Open Access Article
Open Access ArticleMolecular properties affecting the hydration of acid–base clusters†
Nanna
Myllys
 *ab,
Deanna
Myers
a,
Sabrina
Chee
a and
James N.
Smith
*ab,
Deanna
Myers
a,
Sabrina
Chee
a and
James N.
Smith
 a
a
aDepartment of Chemistry, University of California, Irvine, California 92617, USA
bDepartment of Chemistry, University of Jyväskylä, Jyväskylä 40014, Finland. E-mail: nanna.myllys@helsinki.fi
First published on 25th May 2021
Abstract
In the atmosphere, water in all phases is ubiquitous and plays important roles in catalyzing atmospheric chemical reactions, participating in cluster formation and affecting the composition of aerosol particles. Direct measurements of water-containing clusters are limited because water is likely to evaporate before detection, and therefore, theoretical tools are needed to study hydration in the atmosphere. We have studied thermodynamics and population dynamics of the hydration of different atmospherically relevant base monomers as well as sulfuric acid–base pairs. The hydration ability of a base seems to follow in the order of gas-phase base strength whereas hydration ability of acid–base pairs, and thus clusters, is related to the number of hydrogen binding sites. Proton transfer reactions at water–air interfaces are important in many environmental and biological systems, but a deeper understanding of their mechanisms remain elusive. By studying thermodynamics of proton transfer reactions in clusters containing up to 20 water molecules and a base molecule, we found that that the ability of a base to accept a proton in a water cluster is related to the aqueous-phase basicity. We also studied the second deprotonation reaction of a sulfuric acid in hydrated acid–base clusters and found that sulfate formation is most favorable in the presence of dimethylamine. Molecular properties related to the proton transfer ability in water clusters are discussed.
1 Introduction
Atmospheric aerosol particles influence human health and global climate. Airborne particles act as condensation nuclei for clouds and can also directly absorb or scatter incoming radiation, forming a significant but highly uncertain effect on Earth's radiation balance.1 New-particle formation (NPF) from atmospheric vapors is a significant source of ultrafine particles, but the participating vapors as well as the molecular-level mechanisms are not fully resolved.2,3 In the present-day atmosphere that contains high levels of sulfur, sulfuric acid is a key precursor vapor and has been shown to be linked to NPF events in various environments. However, sulfuric acid-driven NPF requires additional stabilizing compounds in order to yield particle formation rates similar to those observed in the atmosphere.4 These compounds include atmospheric bases, organic compounds and ions.5–7 In addition, water can act a stabilizer but, due to its weak binding ability, it cannot be a main driver of NPF. In the upper troposphere, pure sulfuric acid–water clustering may occur as the low temperature stabilizes clusters and other compounds are scarce.8 Water can also participate in particle growth, but it is unlikely to be a major contributor due to its relatively high saturation vapor pressure and small molecular volume.9Because water evaporates rapidly from ions under the high-vacuum conditions in mass spectrometers, its connection to cluster formation is difficult to observe directly.10,11 The ability of sulfuric acid–base clusters to absorb water is believed to be dependent on either the basicity of the base compound or the number of available hydrogen binding sites in the cluster.12–15 Yang et al. have used tandem mass spectrometry coupled to a temperature-controlled ion trap to study water uptake of positively charged sulfuric acid–base clusters.16 They synthesized the hydrated clusters by mass-selecting dry clusters and storing them in an ion trap held at 180 K with a small partial pressure of water vapor. Based on the vibrational spectra, they showed that the binding of water molecules is most favorable for clusters containing base compounds with more NH groups. Thus the hydration ability of a cluster correlates much stronger with the number of hydrogen bond donors than the strength of the base.17 With similar methods, Kreinbihl et al. showed that hydration of ammonium bisulfate clusters has complex temperature dependence and that water can also insert into a bisulfate-bisulfate hydrogen bond.18 Also in negative ion photoelectron spectroscopy studies of bisulfate-oxidized organic compound complex, it has been found that the hydrated cluster stability is directly connected to the total number of the hydrogen bonds formed.19,20
Several studies have used ion mobility spectrometry to probe water uptake by sulfuric acid–dimethylamine (SA–DMA) nanoclusters. Ouyang et al. produced SA–DMA clusters using electrospray ionization (ESI) and investigated their water uptake through a coupled differential mobility analyzer-atmospheric pressure drift tube ion mobility spectrometer (DT-IMS).21 They observed that as relative humidity (RH) increased, the electrical mobility of the nanoclusters decreased, indicating water uptake by the clusters. Based on thermal stability measurements, hydrated clusters are stabilized under ambient conditions by the presence of water molecules. Similar work by Thomas et al. using the same differential mobility analyzer-DT-IMS technique to study charged SA–DMA clusters showed modest water uptake by both positive and negative polarities.22 For the RH range studied (3–30%), they estimated that typically fewer than 10, and possibly fewer than 5, water molecules adsorbed to the nanocluster surface.
