Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Local structure of a highly concentrated NaClO4 aqueous solution-type electrolyte for sodium ion batteries
Ryo
Sakamoto
a,
Maho
Yamashita
b,
Kosuke
Nakamoto
c,
Yongquan
Zhou
d,
Nobuko
Yoshimoto
b,
Kenta
Fujii
b,
Toshio
Yamaguchi
e,
Ayuko
Kitajou
*bf and
Shigeto
Okada
*cf
aInterdisciplinary Graduate School of Engineering Sciences, Kyushu University, 6-1 Kasuga-koen, Kasuga 816-8580, Japan
bGraduate School of Sciences and Technology for Innovation, Yamaguchi University, 2-16-1 Tokiwadai, Yamaguchi Ube, 755-8611, Japan. E-mail: kitajou@yamaguchi-u.ac.jp
cInstitute for Materials Chemistry and Engineering, Kyushu University, 6-1 Kasuga-koen, Kasuga 816-8580, Japan. E-mail: s-okada@cm.kyushu-u.ac.jp
dKey Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources, Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining 810008, China
eDepartment of Chemistry, Faculty of Science, Fukuoka University, 8-19-1 Nanakuma, Jonan, Fukuoka 814-0180, Japan
fElements Strategy Initiative for Catalysts and Batteries (ESICB), Kyoto University, Kyoto, 615-8245, Japan
First published on 19th April 2021
Abstract
Correction for ‘Local structure of a highly concentrated NaClO4 aqueous solution-type electrolyte for sodium ion batteries’ by Ryo Sakamoto et al., Phys. Chem. Chem. Phys., 2020, 22, 26452–26458, DOI: 10.1039/D0CP04376A.
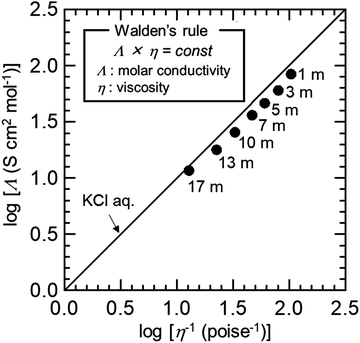
The authors apologise that the Walden plot of NaClO4 aq. solution shown in Fig. 1 and the viscosity and electrical conductivity plot shown in Fig. S1 were incorrect because the concentration used in the calculation was incorrect. The figures are corrected as follows:
 | ||
| Fig. S1 Viscosity and electrical conductivity of the NaClO4 aq. electrolytes at various concentrations. | ||
The corrections shown here do not affect the conclusions in the paper. But, the discussion about Fig. 1 and Fig. S1 on page 26454 should read as follows:
Fig. S1 (ESI†) summarizes the viscosity and the ionic conductivity at each NaClO4 concentration. The viscosity was higher with increasing NaClO4 salt concentration, but the ionic conductivity showed a maximum value at 7 m NaClO4 aq. solution. The 17 m NaClO4 aq. solution had a viscosity of 7.8 mPa s and an ionic conductivity of 108 mS cm−1. The viscosity of the NaClO4 aq. electrolyte was comparable to that of the non-aqueous electrolyte (6.8 mPa s, 1 M NaClO4 in PC), but the ionic conductivity was much higher than that of the non-aqueous electrolyte (6.5 mS cm−1).1 These results suggested that the ionic conductivity of the NaClO4 aq. electrolyte was not impaired even at a high salt concentration. Fig. 1 shows the Walden plot of the NaClO4 aq. solution, and that of KCl aq. solution as an ideal solution. Here, Walden's rule is “Λ × η = const.” (Λ: molar conductivity; η: viscosity). For 1 m NaClO4 aq. solution, it was close to the straight line of the KCl aq. solution, and it was again close to the straight line of KCl aq. solution at 17 m NaClO4 aq. solution. Although NaClO4 was completely dissociated in 1 m NaClO4 aq. solution, ion pairs between the Na+ ions and ClO4− ions were formed in concentrated NaClO4 aq. solution, as shown by the Raman and EPSR results described later. Nevertheless, no significant decrease in ionic conductivity was observed in the concentrated region, suggesting the specific ion-transport mechanism, such as ion hopping mechanism, between vicinal Na+ ions in the orderly solution structure over a long range. Similar behavior was also reported in concentrated LiTFSA aq. solution2 and LiFSA in dinitrile electrolytes.3
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- A. Ponrouch, E. Marchante, M. Courty, J. M. Tarascon and M. R. Palacín, Energy Environ. Sci., 2012, 5, 8572 RSC.
- T. Tsurumura, Y. Hashimoto, M. Morita, Y. Umebayashi and K. Fujii, Anal. Sci., 2019, 35, 289–294 CrossRef CAS.
- Y. Ugata, M. L. Thomas, T. Mandai, K. Ueno, K. Dokko and M. Watanabe, Phys. Chem. Chem. Phys., 2019, 21, 9759 RSC.
| This journal is © the Owner Societies 2021 |