Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aggregation-induced emission leading to two distinct emissive species in the solid-state structure of high-dipole organic chromophores†
Felix
Witte
 *a,
Philipp
Rietsch
b,
Nithiya
Nirmalananthan-Budau
c,
Florian
Weigert
c,
Jan P.
Götze
*a,
Philipp
Rietsch
b,
Nithiya
Nirmalananthan-Budau
c,
Florian
Weigert
c,
Jan P.
Götze
 a,
Ute
Resch-Genger
a,
Ute
Resch-Genger
 c,
Siegfried
Eigler
c,
Siegfried
Eigler
 b and
Beate
Paulus
a
b and
Beate
Paulus
a
aInstitute of Chemistry and Biochemistry, Freie Universität Berlin, Arnimallee 22, 14195 Berlin, Germany. E-mail: jf.witte@fu-berlin.de
bInstitute of Chemistry and Biochemistry, Freie Universität Berlin, Takustr. 3, 14195 Berlin, Germany
cDivision Biophotonics, Federal Institute for Material Research and Testing (BAM), Richard-Willstätter-Straße 11, 12489 Berlin, Germany
First published on 23rd July 2021
Abstract
The concept of aggregation-induced emission represents a means to rationalise photoluminescence of usually nonfluorescent excimers in solid-state materials. In this publication, we study the photophysical properties of selected diaminodicyanoquinone (DADQ) derivatives in the solid state using a combined approach of experiment and theory. DADQs are a class of high-dipole organic chromophores promising for applications in non-linear optics and light-harvesting devices. Among the compounds investigated, we find both aggregation-induced emission and aggregation-caused quenching effects rationalised by calculated energy transfer rates. Analysis of fluorescence spectra and lifetime measurements provide the interesting result that (at least) two emissive species seem to contribute to the photophysical properties of DADQs. The main emission peak is notably broadened in the long-wavelength limit and exhibits a blue-shifted shoulder. We employ high-level quantum-chemical methods to validate a molecular approach to a solid-state problem and show that the complex emission features of DADQs can be attributed to a combination of H-type aggregates, monomers, and crystal structure defects.
Introduction
Understanding the mechanisms and intricacies related to the optical properties of solid-state materials opens the door towards applications in optoelectronic devices,1,2 photosensors,3–5 and bioimaging6–8 and is therefore of broad interest for chemists, physicists, and material scientists alike. Notably, significant advances have been made in the field of luminescent organic solid-state materials.9–15 One major drawback encountered in solid-state structures is so-called aggregation-caused quenching (ACQ) of emission promoted by close molecular packing within a crystal often leading to severe reduction of fluorescence quantum yields (QYs).16–18 Probably the most prominent quenching mechanism in organic crystals involves energy transfer mediated through π–π-stacking in excimers. Quenching mechanisms include Förster resonance energy transfer (FRET) and the short-range Dexter process.19,20 While the former depends on the potentially far-reaching (>10 Å) interaction of molecular (transition) dipoles, decaying with an R−6 dependence, the latter is determined by wavefunction overlap which decays much quicker with an exponential dependence on distance. Without the need for resource-demanding high-level quantum-chemical methods, Radhakrishnan and co-workers have developed and successfully applied a model based on the relative orientation and assembly of isolated diaminodicyanoquinones (DADQs) in the solid state to estimate Förster19 and Dexter20 energy transfer rates and rationalise fluorescence QY trends.21In contrast to ACQ, solid-state materials may also experience QY enhancement due to close molecular packing. In 2001, Luo et al.22,23 introduced the concept of aggregation-induced emission (AIE) presenting a silole compound which for the first time showed improved QYs upon aggregation in solution. Most commonly, the AIE phenomenon can be attributed to intramolecular torsion-induced emission quenching. Seminal works on this field have been conducted by Gierschner, Park, and others.24–27 It is, furthermore, didactically meaningful to differentiate AIE from related phenomena such as aggregation-induced emission enhancement (AIEE),28 which describes an increase in fluorescence quantum yields in the solid of already emissive materials in solution. The discovery of AIE luminogens sparked a rapid increase in interest in this topic and many systems were introduced exploiting the AIE idea.29–31 Approaches to enhance emission in the solid state include the design of materials based on J-aggregates32 and embedded or isolated dimers, which may show diminished non-radiative decay rates due to the rigidity of their microscopic environment.