Water uptake by larger sulfuric acid–base particles has also been studied. Using a hygroscopicity tandem differential mobility analyzer (HTDMA), Hu et al. investigated the hygroscopic growth of several alkylaminium sulfate salts.23 Unlike ammonium sulfate, ethylammonium, diethylammonium, and triethylammonium sulfate particles do not show deliquescence but instead exhibit a monotonic diameter increase with relative humidity. Notably, all particle sizes studied (diameters 40, 100, and 150 nm) for all salts showed increased hygroscopic growth compared to ammonium sulfate, and the hygroscopicity of the particles was not affected by the number of ethyl groups. Ambient measurements suggest that ammonium sulfate is the major form of sulfate in atmospheric particles, but reactions of gaseous amines on particle surfaces can lead to displacement of ammonia. Calculations of hygroscopic growth of triethylammonium sulfate–ammonium sulfate mixed particles using the Zdanovskii–Stokes–Robinson (ZSR) mixing rule suggest that growth occurs at low RH and is monotonic like triethylammonium sulfate. These results indicate that the displacement of ammonia by amines in atmospheric particles will greatly enhance their hygroscopicity.
Here we investigate the molecular properties that impact the hydration ability of a base molecule or an acid–base heterodimer. Using a relatively large number of water molecules in a cluster (up to 20), we have studied the protonation of a base molecule as well as sulfate formation in a water cluster. We have estimated how different stable acid–base particles hydrate in atmospheric conditions. To gain insights into how varying molecular properties affect to the role of water, we have studied sulfuric acid together with ammonia, dimethylamine, guanidine and trimethylamine oxide, which cover a wide range of structural properties (e.g. number of available hydrogen-bonds, volatilities, dipole moments, gas-phase basicities, and aqueous-phase basicities).24–27
2 Computational details
We studied heterodimers of sulfuric acid (SA) with ammonia (AMM), dimethylamine (DMA), guanidine (GUA) and trimethylamine oxide (TMAO), where heterodimers were hydrated with 0–20 water molecules. Monomer hydration was also studied. To find the global minimum energy cluster structures, we explored the potential energy surface of all the acid–base–water clusters using a systematic configurational sampling technique.28 To create the initial cluster structures, we used 3000 random guesses and 100 exploration loops, with a scout limit of 4 in the ABCluster program,29,30 and for each building block combination we saved 2000 of the lowest energy structures that were subsequently optimized by the tight-binding method GFN2-xTB with a very tight optimization criteria.31 Based on the electronic energies, radius of gyration, and dipole moments, we separated different conformers, which were then optimized using the ωB97X-D/6-31+G* level of theory.32,33 Based on the obtained electronic energies, we selected structures with a maximum of N kcal mol−1 from the lowest electronic energy (where N is the number of molecules in the cluster). For remaining structures, the ωB97X-D/6-31++G** level of theory was used for final optimization and vibrational frequency calculation using Gaussian 16 RevA.03.34,35 We selected 2–5 of the lowest Gibbs free energy structures, for which we performed single point energy calculations using the highly accurate DLPNO-CCSD(T)/aug-cc-pVTZ level of theory with tight pair natural orbital criteria, tight self consistent field criteria, and integration grid 4 as implemented in Orca version 4.2.1.36–41 For each clusters we identified the global minimum Gibbs free energy structure at the DLPNO-CCSD(T)/aug-cc-pVTZ//ωB97X-D/6-31++G** level.42 Calculated thermochemical data are further used to study population dynamics of water clusters by Atmospheric Cluster Dynamics Code (ACDC).43 Enthalpies and entropies for the minimum energy clusters are available in the ESI.† The ACDC code is available from the authors upon request.3 Results and discussion
3.1 Hydration of monomers
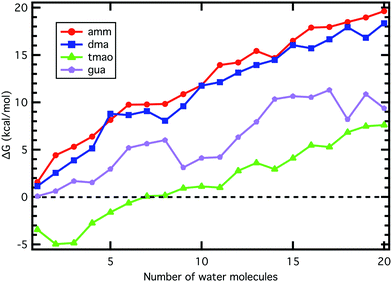
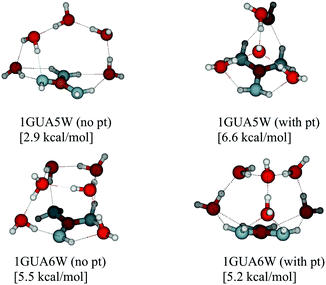
Acid and base molecules may exist in the atmosphere as isolated monomers or they may be clustered with water molecules. The hydration ability is dependent on the molecular properties of a monomeric compound as well as the environment (RH and T). Possible monomeric properties affecting hydration ability can be studied by calculating thermochemistry of clusters and the environmental effects can be accounted by cluster dynamics simulations.The majority of water addition reactions to any of the base monomers are endergonic, as shown by the increased ΔG value as a function of water molecule number in Fig. 1. There are a few exceptions where the addition free energy is favorable, for instance, the formation of 1GUA9W and 1GUA18W clusters. This can be either a result of an additional stabilization effect in cluster structure such as increased number of hydrogen bonds (see Fig. 2) or an artifact caused by locating a wrong global minimum structure.
