Ghosh, J. Lim, R. Narayan and S. O. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 22253–22260 CrossRef CAS.
- C. Wan, Y. Jiao, W. Bao, H. Gao, Y. Wu and J. Li, J. Mater. Chem. A, 2019, 7, 9556–9564 RSC.
- Z. Pan, M. Liu, J. Yang, Y. Qiu, W. Li, Y. Xu, X. Zhang and Y. Zhang, Adv. Funct. Mater., 2017, 27, 1701122 CrossRef.
- W. Sun, G. Gao, Y. Du, K. Zhang and G. Wu, J. Mater. Chem. A, 2018, 6, 9938–9947 RSC.
- H. Zhang, A. Xie, C. Wang, H. Wang, Y. Shen and X. Tian, ChemPhysChem, 2014, 15, 366–373 CrossRef CAS.
- Y. Xu, Z. Lin, X. Huang, Y. Liu, Y. Huang and X. Duan, ACS Nano, 2013, 7, 4042–4049 CrossRef CAS.
- L. Liu, Y. Yan, Z. Cai, S. Lin and X. Hu, Adv. Mater. Interfaces, 2018, 5, 1701548 CrossRef.
- D. Lin, Y. Liu and Y. Cui, Nat. Nanotechnol., 2017, 12, 194–206 CrossRef CAS.
- D. Tie, S. Huang, J. Wang, J. Ma, J. Zhang and Y. Zhao, Energy Storage Mater., 2019, 21, 22–40 CrossRef.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS.
- G. Nagaraju, S. Chandra Sekhar, L. Krishna Bharat and J. S. Yu, ACS Nano, 2017, 11, 10860–10874 CrossRef CAS.
- R. Liu, L. Ma, G. Niu, X. Li, E. Li, Y. Bai and G. Yuan, Adv. Funct. Mater., 2017, 27, 1701635 CrossRef.
- Y. Zhao, H. Ma, S. Huang, X. Zhang, M. Xia, Y. Tang and Z. F. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 22997–23066 CrossRef CAS.
- Z. Gao, C. Chen, J. Chang, L. Chen, P. Wang, D. Wu, F. Xu and K. Jiang, Chem. Eng. J., 2018, 343, 572–582 CrossRef CAS.
- Y. Chen, B. Xu, J. Wen, J. Gong, T. Hua, C.-W. Kan and J. Deng, Small, 2018, 14, 1704373 CrossRef.
- N. Chen, C. Han, R. Shi, L. Xu, H. Li, Y. Liu, J. Li and B. Li, Electrochim. Acta, 2018, 283, 36–44 CrossRef CAS.
- H. Sun, L. Mei, J. Liang, Z. Zhao, C. Lee, H. Fei, M. Ding, J. Lau, M. Li, C. Wang, X. Xu, G. Hao, B. Papandrea, I. Shakir, B. Dunn, Y. Huang and X. Duan, Science, 2017, 356, 599–604 CrossRef CAS.
- S. Choi, S. I. Han, D. Kim, T. Hyeon and D. H. Kim, Chem. Soc. Rev., 2019, 48, 1566–1595 RSC.
- J. Huang, J. Zeng, B. Liang, J. Wu, T. Li, Q. Li, F. Feng, Q. Feng, M. J. Rood and Z. Yan, ACS Appl. Mater. Interfaces, 2020, 12, 16822–16830 CrossRef CAS.
- C. Yang, W. Liu, N. Liu, J. Su, L. Li, L. Xiong, F. Long, Z. Zou and Y. Gao, ACS Appl. Mater. Interfaces, 2019, 11, 33165–33172 CrossRef CAS.
- X. Hou, R. Zhang and D. Fang, Ceram. Int., 2020, 46, 2122–2127 CrossRef CAS.
- T. R. Ray, J. Choi, A. J. Bandodkar, S. Krishnan, P. Gutruf, L. Tian, R. Ghaffari and J. A. Rogers, Chem. Rev., 2019, 119, 5461–5533 CrossRef CAS.
- K. Kruusamäe, A. Punning, A. Aabloo and K. Asaka, Actuators, 2015, 4, 17–38 CrossRef.
- D. Chen and Q. Pei, Chem. Rev., 2017, 117, 11239–11268 CrossRef CAS.
- Q. Zheng, B. Shi, Z. Li and Z. L. Wang, Adv. Sci., 2017, 4, 1700029 CrossRef.