In this account, we examine the optical properties of selected solid-state DADQ derivatives. DADQs are a class of organic compounds promising for application in non-linear optics and light-harvesting devices due to their large dipole moments, redox activity, and fluorescence properties. We study four C2 symmetric DADQ derivatives using a combination of fluorescence experiments and state-of-the-art quantum-chemical approaches. Due to an interplay of AIE and ACQ effects, a reverse trend between fluorescence QYs in solution and in the solid state is observed with respect to the substituents. We show that this can be related to the degree of π–π-stacking within the crystals which is furthermore supported by energy transfer rates computed according to the ideas of Radhakrishnan and co-workers.33
We present an unexpected correlation between fluorescence lifetimes and emission wavelengths in solid-state DADQs. Lifetimes increase with increasing emission wavelength in a way that is incompatible with a two-state system and thus provide a hint for the existence of (at least) one more emissive species. This observation is closely related to dual emission phenomena such as the twisted intramolecular charge-transfer and the interested reader is referred to a recent review by Gierschner et al.34 For two of our compounds, we observe a blue-shifted shoulder of the main emission peak. Additionally, fluorescence intensities are still detectable close to 100 nm beyond the main peak in the long-wavelength region.
We show that instead of computing the optical properties of entire crystal structures, which would necessitate resource-draining evaluation of Greens functions or Bethe–Salpeter equations, a molecular approach based on monomers and dimers embedded in the crystal is sufficient for a proper theoretical analysis. Our protocol is based on a combination of QM/MM (quantum-mechanics/molecular-mechanics) calculations and DFT/MRCI35–37 (density functional theory/multireference configuration interaction). Finally, we reveal that the main emission peak can be attributed to monomers, while short-wavelength emission associated with much shorter lifetimes are caused by H-type dimers. Lastly, our calculations suggest that long lifetimes measured at the low-energy end of the spectra can be explained by crystal defects.
Results and discussion
Synthesis
The synthesis of compounds 1–4 is illustrated in Scheme 1. The DADQs differ in their amino substituent: 1 for neopentyl, 2 for methyl, and 3 for para-methylbenzoate. Compound 4 is defined by a benzene instead of an ethyl bridge between the nitrogen atoms of the diamino group. All substances were prepared according to literature by coupling 7-pyrrolidino-7,8,8-tricyanoquinomethane (PTCNQ) and the respective diamine.38,39 | ||
| Scheme 1 Synthetic route to DADQ derivatives starting from TCNQ by reaction with pyrrolidine and subsequent reaction with the respective primary or secondary diamine. (i) 0.8 eq. pyrrolidine, 70 °C, 4 hours. (ii) 1 eq. of the respective diamine, 70 °C, 2–20 hours. Detailed protocols, yields and characterisation of the compounds can be found in the ESI.† | ||
QY measurements in solution and in the solid state
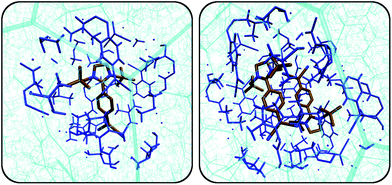
