Solubilities in aqueous nitrate solutions that appear to reverse the law of mass action
Abstract
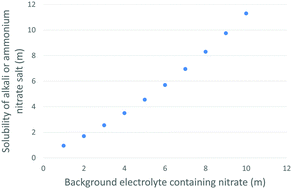
Non-ideal aqueous electrolyte solutions have been studied since the start of the application of thermodynamics to chemistry in the late 19th century. The present study examines some of the most extreme non-ideal behavior ever observed: solubilities of alkali and NH4+ nitrate salts in water that appear to behave the opposite of how the Law of Mass Action would predict. A literature review discovered that the solubilities of NH4NO3 and many alkali nitrate salts increases when another nitrate-bearing electrolyte is added to solution. These occurrences were in concentrated solutions with insufficient water to provide all ions their preferred hydration number without sharing waters between ions. This water deficit results in the formation of contact ion-pairs as well as larger ion-clusters. These ion-clusters may be favored when there is more than one type of monovalent cation present.
- This article is part of the themed collections: 2021 PCCP HOT Articles and PCCP Perspectives


 Please wait while we load your content...
Please wait while we load your content...