Single-conformation spectroscopy of cold, protonated DPG-containing peptides: switching β-turn types and formation of a sequential type II/II′ double β-turn†
Abstract
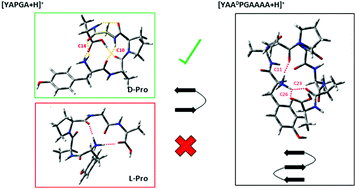
D-Proline (DPro, DP) is widely utilized to form β-hairpin loops in engineered peptides that would otherwise be unstructured, most often as part of a DPG sub-unit that forms a β-turn. To observe whether DPG facilitated this effect in short protonated peptides, conformation specific IR–UV double resonance photofragment spectra of the cold (∼10 K) protonated DP and LP diastereomers of the pentapeptide YAPGA was carried out in the hydride stretch (2800–3700 cm−1) and amide I/II (1400–1800 cm−1) regions. A model localized Hamiltonian was developed to better describe the 1600–1800 cm−1 region commonly associated with the amide I vibrations. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch fundamentals experience extensive mixing with the N–H bending fundamentals of the NH3+ group in these protonated peptides. The model Hamiltonian accounts for experiment in quantitative detail. In the DP diastereomer, all the population is funneled into a single conformer which presented as a type II β-turn with A and DP in the i + 1 and i + 2 positions, respectively. This structure was not the anticipated type II′ β-turn across DPG that we had hypothesized based on solution-phase propensities. Analysis of the conformational energy landscape shows that both steric and charge-induced effects play a role in the preferred formation of the type II β-turn. In contrast, the LP isomer forms three conformations with very different structures, none of which were type II/II′ β-turns, confirming that LPG is not a β-turn former. Finally, single-conformation spectroscopy was also carried out on the extended peptide [YAADPGAAA + H]+ to determine whether moving the protonated N-terminus further from DPG would lead to β-hairpin formation. Despite funneling its entire population into a single peptide backbone structure, the assigned structure is not a β-hairpin, but a concatenated type II/type II′ double β-turn that displaces the peptide backbone laterally by about 7.5 Å, but leaves the backbone oriented in its original direction.
O stretch fundamentals experience extensive mixing with the N–H bending fundamentals of the NH3+ group in these protonated peptides. The model Hamiltonian accounts for experiment in quantitative detail. In the DP diastereomer, all the population is funneled into a single conformer which presented as a type II β-turn with A and DP in the i + 1 and i + 2 positions, respectively. This structure was not the anticipated type II′ β-turn across DPG that we had hypothesized based on solution-phase propensities. Analysis of the conformational energy landscape shows that both steric and charge-induced effects play a role in the preferred formation of the type II β-turn. In contrast, the LP isomer forms three conformations with very different structures, none of which were type II/II′ β-turns, confirming that LPG is not a β-turn former. Finally, single-conformation spectroscopy was also carried out on the extended peptide [YAADPGAAA + H]+ to determine whether moving the protonated N-terminus further from DPG would lead to β-hairpin formation. Despite funneling its entire population into a single peptide backbone structure, the assigned structure is not a β-hairpin, but a concatenated type II/type II′ double β-turn that displaces the peptide backbone laterally by about 7.5 Å, but leaves the backbone oriented in its original direction.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...