Fig. 1 depicts excerpts of the crystal structures either obtained from X-ray diffraction measurements (1–3) or from a quantum-chemical solid-state optimisation (4) (see Sections S3 and S6 in the ESI† for details). QYs in solution (ACN) and in the solid state are given below the figures. In solution, compound 4 is the only molecule showing high fluorescence QY (92%). The QY differences in solution are related to energetic barriers of intramolecular rotation around the dihedral angles near the dicyano and the amino moieties, as we have shown in an earlier publication.38 Especially rotation of the dicyano moiety provides a mechanism of fluorescence deactivation. In the solid state, this rotational mode is obviously immobilised and cannot contribute to emission quenching (ΔErot ≈ 700 kJ mol−1, Fig. S6, ESI†). | ||
| Fig. 1 Crystal structure excerpts, QYs in the solid state and in solution (ACN), and selected distances of compounds 1–4. Note that compound 4 was obtained from a periodic DFT calculation at the PBE-D3(BJ) level (see Section S6.2 in the ESI†). The increasing degree of π–π-overlap from 1 to 4 can be appreciated from the decreasing π–π-stacking distance (depicted in green). Hydrogen atoms were omitted for clarity. | ||
Solid-state fluorescence QYs range from 18% for 1 to less than 1% for 4. The QY trend observed in the solid state can be explained by the degree of π–π-stacking in the crystal structures. From the distances listed in Fig. 1, it is clear that there is practically no effective overlap between the π-systems in 1, while molecules in the crystal structure of 4 are efficiently stacked. The plane-to-plane (π–π-stacking) distance decreases quite significantly from 8.3 Å in 1 to 3.1 Å in 4. To account for the difference between 3 and 4, which virtually have the same π–π-stacking distance, the centres of mass (COM) and the centres of the quinone systems (aryl centroids) are taken into account. As can be deduced from the crystal structures, these latter distances are much larger in the case of 3. As we will see later, our observations can be related to energy transfer rates in the spirit of Förster and Dexter modes (Fig. 5 down below).21
Dependence of lifetime and emission wavelength
Lifetimes τ for selected emission wavelengths λem for compounds 1, 2, and 3 are given in Table 1. A detailed spectroscopic analysis of compound 1 is illustrated in Fig. 2 (Fig. S3 and S4 for 2 and 3, respectively, ESI†). As can be seen in Fig. 2a, there is a strong dependence of τ and λem, which is in correlation with the respective emission spectrum (Fig. 2b) and is depicted in Fig. 2c. From Fermis golden rule it can be shown that in a two-level system there should be a linear relationship between τ and λem2:40| k ∝ τ−1 ∝ ω3M2 ∝ ω2fosc ∝ λem−2 → τ ∝ λem2 | (1) |
| 1 | 0.86 (435) | 3.60 (495) | 4.31 (555) |
| 2 | 1.20 (410) | 2.13 (470) | 3.44 (530) |
| 3 | 0.46 (460) | 0.60 (480) | 3.95 (500) |
The main emission peak of 1 has a significantly longer lifetime than the high-energy (short wavelength) transition indicating a smaller oscillator strength according to eqn (1). However, their intensities in the emission spectrum are reversed. This may be caused by a larger degree of non-radiative quenching for the high-energy signal. The largest portion of the QY is, hence, unsurprisingly, due to the main peak. A Gaussian curve fit inside the experimental emission spectrum of 1 estimates a contribution of 3% to the overall QY from the high-energy shoulder (Fig. S5, ESI†). In the following sections, we show that these observations can be rationalised by the different optical properties obtained by treating isolated monomers and dimers embedded in the crystal structure. We subsequently apply a combination of energy transfer rate calculations, periodic quantum-chemical methods, and the DFT/MRCI approach to examine the electronic and optical properties of DADQs in the solid state and investigate what may be the origin of the two emitting species.