- F. Yi, H. Ren, J. Shan, X. Sun, D. Wei and Z. Liu, Chem. Soc. Rev., 2018, 47, 3152–3188 RSC.
- J. Nie, X. Chen and Z. L. Wang, Adv. Funct. Mater., 2019, 29, 1806351 CrossRef CAS.
- Z. Meng, R. M. Stolz, L. Mendecki and K. A. Mirica, Chem. Rev., 2019, 119, 478–598 CrossRef CAS.
- T.-C. Wu, J. Dai, G. Hu, W.-B. Yu, O. Ogbeide, A. De Luca, X. Huang, B.-L. Su, Y. Li, F. Udrea and T. Hasan, Sens. Actuators, B, 2020, 321, 128446 CrossRef CAS.
- T. C. Wu, A. De Luca, Q. Zhong, X. Zhu, O. Ogbeide, D. S. Um, G. Hu, T. Albrow-Owen, F. Udrea and T. Hasan, npj 2D Mater. Appl., 2019, 3, 42 CrossRef.
- G. Gan, X. Li, S. Fan, L. Wang, M. Qin, Z. Yin and G. Chen, Eur. J. Inorg. Chem., 2019, 3126–3141 CrossRef CAS.
- J. E. Amonette and J. Matyáš, Microporous Mesoporous Mater., 2017, 250, 100–119 CrossRef CAS.
- C. Parra-Cabrera, C. Achille, S. Kuhn and R. Ameloot, Chem. Soc. Rev., 2018, 47, 209–230 RSC.
- W. J. Yang, A. C. Y. Yuen, A. Li, B. Lin, T. B. Y. Chen, W. Yang, H. D. Lu and G. H. Yeoh, Cellulose, 2019, 26, 6449–6476 CrossRef CAS.
- J. Ge, H. Y. Zhao, H. W. Zhu, J. Huang, L. A. Shi and S. H. Yu, Adv. Mater., 2016, 28, 10459–10490 CrossRef CAS.
- Y. Yang, X. Li, X. Zheng, Z. Chen, Q. Zhou and Y. Chen, Adv. Mater., 2018, 30, 1704912 CrossRef.
- C. A. García-González, T. Budtova, L. Durães, C. Erkey, P. Del Gaudio, P. Gurikov, M. Koebel, F. Liebner, M. Neagu and I. Smirnova, Molecules, 2019, 24, 1815 CrossRef.
- J. Ge, M. Li, Q. Zhang, C. Z. Yang, P. H. Wooley, X. Chen and S. Y. Yang, Int. J. Polym. Sci., 2013, 402859 Search PubMed.
- S. Asha, A. N. Ananth, S. P. Jose and M. A. J. Rajan, Appl. Nanosci., 2018, 8, 395–405 CrossRef CAS.
- A. Muñoz-Ruíz, D. M. Escobar-García, M. Quintana, A. Pozos-Guillén and H. Flores, J. Nanomater., 2019, 2875375 Search PubMed.
- R. Ghafari, M. Jonoobi, L. M. Amirabad, K. Oksman and A. R. Taheri, Int. J. Biol. Macromol., 2019, 136, 796–803 CrossRef CAS.
- S. Dong, Y. N. Zhang, J. Wan, R. Cui, X. Yu, G. Zhao and K. Lin, J. Mater. Chem. B, 2020, 8, 368–379 RSC.
- M. Mohammadian, T. S. Jafarzadeh Kashi, M. Erfan and F. P. Soorbaghi, J. Drug Delivery Sci. Technol., 2018, 44, 205–212 CrossRef CAS.
- K. C. W. Wu, Y. Yamauchi, C. Y. Hong, Y. H. Yang, Y. H. Liang, T. Funatsu and M. Tsunoda, Chem. Commun., 2011, 47, 5232–5234 RSC.
- S. Wei, Y. C. Ching and C. H. Chuah, Carbohydr. Polym., 2020, 231, 115744 CrossRef CAS.
- A. Veronovski, G. Tkalec, Z. Knez and Z. Novak, Carbohydr. Polym., 2014, 113, 272–278 CrossRef CAS.
- D. A. S. Agostinho, A. I. Paninho, T. Cordeiro, A. V. M. Nunes, I. M. Fonseca, C. Pereira, A. Matias and M. G. Ventura, Mater. Chem. Phys., 2020, 253, 123290 CrossRef CAS.
