Mechanism and kinetics for the reaction of methyl peroxy radical with O2†
Abstract
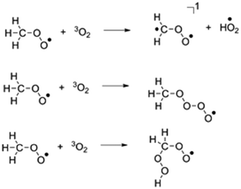
Quantum chemical calculations and dynamics simulations were performed to study the reaction between methyl peroxy radical (CH3O2) and O2. The reaction proceeds through three different pathways (1) H-atom abstraction, (2) O2 addition and (3) concerted H-atom shift and O2 addition reactions. The concerted H-atom shift and O2 addition pathway is the most favourable reaction both kinetically and thermodynamically. The major product channel formed from these reactions is H2CO + OH + O2. Trajectory calculations also confirm that H2CO + OH + O2 is the main product channel. An estimated rate constant expression for this reaction from master equation calculations is 4.20 × 1013 e−8676/T cm3 mole−1 s−1.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...