 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: The corrosion inhibition of stainless steel by ferrocene–polyoxometalate hybrid molecular materials – experimental and first principles studies
Sruthi
G.
 a,
Shakeela
K.
b,
Shanmugam
R.
a and
Ranga Rao
G.
a,
Shakeela
K.
b,
Shanmugam
R.
a and
Ranga Rao
G.
 *a
*a
aDepartment of Chemistry and DST-IITM Solar Energy Harnessing Centre (DSEHC), Indian Institute of Technology Madras, Chennai 600036, India. E-mail: grrao@iitm.ac.in; Fax: +91 44 2257 4202; Tel: +91 44 2257 4226
bDepartment of Chemistry, B.S. Abdur Rahman Crescent Institute of Science and Technology, Vandalur, Chennai 600048, India
First published on 24th June 2021
Abstract
Correction for ‘The corrosion inhibition of stainless steel by ferrocene–polyoxometalate hybrid molecular materials – experimental and first principles studies’ by G. Sruthi et al., Phys. Chem. Chem. Phys., 2020, 22, 3329–3344, DOI: 10.1039/C9CP06284J.
The authors would like to make the changes described below to their published article. An updated version of the article itself with these changes incorporated is available as an additional supplementary information file alongside the original published article.
Correction on page 3331:
The following sentence on page 3331:
“The composition of 316 grade steel is Fe: 1%; C: <0.08%; Cr: 16–18%; Ni: 10–14%; Mo: 2–3%; Mn: <2%; Si: <1%; P: <0.045%; S: <0.03%, with a molecular weight of 20.376 g mol−1 and a density of 7.87 g cm−3 (ASTM F138).”
Should read as:
“The composition of 316 grade steel is Fe: 60–62%; C: <0.08%; Cr: 16–18%; Ni: 10–14%; Mo: 2–3%; Mn: <2%; Si: <1%; P: <0.045%; S: <0.03%, with a molecular weight of 56.0 g mol−1 and a density of 7.87 g cm−3 (ASTM F138).”
Please note that accordingly the corrosion rates are also corrected. However, the corrosion inhibition efficiencies of SS@FcPW and SS@FcPMo reported remain the same.
Corrected Figure 11(c) on page 3338:
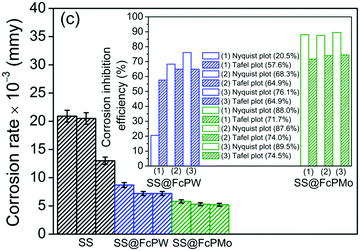 | ||
| Fig. 11 Reproducibility tests with error bars [Fig. 11(c) is corrected, but the inset of Fig. 11(c) has no change]. | ||
Corrected Table on page 3339:
| Electrode | Method | E corr (V) | i corr (μA cm−2) | Corrosion inhibition (η) and corrosion rate | β a (V dec−1) | β c (V dec−1) | R p (ohm cm2) |
|---|---|---|---|---|---|---|---|
| a Instr. From CH Instrument software. b Calc. From Tafel extrapolation method. mmy denotes millimeter per year (mm year−1). | |||||||
| Bare steel (SS) (316 grade) | Instr.a | −0.210 | 2.10 |

|
0.125 | −0.068 | 8804 |
| Calc.b | −0.210 | 1.77 |

|
0.119 | −0.053 | 8995 | |
| SS@ FcPW | Instr. | 0.0100 | 0.62 |

|
0.102 | −0.059 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222 222 |

|
|||||||
| Calc. | 0.0100 | 0.62 | η = 64.9% | 0.108 | −0.059 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 722 |
|

|
|||||||
| SS@ FcPMo | Instr. | 0.0155 | 0.56 | η = 68.3% | 0.084 | −0.088 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 344 344 |
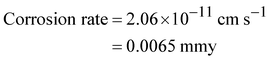
|
|||||||
| Calc. | 0.0155 | 0.46 | η = 74.0% | 0.112 | −0.101 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 194 194 |
|

|
|||||||
Correction on page 3340:
The following sentence on page 3340:
“The corrosion rates and corrosion inhibition efficiencies calculated from the Tafel extrapolation method are as follows: bare SS: 0.013 mmy; SS@FcPW: 0.009 mmy, 32%; and SS@FcPMo: 0.003 mmy, 71%.”
Should read as:
The corrosion rates and corrosion inhibition efficiencies calculated from the Tafel extrapolation method are as follows: bare SS: 0.036 mmy; SS@FcPW: 0.025 mmy, 32%; and SS@FcPMo: 0.011 mmy, 71%.
Correction on page 3342:
The sentence on page 3342:
“From the Pearson method, we can predict the number of electrons being transferred from the inhibitor molecule to the Fe(100) surface using the expression [Δn = (ϕFe − χinhibitor)/2(ηFe − ηinhibitor)], where ϕFe is the work function of Fe(110) and ηFe = 0.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74,75”
74,75”
Should read as:
From the Pearson method, we can predict the number of electrons being transferred from the inhibitor molecule to the Fe(110) surface using the expression [Δn = (ϕFe − χinhibitor)/2(ηFe − ηinhibitor)], where ϕFe is the work function of Fe(110) which is 4.82 eV and ηFe = 0.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74,75
74,75
Correction in Table 4 on page 3342:
Δn value of FcPW is 1.24 (instead of −0.22) and that of FcPMo is 1.42 (instead of −0.15).
Correction on page 3343:
The following sentence on page 3343:
“From the table, the Δn values of FcPW and FcPMo are −0.22 and −0.15, respectively. These negative values indicate that both the inhibitor molecules FcPW and FcPMo act as electron acceptors from the vacant d-orbital of Fe metal on the surface of the stainless steel plate. The electron-accepting nature of the hybrid molecules increases the adsorption of the inhibitor molecules on the metal surface, therefore inhibiting the corrosion mechanism.”
Should read as:
“From the table, the Δn values for FcPW and FcPMo are 1.24 and 1.42, respectively. These values indicate that the fraction of electrons is transferred from the inhibitor molecules FcPW and FcPMo to the vacant d-orbital of Fe metal on the surface of stainless steel plate. The fraction of electrons transferred in the case of SS@FcPMo is more than that of SS@FcPW. So, there is increased adsorption of the inhibitor molecule on the metal surface, and therefore enhanced corrosion inhibition of stainless steel in the case of SS@FcPMo.”
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © the Owner Societies 2021 |
