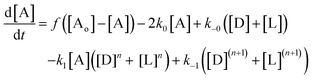Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Spontaneous mirror symmetry breaking: an entropy production survey of the racemate instability and the emergence of stable scalemic stationary states
Josep M.
Ribó
*ab and
David
Hochberg
*c
aDepartment of Organic Chemistry, University of Barcelona, E-08028 Barcelona, Catalonia, Spain. E-mail: jmribo@ub.edu
bInstitute of Cosmos Science (IEEC-UB), University of Barcelona, E-08028 Barcelona, Catalonia, Spain
cDepartment of Molecular Evolution, Centro de Astrobiology (CSIC-INTA), E-28850 Torrejón de Ardoz, Madrid, Spain. E-mail: hochbergd@cab.inta-csic.es
First published on 6th July 2021
Abstract
Correction for ‘Spontaneous mirror symmetry breaking: an entropy production survey of the racemate instability and the emergence of stable scalemic stationary states’ by Josep M. Ribó et al., Phys. Chem. Chem. Phys., 2020, 22, 14013–14025, DOI: 10.1039/D0CP02280B.
The authors would like to correct two typographical errors in the published article.
(1) There is an error in the ordinary differential equation set (4) on page 14015. The first differential equation should read as follows:
(2) In the caption of Scheme 1 on page 14015, the rate constant k−1 = 1 × 10−4 should be replaced by k−1 = 1 × 10−1. Therefore, the caption is corrected to:
Scheme 1. Open system model used in the simulations reported here corresponding to a continuous open flow reactor assuming instant and perfect diffusion of the all species in solution. The reaction rate constants were fixed to k0 = 1 × 10−3, k−0 = 1 × 10−7, k1 = 1 × 103 and k−1 = 1 × 10−1, the chemical mass entry of resources [Ao] = 1 × 10−1 mol L−1 and the flow entry/exit rate constant f = 1 × 10−3. f is the ratio between volume of solution entry/exit in the reactor and the reactor volume V that corresponds to reaction rate constants of the pseudo-reactions or matter exchange flows with the surroundings. The results reported here refer to the range 0.1 ≤ n ≤ 2.0 (and in steps of Δn = 0.1)
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © the Owner Societies 2021 |