Our results indicate that the ability of a base to accept a proton in a small water cluster has a stronger connection to the aqueous phase than gas-phase basicity as GUA is the strongest base in aqueous phase but TMAO is the strongest one in gas phase. However, the total hydration ability is likely to be connected to the gas-phase base strength.
3.2 Water and self stabilization
Ions can have a remarkable role in stabilizing atmospheric particle formation but, unlike neutral compounds, they are unlikely to exist as isolated monomers in the gas phase. Here we have investigated whether a water cluster or a homomolecular base cluster offers a better stabilizing environment for a protonated base. This has been done by studying the strength of cation–base and cation–water interactions, and comparing the ability of a surrounding base or water cluster to stabilize the protonated base. Additionally, we have compared the interactions between a neutral base with a surrounding base cluster or a water cluster and discussed properties related those results.| n | Base environment | Water environment | ||
|---|---|---|---|---|
| AMM | AMM(+) | AMM | AMM(+) | |
| 2 | 3.7 | −19.1 | 1.6 | −13.4 |
| 3 | 8.0 | −31.8 | 4.4 | −22.7 |
| 4 | 11.9 | −38.1 | 5.3 | −27.7 |
| 5 | 16.2 | −41.2 | 6.4 | −32.5 |
| 6 | 21.1 | −40.3 | 8.1 | −33.5 |
| n | Base environment | Water environment | ||
|---|---|---|---|---|
| DMA | DMA(+) | DMA | DMA(+) | |
| 2 | 3.9 | −15.7 | 1.1 | −9.5 |
| 3 | 5.5 | −24.6 | 2.5 | −16.2 |
| 4 | 5.8 | −23.0 | 3.9 | −18.1 |
| 5 | 12.8 | −21.5 | 5.1 | −20.5 |
| 6 | 16.9 | −18.5 | 8.8 | −22.0 |
| n | Base environment | Water environment | ||
|---|---|---|---|---|
| TMAO | TMAO(+) | TMAO | TMAO(+) | |
| 2 | −3.6 | −33.9 | −3.5 | −7.5 |
| 3 | −4.1 | −46.4 | −5.0 | −10.5 |
| 4 | −6.1 | −46.1 | −4.8 | −13.5 |
| 5 | −7.1 | −53.6 | −2.7 | −14.0 |
| 6 | −16.2 | −60.4 | −1.6 | −12.8 |
| n | Base environment | Water environment | ||
|---|---|---|---|---|
| GUA | GUA(+) | GUA | GUA(+) | |
| 2 | −2.2 | −19.5 | 0.1 | −8.0 |
| 3 | −3.1 | −31.9 | 0.6 | −12.8 |
| 4 | −10.5 | −39.2 | 1.7 | −16.6 |
| 5 | −10.9 | −46.8 | 1.5 | −18.7 |
| 6 | −13.2 | −48.4 | 2.9 | −20.6 |
The comparison of base–base and base–water interactions in Table 1 shows that neutral AMM and DMA interact stronger with water than themselves. For ammonia the AMM–W interaction is 2.1 kcal mol−1 more favorable than AMM–AMM and for dimethylamine the interaction with water is 2.8 kcal mol−1 more favorable than with itself. TMAO and GUA interact stronger with themselves than with water, with Gibbs free energy difference of 0.1 and 2.3 kcal mol−1 in 2-component clusters, respectively. The difference in base–base and base–water interaction strengths might be related to the fact that AMM and DMA have smaller dipole moments than water, whereas TMAO and GUA have larger dipole moments, making them capable of forming stronger dipole–dipole interactions with themselves than with water.