- T. Athamneh, A. Amin, E. Benke, R. Ambrus, C. S. Leopold, P. Gurikov and I. Smirnova, J. Supercrit. Fluids, 2019, 150, 49–55 CrossRef CAS.
- I. De Marco, R. Iannone, S. Miranda and S. Riemma, Int. J. Life Cycle Assess., 2018, 23, 1228–1239 CrossRef CAS.
- X. Chen, X. Xu, W. Li, B. Sun, J. Yan, C. Chen, J. Liu, J. Qian and D. Sun, ACS Appl. Bio Mater., 2018, 1, 42–50 CrossRef CAS.
- Z. Ulker and C. Erkey, J. Controlled Release, 2014, 177, 51–63 CrossRef CAS.
- C. A. García-González, M. Alnaief and I. Smirnova, Carbohydr. Polym., 2011, 86, 1425–1438 CrossRef.
- T. Silvetti, L. Merlini, M. Brasca and Y. M. Galante, Appl. Microbiol. Biotechnol., 2018, 102, 2683–2692 CrossRef CAS.
- W. Mueannoom, A. Srisongphan, K. M. G. Taylor, S. Hauschild and S. Gaisford, Eur. J. Pharm. Biopharm., 2012, 80, 149–155 CrossRef CAS.
- G. Sharma, W. Mueannoom, A. B. M. Buanz, K. M. G. Taylor and S. Gaisford, Int. J. Pharm., 2013, 447, 165–170 CrossRef CAS.
- C. S. Ponseca, P. Chábera, J. Uhlig, P. Persson and V. Sundström, Chem. Rev., 2017, 117, 10940–11024 CrossRef CAS.
- J. Deng, Y. Su, D. Liu, P. Yang, B. Liu and C. Liu, Chem. Rev., 2019, 119, 9221–9259 CrossRef CAS.
- C. S. Hu, H. J. Li, J. Y. Wang, A. Haleem, X. C. Li, M. Siddiq and W. D. He, ACS Appl. Energy Mater., 2019, 2, 7554–7563 CrossRef CAS.
- P. Mu, Z. Zhang, W. Bai, J. He, H. Sun, Z. Zhu, W. Liang and A. Li, Adv. Energy Mater., 2019, 9, 1802158 CrossRef.
- P. Mu, W. Bai, Z. Zhang, J. He, H. Sun, Z. Zhu, W. Liang and A. Li, J. Mater. Chem. A, 2018, 6, 18183–18190 RSC.
- Y. Fu, G. Wang, T. Mei, J. Li, J. Wang and X. Wang, ACS Sustainable Chem. Eng., 2017, 5, 4665–4671 CrossRef CAS.
- L. Tang, C. Jia, Y. Xue, L. Li, A. Wang, G. Xu, N. Liu and M. Wu, Appl. Catal., B, 2017, 219, 241–248 CrossRef CAS.
- Y. Hou, Z. Wen, S. Cui, X. Feng and J. Chen, Nano Lett., 2016, 16, 2268–2277 CrossRef CAS.
- X. D. Gao, X. M. Li, X. Y. Gan, Y. Q. Wu, R. K. Zheng, C. L. Wang, Z. Y. Gu and P. He, J. Mater. Chem., 2012, 22, 18930–18938 RSC.
- L. Zhao, B. Bhatia, S. Yang, E. Strobach, L. A. Weinstein, T. A. Cooper, G. Chen and E. N. Wang, ACS Nano, 2019, 13, 7508–7516 CrossRef CAS.
- P. Tao, G. Ni, C. Song, W. Shang, J. Wu, J. Zhu, G. Chen and T. Deng, Nat. Energy, 2018, 3, 1031–1041 CrossRef.
- L.-H. Chen, M.-H. Sun, Z. Wang, W. Yang, Z. Xie and B.-L. Su, Chem. Rev., 2020, 120, 11194–11294 CrossRef CAS.
- J. F. Xing, M. L. Zheng and X. M. Duan, Chem. Soc. Rev., 2015, 44, 5031–5039 RSC.
- T. A. Schaedler, A. J. Jacobsen, A. Torrents, A. E. Sorensen, J. Lian, J. R. Greer, L. Valdevit, W. B. Carter, Q. Ge, J. A. Jackson, S. O. Kucheyev, N. X. Fang and C. M. Spadaccini, Science, 2011, 334, 1373–1377 CrossRef.
- M. A. Skylar-Scott, J. Mueller, C. W. Visser and J. A. Lewis, Nature, 2019, 575, 330–335 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |