Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Probing the influence of Zn and water on solvation and dynamics in ethaline and reline deep eutectic solvents by 1H nuclear magnetic resonance†
Yasmeen M.
AlZahrani
and
Melanie M.
Britton
 *
*
School of Chemistry, University of Birmingham, Edgbaston, B15 2TT, UK. E-mail: m.m.britton@bham.ac.uk
First published on 17th September 2021
Abstract
A range of ethaline and reline deep eutectic solvents (DESs) have been investigated in the absence and presence of Zn (0–0.3 M) and water (0–29 wt%) by one-dimensional 1H NMR spectroscopy, two-dimensional 1H–1H nuclear Overhauser effect and exchange spectroscopy, 1H T1 NMR relaxation times and 1H NMR diffusion. The role of zinc and water in controlling solvation and microstructure in reline and ethaline were investigated. We show that in ethaline there is proton exchange between hydroxyl groups in ethaline glycol and choline chloride. The rate of exchange between these protons is found to significantly increase in the presence of Zn, but decreases with increasing water content. In the case of reline, no proton exchange is observed between the amide protons in urea and hydroxyl protons in choline chloride. However, the addition of water decreases the viscosity of the system, as well as changes the distance between amide and hydroxyl protons in urea and choline chloride, respectively. The addition of Zn does not appear to change the interactions between urea and choline chloride species, but does reduce the rate of exchange between water and hydroxyl protons in reline formulations containing water.
Introduction
In recent years, there has been increasing interest in the use of deep eutectic solvents (DESs) as a replacement for aqueous and organic electrolytes in many electrochemical applications, including metal electrodeposition,1–3 metal electropolishing4,5 and batteries.6 This increasing interest largely arises from their high chemical stability, wide potential window, low flammability and volatility. Another important benefit of DESs is that they can be formed by mixing, typically, inexpensive, low-toxicity, biodegradable components, and, hence, also offer economic and environmental benefits.7–9A widely investigated class of DES is based on the quaternary ammonium salt choline chloride (ChCl).6,10,11 This class includes ethaline, which is a mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of ChCl and ethylene glycol (EG), and reline, a 1
2 molar ratio of ChCl and ethylene glycol (EG), and reline, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio mixture of ChCl and urea (U).12 While multiple interactions contribute to the intermolecular network within DESs, including van der Waals interactions, hydrogen bonding and/or ionic bonding,13 it is hydrogen bonding that is considered to be the primary cause of their melting point depression and physicochemical properties, such as viscosity and conductivity.8 Ethaline and reline have been investigated as more sustainable electrolytes in the electrodeposition of Zn, as an anti-corrosion layer.3,11,14,15 However, zinc deposits have been found to have different morphologies, depending on the DES used, which has been attributed to differences in the hydrogen bond network between ethaline and reline systems15 and zinc ion species, which play an important role in the mechanisms of deposition.3 Abbott et al.15 proposed that the predominant zinc species in both ethaline and reline systems is [ZnCl4]2−. Reline has also been investigated as a novel electrolyte for rechargeable zinc-batteries.6 Reversible plating/stripping of zinc species has been observed, without hydrogen evolution or the formation of a passivation layer on the zinc electrode.6 However, despite this initial interest in these DES systems for Zn-electrochemical applications, there are several challenges currently preventing their commercialisation. Primarily, these are associated with poor physical properties, such as their typically high viscosity, and, hence, lower conductivity.12 Also, a lack of understanding of the species formed has limited the optimisation of DES formulations.16
2 molar ratio mixture of ChCl and urea (U).12 While multiple interactions contribute to the intermolecular network within DESs, including van der Waals interactions, hydrogen bonding and/or ionic bonding,13 it is hydrogen bonding that is considered to be the primary cause of their melting point depression and physicochemical properties, such as viscosity and conductivity.8 Ethaline and reline have been investigated as more sustainable electrolytes in the electrodeposition of Zn, as an anti-corrosion layer.3,11,14,15 However, zinc deposits have been found to have different morphologies, depending on the DES used, which has been attributed to differences in the hydrogen bond network between ethaline and reline systems15 and zinc ion species, which play an important role in the mechanisms of deposition.3 Abbott et al.15 proposed that the predominant zinc species in both ethaline and reline systems is [ZnCl4]2−. Reline has also been investigated as a novel electrolyte for rechargeable zinc-batteries.6 Reversible plating/stripping of zinc species has been observed, without hydrogen evolution or the formation of a passivation layer on the zinc electrode.6 However, despite this initial interest in these DES systems for Zn-electrochemical applications, there are several challenges currently preventing their commercialisation. Primarily, these are associated with poor physical properties, such as their typically high viscosity, and, hence, lower conductivity.12 Also, a lack of understanding of the species formed has limited the optimisation of DES formulations.16
In order to overcome poor physical properties in DESs, the addition of water has been investigated.12,17–20 Computer simulations have revealed that the addition of water, to ethaline or reline DESs, decreases the number of hydrogen bonds formed and consequently lowers the viscosity of both DES systems.20 However, as more water is added, it has been observed12 that ChCl-based DESs start to lose their DES structure, and form aqueous solutions, above a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equivalent of water:chloride. This transition is also reported to change the electrochemical properties of ChCl-based DESs, in particular, by narrowing their potential window.21,22 Cyclic voltammetry (CV) experiments have shown that reline retains a wide potential window until the amount of added water reaches 9 wt%.21 The effects of adding water to reline21 and ethaline,22 for electroplating Cu22 and Ni21 have been investigated. It has been observed that the addition of 15 wt% water in ethaline, for Cu electroplating, results in a reduction in the ethaline viscosity without reducing the wide potential of the electolyte.22 Adding 6 wt% of water to reline, for nickel electroplating, lowers the reline viscosity, and suppresses nickel dendritic growth, resulting in a smooth, dense nickel deposit.21
1 equivalent of water:chloride. This transition is also reported to change the electrochemical properties of ChCl-based DESs, in particular, by narrowing their potential window.21,22 Cyclic voltammetry (CV) experiments have shown that reline retains a wide potential window until the amount of added water reaches 9 wt%.21 The effects of adding water to reline21 and ethaline,22 for electroplating Cu22 and Ni21 have been investigated. It has been observed that the addition of 15 wt% water in ethaline, for Cu electroplating, results in a reduction in the ethaline viscosity without reducing the wide potential of the electolyte.22 Adding 6 wt% of water to reline, for nickel electroplating, lowers the reline viscosity, and suppresses nickel dendritic growth, resulting in a smooth, dense nickel deposit.21
The study of speciation and solvation, and the effect of additives such as water, on the chemical and physical properties of DES have been investigated computationally, using quantum mechanics (QM) simulations,18,23 and experimentally, using nuclear magnetic resonance (NMR),24 Fourier-transform infra-red (FTIR) and Raman spectroscopies.25 In these studies, it has been proposed that the addition of water affects the hydrogen bond network of DESs, by forming new hydrogen bonds between water and DESs components, which subsequently affect the properties of the DES, such as viscosity.18,23 Moreover, it has been observed that the number of hydrogen bonds formed between water and EG, in ethaline, are comparatively higher than those formed between water and U in the reline system.25 In the case of reline, while water can hydrogen bond with ChCl and U,19,23 it has been found that water molecules also occupy the interstices within the hydrogen bond network, reducing the number of hydrogen bonds between water and ChCl or U.25 Following investigation by diffusion NMR, D’Agostino et al.12 proposed that, in the presence of 20 wt% of water, ethaline forms a homogenous ethaline–water mixture, while reline forms a non-homogeneous reline–water mixture with a rich-water region. Zinc speciation in ethaline and reline has been studied using mass spectrometry.26 However, as this is typically an ex situ technique, it is not able to provide information on speciation during electrochemical processes. Non-invasive, in situ techniques have been explored, which are able to provide information on speciation and dynamics of metal ions in DES electrolytes.26 Furthermore, there is growing interest in developing operando techniques for observing speciation and dynamics, in situ and in real time, under working conditions. Such techniques have been demonstrated using 1H NMR T1 relaxation times to determine Zn speciation in aqueous electrolytes during Zn corrosion and discharging of a Zn-battery.27,28
In this paper, a range of ethaline and reline DESs have been investigated, in the absence and presence of Zn (0–0.3 M) and water (0–29 wt%). Molecular interactions and dynamics have been investigated using one-dimensional (1D) 1H NMR and two-dimensional (2D) 1H–1H nuclear Overhauser exchange (NOESY) and exchange (EXSY) NMR spectroscopy. The influence of zinc and water, on the mobility and microstructure with each DES system, has been investigated using 1H NMR T1 relaxation time and diffusion measurements. We demonstrate that the zinc species are different in ethaline and reline, and the interaction between zinc and water is also different in ethaline and reline.
Experimental details
Materials and samples preparation
Choline chloride (ChCl, 99%), ethylene glycol (EG, 99.8%), urea (U, 99.9%) and zinc chloride (ZnCl2, 99.99%) were supplied by Sigma-Aldrich. All components were used without further purification, but dried in a vacuum oven under reduced pressure (100 mbar), at 80 °C (ChCl, EG and U) or at 120 °C (ZnCl2), for a minimum of 24 hours and were stored in a glove-box under argon atmosphere. In a glove-box, ethaline was prepared by mixing ChCl and EG in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio and reline was prepared by mixing ChCl and U in a 1
2 molar ratio and reline was prepared by mixing ChCl and U in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio before sonication and heating to 60 °C until homogenous clear colourless liquids were formed. Solutions of zinc in ethaline or reline were prepared in a glove-box by dissolving dry ZnCl2 in either ethaline or reline, over a range of ZnCl2 concentrations (0.1, 0.2 and 0.3 M in ethaline and 0.3 M in reline) at 25 °C. A range of concentrations of water were prepared by adding water (Nanopure filtered, resistivity 18 MΩ cm), to ethaline and reline, in the presence and absence of 0.3 M ZnCl2. Solutions of water in ethaline, at concentrations of 2.7, 5.5, 8.3, 16 and 29.2 wt%, were prepared by adding 26, 52, 79, 151 and 277 μl, respectively, of water to 850 μl of ethaline. Solutions of water in reline, at concentrations of 5.5, 8.3 and 26.2 wt%, were prepared by adding by adding 58.5, 88 and 279 μl of water, respectively, to 850 μl of reline. Samples were put, immediately after preparation, into 5 mm Wilmad® NMR tubes fitted with J Young valves, to prevent the absorption of additional water. NMR measurements were performed <12 h after sample preparation. The amount of water in each sample was confirmed using 1H NMR spectroscopy.
2 molar ratio before sonication and heating to 60 °C until homogenous clear colourless liquids were formed. Solutions of zinc in ethaline or reline were prepared in a glove-box by dissolving dry ZnCl2 in either ethaline or reline, over a range of ZnCl2 concentrations (0.1, 0.2 and 0.3 M in ethaline and 0.3 M in reline) at 25 °C. A range of concentrations of water were prepared by adding water (Nanopure filtered, resistivity 18 MΩ cm), to ethaline and reline, in the presence and absence of 0.3 M ZnCl2. Solutions of water in ethaline, at concentrations of 2.7, 5.5, 8.3, 16 and 29.2 wt%, were prepared by adding 26, 52, 79, 151 and 277 μl, respectively, of water to 850 μl of ethaline. Solutions of water in reline, at concentrations of 5.5, 8.3 and 26.2 wt%, were prepared by adding by adding 58.5, 88 and 279 μl of water, respectively, to 850 μl of reline. Samples were put, immediately after preparation, into 5 mm Wilmad® NMR tubes fitted with J Young valves, to prevent the absorption of additional water. NMR measurements were performed <12 h after sample preparation. The amount of water in each sample was confirmed using 1H NMR spectroscopy.
NMR measurements
NMR data were collected on a Bruker AVANCE III HD 300 spectrometer equipped with a 7 T vertical wide-bore superconducting magnet, operating at a proton resonance frequency of 300.13 MHz, with a 10 mm 1H diff30 radiofrequency (RF) coil. NMR experiments were performed at 293 ± 0.3 K, controlled by the temperature of the water-cooled gradient coils. The 90° RF pulse was calibrated for each sample and found to be 20 ± 1 μs.1H NMR spectra were acquired using a pulse-acquire sequence, with a repetition time of 6 s. The chemical shift of peaks were calibrated to an external reference of TMS in deuterated chloroform, which was put in a 10 mm NMR tube, with the sample in a 5 mm NMR tube inside. 2D 1H–1H NOESY experiments were acquired using the sequence, [90° − τ1 − 90° − τmix − 90° − acq], with 256 point in the F1 direction and 2048 in the F2 direction, with a repetition time of 2 s, 16 signal averages and 16 dummy scans. The mixing time, τmix, was increased from 0 to 300 ms, over a series of six experiments. Proton exchange rates (kex) were calculated,29 by fitting, in Kaleidagraph,30 the signal intensity of exchange peaks (IAB and IBA), as a function of mixing time (τmix), to eqn (1):
IAB = IBA = PAPB(1 − exp(−kexτmix))![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−R1τmix) exp(−R1τmix) | (1) |
 ,
,  and R1 = (R1A + R1B), IA and IB are the intensities at τmix = 0 ms, and R1A, R1B are the relaxation rates of species A and B. In the fitting, an average value of IAB and IBA was used. Exchange peaks were identified as having the same phase as the diagonal peaks, which, in this study, are plotted positively. Cross-peaks arising from the nOe have a phase depending on the molecular size of species and viscosity of the solvent.31 In the case of small molecules in low viscosity DES, where tumbling rates are faster, the nOe cross-peaks are expected to have the opposite sign to diagonal peaks and are negative where diagonal peaks are positive. For molecules in high viscosity solvents, where molecular tumbling is slow, the nOe cross-peaks are expected to be positive, where diagonal peaks are also positive.
and R1 = (R1A + R1B), IA and IB are the intensities at τmix = 0 ms, and R1A, R1B are the relaxation rates of species A and B. In the fitting, an average value of IAB and IBA was used. Exchange peaks were identified as having the same phase as the diagonal peaks, which, in this study, are plotted positively. Cross-peaks arising from the nOe have a phase depending on the molecular size of species and viscosity of the solvent.31 In the case of small molecules in low viscosity DES, where tumbling rates are faster, the nOe cross-peaks are expected to have the opposite sign to diagonal peaks and are negative where diagonal peaks are positive. For molecules in high viscosity solvents, where molecular tumbling is slow, the nOe cross-peaks are expected to be positive, where diagonal peaks are also positive.
Spin–lattice (T1) NMR relaxation times were measured using an inversion recovery experiment,31 [180° − τ − 90° − acq]n, with a repetition time of 6 s and 8 signal averages. A series of spectra (n = 12) were collected with logarithmically spaced time delays, τ, ranging from 5 × 10−6 s to 6 s. T1 relaxation times were determined by fitting the normalised signal intensity (I(τ)/I(0)), as a function of time, to eqn (3).
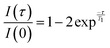 | (3) |
Self-diffusion co-efficients (D) were measured using a pulsed gradient stimulated echo (PGSTE) sequence31 with 16 gradient steps. Diffusion measurements of dry ethaline samples were collected with a maximum gradient (Gmax) of 300 G cm−1, pulse duration (δ) of 2 ms, observation time (Δ) of 60 ms, and repetition time of 2 s. Diffusion measurements of dry reline samples were collected using Gmax = 600 G cm−1, δ = 2 ms, Δ = 100 ms, with a repetition time of 2 s. Diffusion measurements for ethaline and reline systems containing water were collected using Gmax = 300 G cm−1, δ = 2 ms, Δ = 30 ms, and a repetition time of 4 s. The average self-diffusion co-efficients (D) were determined by fitting the normalised signal intensity as a function of gradient strength, (I(G)/I(0)), to the Stejskal–Tanner32eqn (4). Where a single diffusion co-efficient was not sufficient to fit the data, fitting to a bi-exponential function was performed.
 | (4) |
Results
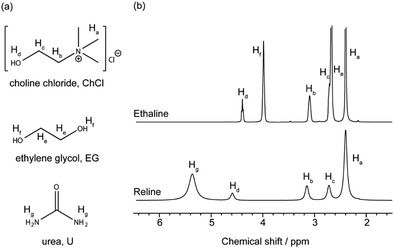
Fig. 1 shows the 1H NMR spectra for dry ethaline and reline DESs, along with the molecular structures and proton labelling scheme of constituent species in each DES. The broad line widths observed for peaks in the 1H NMR spectrum of reline are indicative of the higher viscosity for reline compared to ethaline.1H NMR spectra for dry ethaline and reline, with increasing ZnCl2 concentration, are shown in Fig. 2. For both reline and ethaline, no visible change in viscosity was observed upon the addition of zinc. This observation is consistent with the 1H NMR spectra in Fig. 2, which do not show a change in line width for the peaks of the non-exchanging protons (Ha, Hb, Hc and He; Table S1 in the ESI†). In the ethaline system, the line widths for hydroxyl protons in choline cation (Ch+) and EG (Hd and Hf respectively) are observed to increase gradually, as the concentration of Zn increases. This is matched by a slight upfield shift of the peak for the hydroxyl proton in Ch+ (Hd). However, in the reline system, the line width and chemical shift for the Hd peak do not appear to be affected by the addition of zinc.
 | ||
| Fig. 2 A series of 1H NMR spectra for dry (a) ethaline and (b) reline as a function of ZnCl2 concentration, at 293 K. Molecular structures and numbering scheme of peak assignments were presented in Fig. 1. Associated line widths can be found in Table S1 in the ESI.† | ||
Fig. 3 shows 1H NMR spectra for ethaline, in the presence and absence of Zn and water. There is a gradual shift for all hydroxyl proton peaks (Hd, Hf and Hw) downfield, as the water content increases. Also, the addition of water narrows the line width for (Ha, Hb, Hc and He) peaks, irrespective of whether Zn is present or not. However, the presence of zinc broadens the Hd, Hf and Hw peaks.
 | ||
| Fig. 3 A series of 1H NMR spectra for ethaline system in the absence (a) and presence (b) of 0.3 M ZnCl2 as a function of water content (Hw), at 293 K. Molecular structures and numbering scheme of peak assignments were presented in Fig. 1. Associated line widths can be found in Table S2 in the ESI.† | ||
Fig. 4 shows 1H NMR spectra for reline, in the presence and absence of Zn and water. The presence of Zn does not appear to have a significant effect on the 1H NMR spectra. However, the presence of water leads to a significant reduction in line width of the urea peak (Hg), which also shifts upfield as the water content increases. The water peak (Hw) shifts gradually to higher chemical shift, as water content increases. As the water content increases, both in the presence and absence of Zn, there is a slight reduction in the line width of the Ha, Hb, Hc and Hg peaks. The addition of water broadens, and slightly shifts down field, the peak for the hydroxyl protons in Ch+ (Hd). At the highest water content, the Hd peak overlaps completely with Hw peak, in the absence of Zn (Fig. 4a), whereas in the presence of Zn (Fig. 4b), the Hd peak remains at 4.6 ppm. However, the presence of zinc affects the line width of Hd and Hw peaks, which become narrower in the presence of Zn.
 | ||
| Fig. 4 A series of 1H NMR spectra for reline system in the absence (a) and presence (b) of 0.3 M ZnCl2 as a function of water content (Hw), at 293 K. Molecular structures and numbering scheme of peak assignments were presented in Fig. 1. Associated line widths can be found in Table S3 in the ESI.† | ||
Fig. 5 shows 2D NOESY spectra for dry ethaline, in the presence and absence of ZnCl2. In the absence of Zn, intense exchange (positive) peaks are observed between the hydroxyl protons (Hd and Hf) of Ch+ and EG (Fig. 5a). The hydroxyl proton peaks, and exchange peaks, broaden in the presence of Zn (Fig. 5b), indicating increased exchange. 2D NOESY spectra for ethaline with 8.3 wt% water, in the presence and absence Zn, are shown in Fig. 6. Exchange peaks are observed between water (Hw) and the hydroxyl protons in Ch+ and EG (Hd and Hf). In the presence of Zn, however, these exchange peaks are broader. In addition to the exchange peaks, nOe (negative) cross-peaks are also observed between the other protons in EG and Ch+, which appear to become less intense in the presence of Zn.
 | ||
| Fig. 5 1H–1H NOESY NMR spectra for dry ethaline samples in the absence (a) and presence (b) of 0.3 M ZnCl2, for a mixing time τm 300 ms. Positive peaks are black, negative peaks are red. | ||
Fig. 7–9 show 2D NOESY spectra for reline with 0, 8.3 and 26 wt% water, respectively, in the presence and absence of ZnCl2. The higher viscosity of reline at 0 and 8.3 wt% water has led to all cross-peaks being positive.33 In the dry reline system (Fig. 7), cross-peaks peaks are observed between the hydroxyl protons in Ch+ (Hd) and the amide protons in U (Hg). However, as the viscosity of this system is high, it is not possible to identify whether these cross-peaks arise from exchange or nOe interactions. In the presence of water (Fig. 8), no cross-peaks are observed between these protons, indicating no exchange nor spatial proximity. The spectra in Fig. 8 show cross-peaks between Hg protons and the aliphatic protons in Ch+ (Ha, Hb and Hc). These are negative and hence arise from the nOe, indicating their close spatial-proximity. Table 1 shows the proton exchange rates (kex), for both reline and ethaline, determined from the 2D 1H–1H NOESY spectra. Proton exchange is observed between the hydroxyl protons in Ch+ (Hd) and water protons (Hw) in Fig. 8 and 9, as has been observed previously.24 This exchange appears to increase with increasing water concentration (Fig. 4), resulting in a coalesced peak in the NOESY spectrum (Fig. 9) and a cross peak between Hd,w and the amide protons Hg.
| Water wt% | [Zn2+] = 0 M | [Zn2+] = 0.3 M |
|---|---|---|
| Ethaline | ||
| 0 | k ex (Hf–Hd) 11.36 s−1 | — |
| 8.3 | k ex (Hf–Hd) 8.66 s−1 | k ex (Hd,f–Hw) 77.8 s−1 |
| k ex (Hw–Hf) 5.71 s−1 | ||
| k ex (Hw–Hd) 1.03 s−1 | ||
| 29 | k ex (Hf–Hd) 7.96 s−1 | k ex (Hd,f–Hw) 124.8 s−1 |
| k ex (Hw–Hf) 29.3 s−1 | ||
| k ex (Hw–Hd) 10.8 s−1 | ||
| Reline | ||
| 8.3 | k ex (Hd–Hw) 77 s−1 | k ex (Hd–Hw) 24 s−1 |
Diffusion co-efficients for Ch+, in dry ethaline and reline, are shown in Table 2. It can be seen that, Ch+ has a lower mobility in reline than ethaline, as expected because of the higher viscosity in reline. The diffusion coefficient for Ch+ is largely unaffected by the presence of zinc. However, in ethaline, the diffusion co-efficient for the hydroxyl (Hd) and methyl protons (Ha), in Ch+, are the same in the absence of Zn, but are different in the presence of Zn.
| System | Diffusion co-efficient/10−11 m2 s−1 | |
|---|---|---|
| Ha | Hd | |
| Ethaline | 1.92 ± 0.06 | 1.90 ± 0.07 |
| Ethaline + 0.3 M ZnCl2 | 1.76 ± 0.01 | 2.23 ± 0.07 |
| Reline | 0.05 ± 0.01 | 0.06 ± 0.01 |
| Reline + 0.3 M ZnCl2 | 0.06 ± 0.01 | 0.06 ± 0.01 |
Diffusion co-efficients for ethaline species, with increasing water content, in the absence and presence of ZnCl2, are presented in Tables 3 and 4, respectively. These data show that diffusion co-efficients, for all species, increase with increasing water content. At lower concentrations of water (≤8.3 wt%), two diffusion co-efficients are observed for water. However, at high concentrations of water (29 wt%), only a single diffusion co-efficient is observed.
| Water (wt%) | Diffusion co-efficient/10−11 m2 s−1, (contribution %) | ||
|---|---|---|---|
| Ha | He | Hw | |
| 5.5 | 3.12 ± 0.01 | 5.46 ± 0.03 | 3.40 ± 0.08 (5.1 ± 0.1%) |
| 15.47 ± 0.04 (94.9 ± 0.1%) | |||
| 8.3 | 3.66 ± 0.01 | 6.33 ± 0.14 | 4.23 ± 0.01 (14.1 ± 1.1%) |
| 16.25 ± 0.49 (85.9 ± 1.1%) | |||
| 29.2 | 8.91 ± 0.07 | 14.61 ± 1.00 | 30.75 ± 0.46 |
| Water wt% | Diffusion co-efficient/10−11 m2 s−1, (contribution %) | ||
|---|---|---|---|
| Ha | He | Hw | |
| 5.5 | 2.91 ± 0.03 | 5.19 ± 0.01 | 1.72 ± 0.23 (13.1 ± 3.4%) |
| 14.22 ± 0.25 (86.9 ± 3.4%) | |||
| 8.3 | 3.54 ± 0.06 | 6.34 ± 0.20 | 3.28 ± 0.49 (57.0 ± 23.7%) |
| 12.75 ± 4.94 (43.0 ± 23.7%) | |||
| 29.2 | 8.90 ± 0.45 | 16.24 ± 2.00 | 9.60 ± 0.71 (10.8 ± 2.6%) |
| 31.75 ± 0.78 (89.2 ± 2.6%) | |||
Diffusion co-efficients for Ch+, U and water in reline, with increasing water content and in the absence and presence of ZnCl2, are presented in Tables 5 and 6, respectively. It can be seen that the diffusion co-efficients of both Ch+ and U increase with the addition of water, as expected. Two diffusion co-efficients are observed for water for all water concentrations. The diffusion co-efficients reported in our study are comparable with those reported by D’Agostino et al.12 However, only a single diffusion coefficient was observed for water in their study.
| Water (wt%) | Diffusion co-efficient/10−11 m2 s−1, (contribution %) | ||
|---|---|---|---|
| Ha | Hg | Hw | |
| 5.5 | 0.60 ± 0.04 | 1.02 ± 0.07 | 0.93 ± 0.06 (20.0 ± 1.2%) |
| 4.57 ± 0.21 (80.0 ± 1.2%) | |||
| 8.3 | 1.18 ± 0.15 | 1.98 ± 0.23 | 1.77 ± 0.13 (13.0 ± 1.1%) |
| 7.83 ± 0.49 (87.0 ± 1.1%) | |||
| 26.2 | 9.26 ± 0.45 | 14.33 ± 0.61 | 12.93 ± 0.04 (3.2 ± 0.5%) |
| 38.06 ± 0.93 (96.8 ± 0.5%) | |||
| Water (wt%) | Diffusion co-efficient/10−11 m2 s−1, (contribution %) | ||
|---|---|---|---|
| Ha | Hg | Hw | |
| 5.5 | 0.64 ± 0.03 | 1.12 ± 0.05 | 1.08 ± 0.12 (17.0 ± 0.4%) |
| 5.99 ± 0.16 (83.0 ± 0.4%) | |||
| 8.3 | 1.28 ± 0.09 | 2.19 ± 0.15 | 2.22 ± 0.10 (11.7 ± 0.4%) |
| 9.64 ± 0.42 (88.3 ± 0.4%) | |||
| 26.2 | 10.01 ± 0.25 | 15.67 ± 0.7 | 10.11 ± 0.06 (23.8 ± 1.6%) |
| 32.85 ± 0.29 (76.2 ± 1.6%) | |||
Fig. 9 shows the T1 relaxation times for Ch+, in ethaline and reline, in the presence and absence of zinc and water. It can be seen that for ethaline, the T1 NMR relaxation time for the Ha protons in Ch+ increases, monotonically, with water content, but with a slight discontinuity around 8 wt% of water. However, for reline, the T1 relaxation time for the Ha protons in Ch+ decreases on addition of water, but then increases with increasing water content.
Discussion
The viscosity of ethaline is lower than the viscosity of reline, because U is a much stronger HBD than EG, therefore, reline is ten times more viscose than ethaline.15 The effect of zinc on the viscosity of reline and ethaline has been studied previously.15 It has been shown that the viscosity of ethaline is largely unaffected by the addition of Zn, but for reline, a significant decrease in viscosity was observed with increasing Zn.15 The effect is expected to be minimised for ethaline because it already has a low viscosity (∼22 cP for [ZnCl2] = 0 to 0.3 mol dm−3), compared to reline (∼800 cP to 280 cP for [ZnCl2] = 0 to 0.3 mol dm−3).15 However, the significant change in viscosity for reline, when Zn was added, was not observed in our study. This could be explained by our use of dried ZnCl2, compared to the previous study,15 which used ZnCl2 as obtained. As ZnCl2 is hygroscopic, it is possible the previously observed change in viscosity could be due to the introduction of water. The possible effects of added water to the viscosity of ethaline are not observed, because of its significantly lower viscosity.From previous studies of ethaline,34 it is known that the hydroxyl groups of Ch+ and EG coordinate around the Cl−, bringing the Hd and Hf protons into close proximity. It is expected that this close proximity will facilitate proton exchange, which is observed in this study, by the presence of positive cross-peaks between Hd and Hf protons in the NOESY spectrum of pure ethaline (Fig. 5a). When water is added, it is known17 that water also coordinates with the Cl−, Ch+ and EG. Again, this proximity is expected to facilitate exchange between Hd, Hf and Hw protons. This has been observed previously by diffusion NMR12 and is also observed, in this study, by the positive cross-peaks between Hd, Hf and Hw protons in the NOESY spectrum (Fig. 6a). However, this study has shown, for the first time, that the addition of zinc, to both pure ethaline and ethaline-water systems, increases the rate of exchange between these protons (Table 1). This increase in proton exchange explains the increase in diffusion co-efficient for the Hd proton in the presence of Zn (Table 2). These observations indicate that EG, Ch+ and water are predominantly co-ordinated around Zn2+, rather than Cl−.
From previous studies of reline,35 it has been suggested that the hydroxyl group in Ch+ and carbonyl group in U co-ordinate around the Cl− ion. It is expected that such an orientation reduces the opportunity for proton exchange between Hd (U) and Hg (Ch+) protons. This is supported by the diffusion data (Table 2), which show that alkyl and hydroxyl protons in Ch+ diffuse at the same rate. However, the 2D 1H–1H NOESY spectrum shows a cross peak between Hd and Hg protons (Fig. 7a). If proton exchange is not the origin of this cross peak, it can arise from the nOe, indicating these protons are in close (≲3 Å) proximity. The addition of Zn does not appear to change the interactions between U and Ch+ species, where 1D 1H (Fig. 4) and 2D 1H–1H NOESY (Fig. 7) spectra and 1H diffusion data (Table 2) are observed to remain largely unchanged.
When water is added to reline, it has been previously observed23 that water coordinates with Cl− and Ch+. This co-ordination brings the Hw and Hd protons into close proximity, facilitating proton exchange between these protons. This is observed in this study in the 2D 1H–1H NOESY spectra (Fig. 8 and 9). In the NOESY spectrum with 8.3% wt water (Fig. 8a), not only is a cross peak observed between Hd and Hw protons, but there is now an absence of an nOe interaction between Hg and Hd protons, indicating a change in co-ordination between U and Ch+ species. The addition of water to reline, in the presence of Zn, does not appear to change the interactions between U or Ch+ species (Fig. 4 and 8) However, the presence of zinc appears to slow the exchange rate between Hd and Hw protons (Table 1). This is also supported by the 1D 1H NMR spectra (Fig. 4b), where, in the presence of Zn, there is a narrowing of the line width of Hd and Hw peaks, which suggests that the presence of Zn slows down proton exchange. It is not clear why this is, but it could be because Zn2+ may compete with Cl− to co-ordinate with either the water or Ch+, or both, and thus reduces the number Hd and Hw protons able to exchange.
For both ethaline and reline, the addition of water reduces the viscosity.12 This can be seen in the narrowing of peaks for non-exchangeable protons (Fig. 3 and 4) and increase in T1 relaxation times (Fig. 10) and diffusion co-efficients, with increasing water (Tables 3–6). However, the T1 relaxation time and diffusion co-efficient data also indicate a phase transition for both reline and ethaline with increasing water concentration. This can be seen in (Fig. 11), where these data are combined into a single plot for each DES. In ethaline, a discontinuity is observed in T1 data around 8.3 wt% water. A more marked discontinuity in the T1 relaxation time is observed for reline, where there is an initial decrease then increase. It should be noted, that a similar discontinuity can also be observed in the diffusion co-efficient data reported by D’Agostino et al.,12 where a wider range of water concentrations were investigated. The origins of this behaviour in reline are most likely to come from a change in the distribution of water within the system. At low concentrations, water molecules embed in the reline network, forming a supermolecular complex. In such structures, there is an associated increase in size, leading to a higher rotational correlation time, for ChCl, and, hence, lower T1 relaxation time.36 However, when the water content increases (>6 wt%), there is an increase in the T1 relaxation time for ChCl, which suggests a reduction in the tumbling rate for ChCl, indicating a change in the structures (solvation) formed within reline. This observation could be indicative of a transition to the heterogeneous distribution of water from discrete microscopic ‘pockets’ of water, within the reline network, proposed by Posada et al.24 and D’Agostino et al.12 at high water concentrations. Ethaline shows more of a monotonic increase in T1 relaxation time, with increasing water content. However, a slight discontinuity can also be observed around 8 wt% of water, which is at a concentration equivalent to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (water
1 (water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl) mole ratio, a concentration that has previously12 been observed to correspond to a transition in behaviour of other deep eutectic solvents.
ChCl) mole ratio, a concentration that has previously12 been observed to correspond to a transition in behaviour of other deep eutectic solvents.
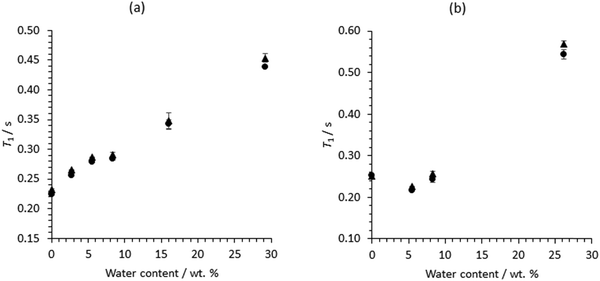 | ||
| Fig. 10 T 1 relaxation times for Ch+ protons (Ha), in (a) ethaline and (b) reline, as a function of water content, (●) in the absence and (▲) presence of 0.3 M ZnCl2. | ||
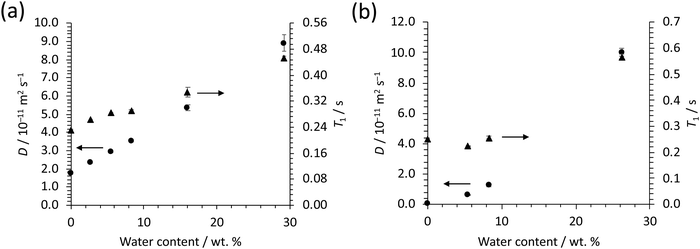 | ||
| Fig. 11 Plot of T1 relaxation times (▲) and diffusion co-efficient (●) for ChCl protons (Ha) in (a) ethaline and (b) reline, as a function of water content. | ||
Lastly, diffusion measurements for both reline and ethaline have shown that water has two diffusion co-efficients, over all water concentrations, in the presence and absence of Zn. This suggests that water exists in two different environments, with no, or slow, exchange between these environments. As we see this bi-exponential diffusion co-efficient for water, over all water concentrations, it is unlikely that these environments are associated with the water within the DES network and interstitial water, which has been observed at >6 wt% water (reline) and >8 wt% (ethaline). It is unclear what the origins are for the bi-exponential diffusion co-efficient of water, and further studies are required.
Conclusions
This paper has investigated the role of Zn and water on solvation, and dynamics, in reline and ethaline DES systems, using 1H NMR spectroscopy, T1 NMR relaxation times, NMR diffusion and 2D NOESY/EXSY spectroscopy. In ethaline, it is found that Zn promotes proton exchange between hydroxyl protons (Hd and Hf) in ChCl and EG, in the presence and absence of water. However, in reline, the presence of Zn was found to have little effect on the interactions between ChCl and U species, but did reduce the proton exchange between hydroxyl (Hd) and water (Hw) protons, in systems containing water. The presence of water was also found to change the interaction between ChCl and U species, removing the nOe cross-peak between amide and hydroxyl protons. These findings reveal key changes in solvation and dynamics, in both reline and ethaline, as a function of water and Zn concentration, proving insight into the role of solvation and dynamics in these electrolytes for a range of Zn electrochemical applications. These data support previous observations of changes in microstructure for both reline and ethaline, with increasing water content. Moreover, the variation in T1 NMR relaxation times, for certain proton environments, in both DES, also demonstrates the potential for Zn electrochemical processes to be visualised by operando magnetic resonance imaging.27,28Data availability
The data generated in this study are available at https://doi.org/10.25500/edata.bham.00000719.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the University of Birmingham and EPSRC (grant EP/K039245/1) for financial support. YAZ thanks the Saudi Arabian Ministry of Higher Education and the chemistry department in Imam Abdulrahman Bin Faisal University for their financial support.References
- F. I. Danilov, V. S. Protsenko, A. A. Kityk, D. A. Shaiderov, E. A. Vasil’eva, U. P. Kumar and C. J. Kennady, Prot. Met. Phys. Chem., 2017, 53, 1131–1138 CrossRef CAS.