Base–base interactions are thermodynamically unfavorable for ammonia and dimethylamine, meaning that the Gibbs free binding energies are increasingly positive for nAMM (from 3.7 to 21.1 kcal mol−1) and nDMA clusters (from 3.9 to 16.9 kcal mol−1), when the number of base molecules n increases from 2 to 6 (see Table 1). For trimethylamine oxide and guanidine, the Gibbs free binding energy is increasingly negative for nTMAO (from −3.6 to −16.2 kcal mol−1) and nGUA (from −2.2 to −13.2 kcal mol−1) clusters, when the number of base molecules n increases from 2 to 6, meaning that the intermolecular interactions between base molecules are favorable. It should be noted that even though the Gibbs free energies for the formation of TMAO or GUA homoclusters are slightly negative, it does not mean that they would have meaningful concentrations under atmospheric conditions. Favorable interactions in TMAO and GUA homoclusters might be correlated with their low vapor pressures and high dipole moments, whereas positive free energies of AMM and DMA homoclusters could be related their high volatility and low dipole moments. Water–water interactions are stronger than those for AMM or DMA but weaker than those for TMAO or GUA, and water is moderately volatile with a medium dipole moment.
3.3 Cluster hydration
The hydration ability of clusters has shown to be related to the number of available hydrogen binding sites. As can be seen in Fig. 5, if a base molecule can form at least two hydrogen bonds, a water molecule can bridge the acid and base molecules while the acid and base still have one direct interaction. In the case of TMAO, which can form only one hydrogen bond, water is hydrogen bonded just with SA, and the direct SA–TMAO interaction remains.The more favorable reaction energies of an addition of a water molecule to the SA–GUA and SA–AMM clusters compared to SA–TMAO or SA–DMA clusters can be assumed to be related to the availability of hydrogen binding sites. For instance, in acid–base clusters having six water molecules, we found that there are 25 hydrogen bonds in SA–GUA and 23 in SA–AMM clusters whereas SA–DMA has 21 and SA–TMAO 20 hydrogen bonds. In clusters containing 12 water molecules, there are 25, 23, 21 and 20 hydrogen bonds in SA clusters with GUA, AMM, DMA and TMAO, respectively. For H-bond definition we have used default settings in Molden, i.e., H-bond length can be from 1.50 to 3.15 Å and H-bond angle can be from 145 to 215°. It should be noted that the same H-bond parameters for different systems might not an appropriate choice in some cases, but rather they should be separately defined for each system.50 Thus, we additionally viewed the structures to make sure that the results make sense chemically. Global minimum structures of acid–base clusters with 6 and 12 water molecules are shown in Fig. 7.
 | ||
| Fig. 7 Molecular structures of SA clusters with AMM, DMA, TMAO and GUA, respectively, with 6 (top) and 12 (bottom) water molecules. | ||
While it is thought that water can participate in aerosol particle formation, particle formation rates from acid–base chemistry have been shown to have a minimal dependence on relative humidity.51,52 Olenius et al. showed that hydration has maximum of an order of magnitude effect on the particle formation rates of sulfuric acid with ammonia or methylamine and negligible effect in the case of dimethylamine or trimethylamine.14 As the particle formation of SA with GUA or TMAO is at the kinetic limit, hydration cannot increase the particle formation rates notably. However it is also possible that particle formation under high relative humidity is suppressed and that option cannot be excluded in this study but further studies are needed.
 | ||
| Fig. 8 Molecular structures of SA clusters with AMM, DMA, TMAO and GUA, respectively, with 4 water molecules. Notable is the interaction between water and OH group of a bisulfate in DMA cluster. | ||
Fig. 9 shows the molecular structures of acid–base pairs in a cluster with 20 water molecules, where the second proton transfer reaction from bisulfate to water has occurred and SO42− has formed. Sulfate is one of the most kosmotropic Hofmeister ions, having an order-making effect on water structure.56–58 Indeed it can be seen that in all cluster structures, SO42− locates on the center of the cluster, maximising the number of hydrogen bonds. Similar results have found experimentally in photoelectron studies by Wang et al.59 It is notable that there is no direct interaction between the sulfate and the protonated base in a hydrated cluster structure, but one or more water molecules bridge them. Also, the protonated base cation seems to prefer to be located on the surface of a cluster. These kinds of structural effects are likely to stabilize the hydrated cluster in which the proton transfer reactions have occurred.
3.4 Estimation of a stable particle hydration
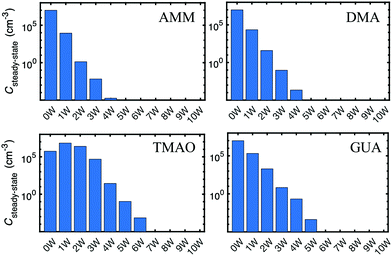
We have simulated population dynamics of acid–base particle hydration, with an assumption that acid–base system does not evaporate. This was done to estimate abilities of “stable particles” to hydrate. Hydration free energies of non-evaporating acid–base clusters are given in Fig. 10.We have simulated the hydration distributions of stable acid–base particles with conditions where the total particle concentration is 105 cm−3, temperature is 273 K, and relative humidity is 100%. Steady-state concentrations for dry and hydrated acid–base particles are given in Fig. 11. In the case of SA–AMM and SA–DMA particles, hydrated particles (2–3 water molecules in the case of AMM and 1–3 in the case of DMA) have slightly higher concentrations than dry particles. This implies that SA–AMM and SA–DMA particles could slightly grow via water additions, which could possibly affect particle composition.60 In the case of SA–TMAO particles, dry particles have the highest steady-state concentration and those with one water molecule have almost an order of magnitude lower concentration. The concentrations of more hydrated particles decrease gradually. This indicates that SA–TMAO particles are unlikely to absorb water in atmospheric conditions and their hygroscopic growth factor is very low. For SA–GUA particles, the concentrations of hydrated particles are significantly higher than that of dry particles. Particles having 2–4 water molecules have up to three orders of magnitude higher concentrations than dry particles. Also particles with 5–6 water molecules have concentrations similar than dry particles. This implies that atmospheric SA–GUA particles are highly hydrated and their hygroscopic growth factor is high. This is most likely related to the large number of possible hydrogen binding sites in SA–GUA structure and to the absence of sterical blocks such as bulky alkyl groups.
 | ||
| Fig. 11 Hydration distributions of stable sulfuric acid particles with ammonia, dimethylamine, trimethylamine oxide, and guanidine at 273 K at [particle] = 105 cm−3 and RH = 100%. | ||
4 Conclusions
In this paper we have studied the hydration of monomers and heterodimers up to 20 water molecules. Previous quantum chemical studies have often modeled up to 4 or 5 water molecules,13–15,61 which limit the study of proton transfer reactions. Here we have discussed connections of aqueous-phase base strength and proton transfer reactions as well as sulfate formation from heterodimer bisulfate ion in water clusters. Our results indicate that the ability of a base to accept a proton in a water cluster is related to the aqueous-phase basicity whereas the ability of a base molecule to hydrate is connected to the gas-phase base strength. Especially TMAO is very likely to be hydrated due to its zwitterionic character.The ability of a cluster to be hydrated is related to the number of possible hydrogen binding sites, meaning that SA–GUA clusters are likely to be strongly hydrated. However, although the effective evaporation rates might vary as a function of relative humidity, we do not think high RH can increase the SA–GUA particle formation rates as that occur at the kinetic limit even at room temperature and low monomer concentrations.62 However, it would be interesting to see what the effect of relative humidity is to SA–TMAO particle formation as the TMAO monomer is likely to be highly hydrated but SA–TMAO cluster does not need water molecules to stabilize it. In that case, it is possible that the presence of high relative humidity would decrease the particle formation rates from SA and TMAO.
By uptake of water vapor, aerosol particles can act as cloud condensation nuclei and thus impact cloud properties. As clouds have an overriding global relevance by regulating Earth's radiative balance, it is clear that understanding how different acid–base particles have different abilities to absorb water is essential. In addition of hygroscopic growth, the absorption of water might lead an aerosol particle to change its chemical composition, morphology, phase or reactivity. Thus it is important to understand what molecular properties have impacts on the hydration ability. While highly accurate quantum chemical calculations are limited to studies of relatively small systems, further studies such as molecular dynamics simulations could assist in understanding how properties of aerosol particles change when particle is hydrated. In addition, laboratory studies are needed to supplement theoretical findings.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding from the U.S. National Science Foundation under Grant No. CHE-1710580 and computational resources from the CSC-IT Center for Science in Espoo, Finland.Notes and references
- IPCC (Intergovernmental Panel on Climate Change), Climate change 2013: The Physical Science Basis, 2013.
- R. Zhang, A. Khalizov, L. Wang, M. Hu and W. Xu, Chem. Rev., 2012, 112, 1957–2011 CrossRef CAS PubMed.
- M. Hallquist, J. C. Wenger, U. Baltensperger, Y. Rudich, D. Simpson, M. Claeys, J. Dommen, N. M. Donahue, C. George, A. H. Goldstein, J. F. Hamilton, H. Herrmann, T. Hoffmann, Y. Iinuma, M. Jang, M. E. Jenkin, J. L. Jimenez, A. Kiendler-Scharr, W. Maenhaut, G. McFiggans, T. F. Mentel, A. Monod, A. S. H. Prévôt, J. H. Seinfeld, J. D. Surratt, R. Szmigielski and J. Wildt, Atmos. Chem. Phys., 2009, 9, 5155–5236 CrossRef CAS.
- M. Kulmala, J. Kontkanen, H. Junninen, K. Lehtipalo, H. E. Manninen, T. Nieminen, T. Petäjä, M. Sipilä, S. Schobesberger, P. Rantala, A. Franchin, T. Jokinen, E. Järvinen, M. Äijälä, J. Kangasluoma, J. Hakala, P. P. Aalto, P. Paasonen, J. Mikkilä, J. Vanhanen, J. Aalto, H. Hakola, U. Makkonen, T. Ruuskanen, R. L. Mauldin, J. Duplissy, H. Vehkamäki, J. Bäck, A. Kortelainen, I. Riipinen, T. Kurtén, M. V. Johnston, J. N. Smith, M. Ehn, T. F. Mentel, K. E. J. Lehtinen, A. Laaksonen, V.-M. Kerminen and D. R. Worsnop, Science, 2013, 339, 943–946 CrossRef CAS PubMed.
- J. Almeida, S. Schobesberger, A. Kürten, I. K. Ortega, O. Kupiainen-Määttä, A. P. Praplan, A. Adamov, A. Amorim, F. Bianchi and M. Breitenlechner, et al. , Nature, 2013, 502, 359–363 CrossRef CAS PubMed.
- K. Lehtipalo, L. Rondo, J. Kontkanen, S. Schobesberger, T. Jokinen, N. Sarnela, A. Kürten, S. Ehrhart, A. Franchin and T. Nieminen, et al. , Nat. Commun., 2016, 7, 11594 CrossRef CAS PubMed.
- J. N. Smith, K. C. Barsanti, H. R. Friedli, M. Ehn, M. Kulmala, D. R. Collins, J. H. Scheckman, B. J. Williams and P. H. McMurry, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6634–6639 CrossRef CAS PubMed.
- F. Arnold and R. Fabian, Nature, 1980, 283, 55–57 CrossRef CAS.
- V. Vaida, J. Chem. Phys., 2011, 135, 020901 CrossRef PubMed.
- S. Schobesberger, H. Junninen, F. Bianchi, G. Lönn, M. Ehn, K. Lehtipalo, J. Dommen, S. Ehrhart, I. K. Ortega, A. Franchin, T. Nieminen, F. Riccobono, M. Hutterli, J. Duplissy, J. Almeida, A. Amorim, M. Breitenlechner, A. J. Downard, E. M. Dunne, R. C. Flagan, M. Kajos, H. Keskinen, J. Kirkby, A. Kupc, A. Kürten, T. Kurtén, A. Laaksonen, S. Mathot, A. Onnela, A. P. Praplan, L. Rondo, F. D. Santos, S. Schallhart, R. Schnitzhofer, M. Sipilä, A. Tomé, G. Tsagkogeorgas, H. Vehkamäki, D. Wimmer, U. Baltensperger, K. S. Carslaw, J. Curtius, A. Hansel, T. Petäjä, M. Kulmala, N. M. Donahue and D. R. Worsnop, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17223–17228 CrossRef CAS PubMed.
- J. N. Smith, D. C. Draper, S. Chee, M. Dam, H. Glicker, D. Myers, A. E. Thomas, M. J. Lawler and N. Myllys, J. Aerosol Sci., 2020, 105733 Search PubMed.
- B. R. Bzdek, J. W. DePalma and M. V. Johnston, Acc. Chem. Res., 2017, 50, 1965–1975 CrossRef CAS PubMed.
- N. T. Tsona, H. Henschel, N. Bork, V. Loukonen and H. Vehkamäki, J. Phys. Chem. A, 2015, 119, 9670–9679 CrossRef CAS PubMed.
- T. Olenius, R. Halonen, T. Kurtén, H. Henschel, O. Kupiainen-Määttä, I. K. Ortega, C. N. Jen, H. Vehkamäki and I. Riipinen, J. Geophys. Res.: Atmos., 2017, 122, 7103–7118 CAS.
- H. Henschel, T. Kurtén and H. Vehkamäki, J. Phys. Chem. A, 2016, 120, 1886–1896 CrossRef CAS PubMed.
- Y. Yang, S. E. Waller, J. J. Kreinbihl and C. J. Johnson, J. Phys. Chem. Lett., 2018, 9, 5647–5652 CrossRef CAS PubMed.
- Y. Yang and C. J. Johnson, Faraday Discuss., 2019, 217, 47–66 RSC.
- J. J. Kreinbihl, N. C. Frederiks and C. J. Johnson, J. Chem. Phys., 2021, 154, 014304 CrossRef CAS PubMed.
- G.-L. Hou, W. Lin and X.-B. Wang, Commun. Chem., 2018, 1, 1–8 CrossRef CAS.
- G.-L. Hou and X.-B. Wang, Acc. Chem. Res., 2020, 779–785 Search PubMed.
- H. Ouyang, S. He, C. Larriba-Andaluz and C. J. Hogan, J. Phys. Chem. A, 2015, 119, 2026–2036 CrossRef CAS PubMed.
- J. M. Thomas, S. He, C. Larriba-Andaluz, J. W. DePalma, M. V. Johnston and C. J. Hogan Jr., Phys. Chem. Chem. Phys., 2016, 18, 22962–22972 RSC.
- D. Hu, C. Li, H. Chen, J. Chen, X. Ye, L. Li, X. Yang, X. Wang, A. Mellouki and Z. Hu, J. Environ. Sci., 2014, 26, 37–43 CrossRef CAS.
- N. Myllys, T. Ponkkonen, S. Chee and J. Smith, Atmosphere, 2020, 11, 35 CrossRef CAS.
- N. Myllys, J. Kubečka, V. Besel, D. Alfaouri, T. Olenius, J. N. Smith and M. Passananti, Atmos. Chem. Phys., 2019, 19, 9753–9768 CrossRef CAS.
- N. Myllys, S. Chee, T. Olenius, M. Lawler and J. N. Smith, J. Phys. Chem. A, 2019, 123, 2420–2425 CrossRef CAS PubMed.
- S. Chee, K. Barsanti, J. N. Smith and N. Myllys, Atmos. Chem. Phys. Discuss., 2021, 2021, 1–27 Search PubMed.
- J. Kubečka, V. Besel, T. Kurtén, N. Myllys and H. Vehkamäki, J. Phys. Chem. A, 2019, 123, 6022–6033 CrossRef PubMed.
- J. Zhang and M. Dolg, Phys. Chem. Chem. Phys., 2015, 17, 24173–24181 RSC.
- J. Zhang and M. Dolg, Phys. Chem. Chem. Phys., 2016, 18, 3003–3010 RSC.
- C. Bannwarth, S. Ehlert and S. Grimme, J. Chem. Theory Comput., 2019, 15, 1652–1671 CrossRef CAS PubMed.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650–654 CrossRef CAS.
- N. Myllys, J. Elm and T. Kurtén, Comput. Theor. Chem., 2016, 1098, 1–12 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian16 Revision A.03, 2016 Search PubMed.
- C. Riplinger and F. Neese, J. Chem. Phys., 2013, 138, 034106 CrossRef PubMed.
- C. Riplinger, B. Sandhoefer, A. Hansen and F. Neese, J. Chem. Phys., 2013, 139, 134101 CrossRef PubMed.
- C. Riplinger, P. Pinski, U. Becker, E. F. Valeev and F. Neese, J. Chem. Phys., 2016, 144, 024109 CrossRef PubMed.
- R. A. Kendall, T. H. Dunning and R. J. Harrison, J. Chem. Phys., 1992, 96, 6796–6806 CrossRef CAS.