Spectroscopic studies of single crystals
In addition to the ensemble measurements which are used for the subsequent quantum-chemical calculations, we assessed the emission features of representative single crystals of compounds 1–4 using a custom-made microscopic setup. The resulting emission spectra measured at an excitation wavelength of 405 nm using different crystals of varying size are shown in Fig. 3. The overall similarity of these emission spectra suggests a good homogeneity of the differently sized crystals and the absence of surface and reabsorption effects. The photoluminescence decay kinetics detected in different emission windows (blue tail of the emission band: 435–475 nm, red tail of the emission band: 525–800 nm, whole spectral window covered by dye emission: 435–800 nm), determined by different sets of longpass and shortpass filters, are shown in the lower panels of the figure. These decay curves reveal shorter lifetimes for detection in the blue tail of the emission band of all crystals compared to the respective red tail and with a good match of the decay kinetics derived for different crystals of the same dye. This trend, which is most pronounced for compound 3, varies slightly between the different dyes studied, where the resulting intensity-weighted lifetimes of the analyses of the fitted decay curves are summarised in Table S5 (ESI†). These findings, i.e., the similar emission spectra and the reduced lifetimes in the blue tail of the emission bands, confirm the absence of reabsorption and surface effects for the crystals studied.Energy transfer rates
To elucidate the differences in solid-state fluorescence QYs of compounds 1–4, we calculated energy transfer rates in close relation to long-range Förster and short-range Dexter coupling modes (visualised in Fig. 4). As proposed by Radhakrishnan and co-workers,21 Förster rates, kF, are determined by the relative orientation and distance of the transition dipole moments of two monomers by | (2) |
kD ∝ cos(θ)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(ϕ)e−r′ = cos(θ) cos(ϕ)e−r′ = cos(θ)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(ϕ)e−r cos(ϕ)e−r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ) cos(θ) | (3) |
While kF links QYs to intermolecular (transition) dipole–dipole interaction, kD can be interpreted as a relative measure to estimate energy transfer through π–π-stacking in the crystal. Fig. 5 correlates relative values of kF and kD with the solid-state QYs of all compounds. Förster energy transfer is most important for compounds 1 and 2, which show large intermolecular separation and π–π-stacking distances. In comparison, Dexter transfer is most prominent in compounds 3 and 4, which display shorter π–π-stacking distances. We note that the π–π-stacking distance of 4.1 Å in compound 4 may well be overestimated as no crystal structure could be obtained for this substance. The solid-state structure of 4 was instead derived from the crystal data of a similar compound (Fig. S9, ESI†). The dimer motifs yielding the largest Förster and Dexter transfer rates are given in Fig. S8 (ESI†) for all compounds.
 | ||
| Fig. 5 Relative Förster and Dexter energy transfer rates of compounds 1–4 (bars, 4 is set to 1.0) with additional indication of the solid-state QY (black line). | ||
Fig. 5 furthermore reveals that kF is unsuited to describe experimental differences with respect to compound 3. It seems that regarding π-interactions becomes increasingly important for 3, while it is not yet as essential in 1 and 2. In general, while Dexter modes dominate the immediate environment of the monomers, most important for 3 and 4, Förster interaction likely contributes as well especially at longer ranges due to the slower R−6 fall-off, which should be the main energy loss mechanism for compounds 1 and 2. Details on how the values were obtained can be found in the ESI† in Section S6.1.
In general, it seems that sterically demanding N-substituents result in more isolated monomer/dimer motifs in the crystal structure, which in turn reduces emission quenching through intermolecular energy transfer. On the other hand, substituents should not be too large, since intramolecular rotations should be prevented as effectively as possible, since they are the most relevant fluorescence deactivation mechanisms in the isolated monomers.38
Please note that this analysis is based on the assumption that the optical properties of DADQs in the solid state can be reduced to the response of a monomer embedded in a crystal environment. In the following, we will refer to this idea as “molecular approach”. In principle, it neglects the possibility of largely delocalised electronic transitions playing an important role in fluorescence experiments. Hence, in the next section, we will justify the molecular approach by analysing the solid-state properties of 1 and 2, for which experimental data for crystal structures, QYs, and lifetimes are available.
A molecular approach to a solid-state problem
Periodic DFT calculations at the PBE-D3(BJ)41–43 level were conducted to analyse the solid-state structure of compound 1 (see ESI† for 2 and Section S6.2 for computational details). A band gap of 2.31 eV at the PBE level and 3.20 eV using the HSE0644 functional (Fig. S10, ESI†) are indicative of a semiconducting material. Calculations at the PBE level produce results in qualitative agreement with the HSE06 level. We note that the latter yields a band gap very similar to the experimentally measured absorption peak of the monomer in solution (3.02 eV in THF38). The bandstructure and the density-of-states (DOS) plots shown in Fig. 6a reveal a molecular, i.e., quite localised, picture of the solid-state electronic structure. The valence band (VB) and conduction band (CB) are energetically separated from the nearest occupied and virtual band by roughly 1.1 eV and 0.7 eV, respectively. A closer look at the VB and CB (Fig. S11, ESI†) shows four interacting bands originating from the four molecules comprising the unit cell. Furthermore, partial charge densities of the VB and CB depicted in Fig. 6b can clearly be interpreted simply as the sum of the in vacuo calculated highest occupied and lowest unoccupied molecular orbitals, respectively (Fig. 6c). All in all, periodic electronic structure calculations present a solid basis for an approach based on small molecular clusters to examine the optical features of DADQs in the solid state.Moreover, to obtain more insight and collect further arguments for (or against) a molecular approach we computed excited states employing the ωB97X-D345 functional within the TD-DFT framework for a tetramer, i.e., the unit cell, and an octamer of compounds 1 and 2, and an additional dodecamer of compound 2. Various dimer conformations were computed for comparison. Larger clusters proved to be unfeasible at this level which prevented us from conducting convergence tests with respect to cluster size. The C-PCM46 solvent model was utilised to mimic the dielectric properties of the crystal around the molecular clusters. The dielectric constant and refractive index necessary for the calculations were extracted from the dielectric tensor obtained at the periodic PBE level (Section S6.2 in the ESI† for details). Molecular structures (Fig. S13 and S14), transition energies, and oscillator strengths (Tables S7–S12) are given in the ESI.†
Fig. 7 depicts the difference densities for the excited states with the highest oscillator strengths of an octamer of 1 (S5) and a dodecamer of 2 (S10). In fact, all electronic transitions are fairly localised on either just a monomer or a dimer resembling an H-type aggregate. This provides a further argument in favour of a molecular approach. Note that in Fig. 7 a transition localised on a monomer (dimer) still involves two (four) molecules, as both the octamer and the dodecamer consist of symmetrically equivalent sub-units. Hence, for clarity, only a part of the clusters is shown.
Certainly, one must be careful in assessing this approach as no experimental absorption data are available. More importantly, however, emission properties of these larger clusters are currently out of reach as this would necessitate excited-state optimisations requiring massive computational resources with the additional problem of modelling the solid-state environment. Still, our periodic DFT results in combination with localised excited states observed in molecular clusters provide sufficient evidence to justify a molecular approach based on monomers and dimers for the description of the electronic and optical properties of DADQs in the solid state. This comes with the major advantage that emission properties become feasible due to the manageable size of monomers and dimers. This will be exploited in the next part, where we will use a combination of the QM/MM method and DFT/MRCI to assess the absorption and emission properties of compounds 1 and 2.
Rationalising solid-state fluorescence properties
In the previous section, we have established that it is sufficient to examine monomers and dimers of our DADQs embedded in their crystal structure to evaluate their photophysical solid-state properties. We calculated monomers and H- and J-like aggregated dimers embedded in the crystal structure of compound 1 and 2 (Fig. 8 and Fig. S19, ESI†). Since compound 3 essentially only contains H-type aggregates (cf.Fig. 1), J-aggregates are likely to play a subordinated role, if we assume that the mechanisms responsible for the emission properties are similar in all compounds. We employed the ONIOM47 scheme to perform a QM/MM optimisation of ground- and excited states using the ωB97XD functional for the high layer and the UFF (universal force field)48 for the low layer. Note that the dispersion part of the functional differs from the one used earlier due to limitations of the employed quantum-chemical programme packages. Afterwards, the DFT/MRCI method in combination with the C-PCM model once again using the dielectric constant obtained from the periodic calculations was employed to compute absorption and emission peaks. A similar approach has been used recently for chalcone-based materials.49 Due to the non-polarisable force field, state crossings with dark charge-transfer states pose a hypothetical problem. However, excited states in DADQ dimers are of local nature which can be deduced from the difference densities of the S1 and S2 states in the H-type dimer displaying the same nodal shape (Fig. S20, ESI†). Details about the DFT/MRCI excited state calculations can be found in Section S6.2 of the ESI.† As can be seen in Table 2, our protocol yields a nice agreement between calculated vertical transitions and experimental emission energies. For compound 1, the main peak at 495 nm is energetically close to the S1 of the monomer, while the shoulder at 438 nm is near the S2 of the H-type dimer. Both emission energies differ by less than 7 nm (0.07 eV, vertical transition lines in Fig. 2b above). J-Aggregates can be assumed to only play a minor role for the emission spectrum, presumably in between the main peak and the high-energy (short wavelength) shoulder (Table S14, ESI†). Fig. 9 illustrates the mechanism and shows that excited-state and ground-state structures optimised in a crystal environment differ only very slightly, as expected. For compound 2, the main peak is exactly reproduced by the calculation likely benefitting from fortuitous error cancellation, while the high-energy component of the spectrum cannot be extracted reliably.As has been addressed earlier, there is a mismatch between emission intensities and fluorescence lifetimes, as shorter lifetimes indicate larger oscillator strengths (eqn (1)) which, in turn, imply larger intensities. In Table 2, we can see that this discrepancy can now be explained by non-radiative fluorescence quenching through internal conversion of the high-energy S2 of the H-type dimer to its dark S1 which lies 34 nm (0.2 eV) lower in energy. Technically, emission from the high-lying S2 violates Kashas rule,50 which states that emission originates from the lowest excited state (S1). However, the two states are very close in energy enabling potentially multiple state crossings or conical intersections that allow for dynamical population exchange. Future analysis would therefore require quantum dynamics which is however not covered in this work.
Depending on the emitting system, the radiative decay pathway of the monomer can vary between quenched or unquenched. We assign the vast majority of the fluorescence QY to the monomers, as those exhibit the largest contribution to the emission according to Table 2. In conclusion, we attribute the general shape of the emission spectrum of 1 to monomers and H-type dimers embedded in the solid-state crystal structure.
One remaining issue is the low-energy (long wavelength) end of the emission spectrum. For compound 1, the longest lifetimes are measured at around 570 nm, almost 80 nm (0.33 eV) red-shifted with respect to the monomer emission peak. This is noteworthy, as lifetime broadening should actually sharpen the signal rather than widening it. Monomers with higher conformational mobility in the crystal should produce larger Stokes shifts and may, thus, explain this observation.
Such structural freedom may be achieved by defects in the solid-state structure. Rather large R-factors of the crystal structures (Table S4, ESI†) support this claim. Hence, we repeated the computational procedure described above for monomers placed inside a defect. As there is no experimental data for the nature of possible defects available, we opted to simply use the cavity formed by the H-type dimer of 1 and put a monomer inside of it. The resulting emission peak is located at 516 nm, red-shifted by 34 nm, making it a potential candidate for the observed long lifetimes near the low-energy end of the emission spectrum. In the most extreme case, without the rigid crystal environment, the monomer loses all of its oscillator strength while constantly increasing the Stokes shift upon relaxation on the S1 (Fig. 10). We can thus assume, that depending on the size of the defect cavity, the monomer may show small amounts of fluorescence at various emission energies beyond the main emission peak.
Conclusion
Using both experimental and computational approaches, we were able to explain the emergence of aggregation-induced emission in an organic crystal system. The solid-state optical properties of four DADQ derivatives were rationalised and the corresponding mechanisms supported by molecular models.Differences in solid-state fluorescence QYs can be related to the efficiency of π–π-stacking interactions within the crystal which were quantified by energy transfer rate calculations according to Radhakrishnan and co-workers. While benzene-bridged, unsubstituted compound 4 experiences aggregation-caused quenching, dineopentyl-substituted derivative 1 is subject to an aggregation-induced emission effect. Furthermore, lifetime measurements at various emission wavelengths reveal multiple emitting species in compounds 1–3, which we attribute to H-type dimers and monomers, while J-aggregates only play a minor role for the optical properties. Our computational protocol based on QM/MM calculations and the DFT/MRCI method to evaluate the photophysical properties of DADQs in the solid state, may serve as a general approach to study optical problems in organic crystals.
In conclusion, our joint study was able to elucidate a wide range of effects occurring in organic crystals, likely not limited to the DADQ compounds studied here. The results may have implications for the design of organic light emitting compounds.
Conflicts of interest
The authors declare no competing interests.Acknowledgements
We thank Tim Küllmey for helpful discussions. The North-German Supercomputing Alliance (Norddeutscher Verbund für Hoch- und Höchstleistungsrechnen) and the computing facilities of Freie Universität Berlin (Zentrale Einrichtung für Datenverarbeitung) are acknowledged for providing computational resources. FW and JPG gratefully acknowledge the Deutsche Forschungsgemeinschaft for financial support through research grant PA 1360/16-1 and project no. 393271229, respectively. URG and NNB gratefully acknowledge financial support by DFG grant RE1203/23-1 and a MI Type 3 Project from BAM.References
- M. M. Stylianakis, Nanomaterials, 2020, 10, 520 CrossRef CAS PubMed.
- X. Wang, Y. Cui, T. Li, M. Lei, J. Li and Z. Wei, Adv. Opt. Mater., 2019, 7, 1801274 CrossRef.
- T. L. Mako, J. M. Racicot and M. Levine, Chem. Rev., 2019, 119, 322–477 CrossRef CAS PubMed.
- R. D. Jansen-van Vuuren, A. Armin, A. K. Pandey, P. L. Burn and P. Meredith, Adv. Mater., 2016, 28, 4766–4802 CrossRef CAS PubMed.
- D. Yang and D. Ma, Adv. Opt. Mater., 2019, 7, 1800522 CrossRef.
- H.-B. Cheng, Y. Li, B. Z. Tang and J. Yoon, Chem. Soc. Rev., 2020, 49, 21–31 RSC.
- Q. Qi, C. Li, X. Liu, S. Jiang, Z. Xu, R. Lee, M. Zhu, B. Xu and W. Tian, J. Am. Chem. Soc., 2017, 139, 16036–16039 CrossRef CAS PubMed.
- C.-B. Ma, Z.-T. Zhu, H.-X. Wang, X. Huang, X. Zhang, X. Qi, H.-L. Zhang, Y. Zhu, X. Deng, Y. Peng, Y. Han and H. Zhang, Nanoscale, 2015, 7, 10162–10169 RSC.
- J. De, S. P. Gupta, S. Sudheendran Swayamprabha, D. K. Dubey, I. Bala, I. Sarkar, G. Dey, J.-H. Jou, S. Ghosh and S. K. Pal, J. Phys. Chem. C, 2018, 122, 23659–23674 CrossRef CAS.
- S. Feuillastre, M. Pauton, L. Gao, A. Desmarchelier, A. J. Riives, D. Prim, D. Tondelier, B. Geffroy, G. Muller, G. Clavier and G. Pieters, J. Am. Chem. Soc., 2016, 138, 3990–3993 CrossRef CAS PubMed.
- I. Bala, N. Singh, R. A. K. Yadav, J. De, S. P. Gupta, D. P. Singh, D. K. Dubey, J.-H. Jou, R. Douali and S. K. Pal, J. Mater. Chem. C, 2020, 8, 12485–12494 RSC.
- A. Adak, T. Panda, A. Raveendran, K. S. Bejoymohandas, K. S. Asha, A. P. Prakasham, B. Mukhopadhyay and M. K. Panda, ACS Omega, 2018, 3, 5291–5300 CrossRef CAS PubMed.
- R. Butkute, R. Lygaitis, V. Mimaite, D. Gudeika, D. Volyniuk, G. Sini and J. V. Grazulevicius, Dyes Pigm., 2017, 146, 425–437 CrossRef CAS.
- S. Mukherjee and P. Thilagar, Angew. Chem., Int. Ed., 2019, 58, 7922–7932 CrossRef CAS PubMed.
- P. Pallavi, V. Kumar, M. W. Hussain and A. Patra, ACS Appl. Mater. Interfaces, 2018, 10, 44696–44705 CrossRef CAS PubMed.
- Y. Huang, J. Xing, Q. Gong, L.-C. Chen, G. Liu, C. Yao, Z. Wang, H.-L. Zhang, Z. Chen and Q. Zhang, Nat. Commun., 2019, 10, 169 CrossRef PubMed.
- W. Z. Yuan, P. Lu, S. Chen, J. W. Y. Lam, Z. Wang, Y. Liu, H. S. Kwok, Y. Ma and B. Z. Tang, Adv. Mater., 2010, 22, 2159–2163 CrossRef CAS PubMed.
- B. Andreiuk, A. Reisch, E. Bernhardt and A. S. Klymchenko, Chem. – Asian J., 2019, 14, 836–846 CrossRef CAS PubMed.
- T. Förster, Ann. Phys., 1948, 437, 55–75 CrossRef.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836–850 CrossRef CAS.