- V. S. Protsenko, L. S. Bobrova, D. E. Golubtsov, S. A. Korniy and F. I. Danilov, Russ. J. Appl. Chem., 2018, 91, 1106–1111 CrossRef CAS.
- A. P. Abbott, J. C. Barron and K. S. Ryder, Trans. Inst. Met. Finish., 2009, 87, 201–207 CrossRef CAS.
- A. P. Abbott, K. J. McKenzie and K. S. Ryder, Ionic Liquids IV, American Chemical Society, 2007, vol. 975, ch. 13, pp. 186–197 Search PubMed.
- A. P. Abbott, G. Capper, K. J. McKenzie, A. Glidle and K. S. Ryder, Phys. Chem. Chem. Phys., 2006, 8, 4214–4221 RSC.
- W. Kao-ian, R. Pornprasertsuk, P. Thamyongkit, T. Maiyalagan and S. Kheawhom, J. Electrochem. Soc., 2019, 166, A1063–A1069 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- E. L. Smith, Trans. Inst. Met. Finish., 2014, 91, 241–248 CrossRef.
- A. P. Abbott, K. El Ttaib, G. Frisch, K. J. McKenzie and K. S. Ryder, Phys. Chem. Chem. Phys., 2009, 11, 4269–4277 RSC.
- A. H. Whitehead, M. Pölzler and B. Gollas, J. Electrochem. Soc., 2010, 157, D328–D334 CrossRef CAS.
- C. D'Agostino, L. F. Gladden, M. D. Mantle, A. P. Abbott, E. I. Ahmed, A. Y. M. Al-Murshedi and R. C. Harris, Phys. Chem. Chem. Phys., 2015, 17, 15297–15304 RSC.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- A. P. Abbott, J. C. Barron, G. Frisch, K. S. Ryder and A. F. Silva, Electrochim. Acta, 2011, 56, 5272–5279 CrossRef CAS.
- A. P. Abbott, J. C. Barron, G. Frisch, S. Gurman, K. S. Ryder and A. Fernando Silva, Phys. Chem. Chem. Phys., 2011, 13, 10224–10231 RSC.
- X. Ge, C. Gu, X. Wang and J. Tu, J. Mater. Chem. A, 2017, 5, 8209–8229 RSC.
- A. T. Celebi, T. J. H. Vlugt and O. A. Moultos, J. Phys. Chem. B, 2019, 123, 11014–11025 CrossRef CAS PubMed.
- T. Zhekenov, N. Toksanbayev, Z. Kazakbayeva, D. Shah and F. S. Mjalli, Fluid Phase Equilib., 2017, 441, 43–48 CrossRef CAS.
- C. Ma, A. Laaksonen, C. Liu, X. Lu and X. Ji, Chem. Soc. Rev., 2018, 47, 8685–8720 RSC.
- J. R. Bezerra-Neto, N. G. Sousa, L. P. M. dos Santos, A. N. Correia and P. de Lima-Neto, Phys. Chem. Chem. Phys., 2018, 20, 9321–9327 RSC.
- C. Du, B. Zhao, X.-B. Chen, N. Birbilis and H. Yang, Sci. Rep., 2016, 6, 1–14 CrossRef PubMed.
- P. E. Valverde, T. A. Green and S. Roy, J. Appl. Electrochem., 2020, 50, 699–712 CrossRef CAS.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Angew. Chem., Int. Ed., 2017, 56, 9782–9785 CrossRef CAS PubMed.
- E. Posada, N. López-Salas, R. J. Jiménez Riobóo, M. L. Ferrer, M. C. Gutiérrez and F. del Monte, Phys. Chem. Chem. Phys., 2017, 19, 17103–17110 RSC.
- A. Pandey and S. Pandey, J. Phys. Chem. B, 2014, 118, 14652–14661 CrossRef CAS PubMed.
- J. C. Barron, Doctor of Philosophy, University of Leicester, 2009.
- A. J. Davenport, M. Forsyth and M. M. Britton, Electrochem. Commun., 2010, 12, 44–47 CrossRef CAS.
- M. M. Britton, P. M. Bayley, P. C. Howlett, A. J. Davenport and M. Forsyth, J. Phys. Chem. Lett., 2013, 4, 3019–3023 CrossRef CAS PubMed.
- I. R. Kleckner and M. P. Foster, Biochim. Biophys. Acta, 2011, 1814, 942–968 CrossRef CAS PubMed.
- KaleidaGraph (Synergy, USA).
- T. D. W. Claridge, High-Resolution NMR Techniques in Organic Chemistry, 2nd edn, Elsevier, Oxford, UK, 2009 Search PubMed.
- E. O. Stejskal and J. E. Tanner, J. Chem. Phys., 1965, 42, 288 CrossRef CAS.
- R. R. Gil and A. Navarro-Vázquez, Modern NMR Approaches to the Structure Elucidation of Natural Products: Volume 2: Data Acquisition and Applications to Compound Classes, The Royal Society of Chemistry, 2017, vol. 2, pp. 1–38 Search PubMed.
- R. Stefanovic, M. Ludwig, G. B. Webber, R. Atkin and A. J. Page, Phys. Chem. Chem. Phys., 2017, 19, 3297–3306 RSC.
- C. R. Ashworth, R. P. Matthews, T. Welton and P. A. Hunt, Phys. Chem. Chem. Phys., 2016, 18, 18145–18160 RSC.
- N. Bloembergen, E. M. Purcell and R. V. Pound, Phys. Rev., 1948, 73, 679–712 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cp03204f |
| This journal is © the Owner Societies 2021 |