- D. G. Liakos, M. Sparta, M. K. Kesharwani, J. M. L. Martin and F. Neese, J. Chem. Theory Comput., 2015, 11, 1525–1539 CrossRef CAS PubMed.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- N. Myllys, J. Elm, R. Halonen, T. Kurtén and H. Vehkamäki, J. Phys. Chem. A, 2016, 120, 621–630 CrossRef CAS PubMed.
- M. J. McGrath, T. Olenius, I. K. Ortega, V. Loukonen, P. Paasonen, T. Kurtén, M. Kulmala and H. Vehkamäki, Atmos. Chem. Phys., 2012, 12, 2345–2355 CrossRef CAS.
- O. Hoegh-Guldberg, P. J. Mumby, A. J. Hooten, R. S. Steneck, P. Greenfield, E. Gomez, C. D. Harvell, P. F. Sale, A. J. Edwards and K. Caldeira, et al. , Science, 2007, 318, 1737–1742 CrossRef CAS PubMed.
- S. Enami, H. Mishra, M. R. Hoffmann and A. J. Colussi, J. Phys. Chem. A, 2012, 116, 6027–6032 CrossRef CAS PubMed.
- A. Ravishankara, Science, 1997, 276, 1058–1065 CrossRef CAS.
- M. Kumar and J. S. Francisco, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 12401–12406 CrossRef CAS PubMed.
- H. Mishra, S. Enami, R. J. Nielsen, M. R. Hoffmann, W. A. Goddard and A. J. Colussi, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10228–10232 CrossRef CAS PubMed.
- S. Biswas, H. Kwon, K. C. Barsanti, N. Myllys, J. N. Smith and B. M. Wong, Phys. Chem. Chem. Phys., 2020, 22, 26265–26277 RSC.
- D. Herschlag and M. M. Pinney, Biochemistry, 2018, 57, 3338–3352 CrossRef CAS PubMed.
- M. L. Dawson, M. E. Varner, V. Perraud, M. J. Ezell, R. B. Gerber and B. J. Finlayson-Pitts, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 18719–18724 CrossRef CAS PubMed.
- A. Kürten, C. Li, F. Bianchi, J. Curtius, A. Dias, N. M. Donahue, J. Duplissy, R. C. Flagan, J. Hakala, T. Jokinen, J. Kirkby, M. Kulmala, A. Laaksonen, K. Lehtipalo, V. Makhmutov, A. Onnela, M. P. Rissanen, M. Simon, M. Sipilä, Y. Stozhkov, J. Tröstl, P. Ye and P. H. McMurry, Atmos. Chem. Phys., 2018, 18, 845–863 CrossRef.
- X.-B. Wang, J. B. Nicholas and L.-S. Wang, J. Chem. Phys., 2000, 113, 10837–10840 CrossRef CAS.
- L. C. Smeeton, J. D. Farrell, M. T. Oakley, D. J. Wales and R. L. Johnston, J. Chem. Theory Comput., 2015, 11, 2377–2384 CrossRef CAS PubMed.
- M. Kulichenko, N. Fedik, K. V. Bozhenko and A. I. Boldyrev, J. Phys. Chem. B, 2019, 123, 4065–4069 CrossRef CAS PubMed.
- F. Hofmeister, Arch. Exp. Pathol. Pharmakol., 1888, 24, 247–260 Search PubMed.
- M. Cacace, E. Landau and J. Ramsden, Q. Rev. Biophys., 1997, 30, 241–277 CrossRef CAS PubMed.
- J. T. OBrien, J. S. Prell, M. F. Bush and E. R. Williams, J. Am. Chem. Soc., 2010, 132, 8248–8249 CrossRef CAS PubMed.
- X.-B. Wang, X. Yang, J. B. Nicholas and L.-S. Wang, Science, 2001, 294, 1322–1325 CrossRef CAS PubMed.
- H. Chen, S. Chee, M. J. Lawler, K. C. Barsanti, B. M. Wong and J. N. Smith, Aerosol Sci. Technol., 2018, 52, 1120–1133 CrossRef CAS.
- F. R. Rasmussen, J. Kubečka, V. Besel, H. Vehkamäki, K. V. Mikkelsen, M. Bilde and J. Elm, J. Phys. Chem. A, 2020, 124, 5253–5261 CrossRef CAS PubMed.
- N. Myllys, T. Ponkkonen, M. Passananti, J. Elm, H. Vehkamäki and T. Olenius, J. Phys. Chem. A, 2018, 122, 4717–4729 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cp01704g |
| This journal is © the Owner Societies 2021 |