- P. Srujana, P. Sudhakar and T. P. Radhakrishnan, J. Mater. Chem. C, 2018, 6, 9314–9329 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, B. Z. Tang, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. Zhu, Chem. Commun., 2001, 1740–1741 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC.
- J. Gierschner and S. Y. Park, J. Mater. Chem. C, 2013, 1, 5818 RSC.
- J. Gierschner, L. Lüer, B. Milián-Medina, D. Oelkrug and H.-J. Egelhaaf, J. Phys. Chem. Lett., 2013, 4, 2686–2697 CrossRef CAS.
- J. Shi, L. E. Aguilar Suarez, S.-J. Yoon, S. Varghese, C. Serpa, S. Y. Park, L. Lüer, D. Roca-Sanjuán, B. Milián-Medina and J. Gierschner, J. Phys. Chem. C, 2017, 121, 23166–23183 CrossRef CAS.
- Z. Guo, C. Yan and W. Zhu, Angew. Chem., Int. Ed., 2020, 59, 9812–9825 CrossRef CAS PubMed.
- B.-K. An, S.-K. Kwon, S.-D. Jung and S. Y. Park, J. Am. Chem. Soc., 2002, 124, 14410–14415 CrossRef CAS PubMed.
- Y. Shen, H. Liu, S. Zhang, Y. Gao, B. Li, Y. Yan, Y. Hu, L. Zhao and B. Yang, J. Mater. Chem. C, 2017, 5, 10061–10067 RSC.
- H. Liu, L. Yao, B. Li, X. Chen, Y. Gao, S. Zhang, W. Li, P. Lu, B. Yang and Y. Ma, Chem. Commun., 2016, 52, 7356–7359 RSC.
- H. Liu, D. Cong, B. Li, L. Ye, Y. Ge, X. Tang, Y. Shen, Y. Wen, J. Wang, C. Zhou and B. Yang, Cryst. Growth Des., 2017, 17, 2945–2949 CrossRef CAS.
- F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376–3410 CrossRef PubMed.
- P. Srujana and T. P. Radhakrishnan, Chem. – Eur. J., 2018, 24, 1784–1788 CrossRef CAS.
- J. Gierschner, S. K. Behera and S. Y. Park, Angew. Chem., Int. Ed., 2021, 60, 2–17 CrossRef.
- I. Lyskov, M. Kleinschmidt and C. M. Marian, J. Chem. Phys., 2016, 144, 034104 CrossRef PubMed.
- M. Kleinschmidt, C. M. Marian, M. Waletzke and S. Grimme, J. Chem. Phys., 2009, 130, 044708 CrossRef PubMed.
- C. M. Marian, A. Heil and M. Kleinschmidt, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2019, 9, e1394 Search PubMed.
- P. Rietsch, F. Witte, S. Sobottka, G. Germer, A. Becker, A. Güttler, B. Sarkar, B. Paulus, U. Resch-Genger and S. Eigler, Angew. Chem., Int. Ed., 2019, 58, 8235–8239 CrossRef CAS PubMed.
- P. Sudhakar and T. P. Radhakrishnan, J. Mater. Chem. C, 2019, 7, 7083–7089 RSC.
- D. Toptygin, J. Fluoresc., 2003, 13, 201–219 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- J.-D. Chai and M. Head-Gordon, J. Chem. Phys., 2008, 128, 084106 CrossRef.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995–2001 CrossRef CAS.
- M. Svensson, S. Humbel, R. D. J. Froese, T. Matsubara, S. Sieber and K. Morokuma, J. Phys. Chem., 1996, 100, 19357–19363 CrossRef CAS.
- A. K. Rappe, C. J. Casewit, K. S. Colwell, W. A. Goddard and W. M. Skiff, J. Am. Chem. Soc., 1992, 114, 10024–10035 CrossRef CAS.
- M. Dommett, M. Rivera, M. T. H. Smith and R. Crespo-Otero, J. Mater. Chem. C, 2020, 8, 2558–2568 RSC.
- M. Kasha, Discuss. Faraday Soc., 1950, 9, 14–19 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2051074. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cp02534a |
| This journal is © the Owner Societies 2021 |