Open Access Article
Open Access ArticleTemperature dependencies of the degradation of NO, NO2 and HONO on a photocatalytic dispersion paint†
Daniela
Pill
,
Peter
Wiesen
and
Jörg
Kleffmann
 *
*
Institute for Atmospheric and Environmental Research, Faculty for Mathematics and Natural Sciences, Bergische Universität Wuppertal, Gaußstr. 20, 42119 Wuppertal, Germany. E-mail: kleffman@uni-wuppertal.de
First published on 29th March 2021
Abstract
The photocatalytic decomposition of nitrogen oxides (NOx) has attracted significant interest as a potential measure of reducing NOx levels in the urban atmosphere. Since photocatalytic activity is highly variable depending on atmospheric conditions, the uptake of NO, NO2 and HONO was studied on a commercial photocatalytic dispersion paint in a flow photoreactor as a function of the relative humidity and temperature. Since the relative humidity is a function of the surface's temperature, here both dependencies were carefully decoupled for the first time. In addition, for the first time the temperature dependence of the whole NOx reaction system including the important intermediate HONO was investigated. While for NO and NO2 strong negative humidity dependencies were observed, the photocatalytic uptake of HONO increased with humidity. For constant relative humidity no temperature dependence of the photocatalytic oxidation of NO was observed, whereas the photocatalytic NO2 uptake decreased with increasing temperature, which is explained by a temperature dependent adsorption equilibrium of the surface active NO2. HONO uptake showed a positive temperature dependence confirming the proposed photocatalysis of nitrite in a layer of adsorbed water on the surface of the photocatalyst. The missing/negative temperature dependencies of the photocatalysis of NO/NO2 are overcompensated by their strong negative relative humidity dependencies, leading to increasing uptake for both pollutants when photocatalytic surfaces are heated by solar irradiation in the atmosphere.
1 Introduction
Nitrogen oxides (NOx = NO + NO2) are important trace species in the atmosphere. NOx control photochemical ozone (O3) formation and the oxidation capacity of the atmosphere, and lead to the acidification of the environment via their final oxidation product nitric acid (HNO3). Since NO2 and its degradation products are also directly harmful, threshold limit values have been introduced in many countries. The relatively low annual average limit value of 40 μg m−3 (∼21 ppb)1 for NO2 in Europe is often exceeded at urban kerbside monitoring stations, although NOx emissions were reduced significantly over the past three decades. Because of the exceedance of NO2 limiting values in German cities even relatively modern Diesel cars (EURO IV and V) were banned from some city centers with a significant economic impact on car manufactures and customers. Thus, other measures to reduce NO2 concentrations are intensively discussed by the city authorities to avoid the unpopular Diesel bans.One of the proposed measures is the photocatalytic degradation of NOx on TiO2 containing surfaces,2–4 leading to the formation of adsorbed nitric acid (HNO3) or nitrate (NO3−), which is finally washed off from the surface by rain.
When a semi-conductor photocatalyst is irradiated by UV-light with an energy higher than the band gap, an electron from the valence band (vb) is transferred to the conduction band (cb) leaving a hole in the valence band:
| TiO2 + hν → hvb+ + ecb−. | (R1) |
| hvb+ + OH−(H2O) → OH(+H+), | (R2) |
| ecb− + O2(+H+) → O2−(HO2). | (R3) |
| NO + HO2 → NO2 + OH, | (R4) |
| NO + OH → HONO, | (R5) |
NO2 formed in reaction (R4) is consecutively oxidized by OH radicals to nitric acid (HNO3) adsorbed as nitrate on the surface:6
| OH + NO2 → HNO3/nitrate. | (R6) |
(a) atmospheric NOx levels will be reduced;
(b) through the accompanying photocatalytic VOC reduction both, direct (NO2 photolysis) and indirect (photochemical smog) O3 formation will decrease;
(c) while the absolute level of HNO3 formation is not affected, HNO3 formed on photocatalytic surfaces will not damage plants and the respiratory systems of animals and humans like naturally formed HNO3 in the gas phase and, finally,
(d) if the rain wash off is further cleaned in wastewater treatment plants, even the total levels of nitrate could be reduced.
However, it is also well documented that photocatalysis can lead to the significant formation of harmful intermediates such as nitrous acid (HONO)6,11–15 or oxygenated VOCs, like formaldehyde (HCHO),16–21 if these commercially available surfaces are not properly designed. In addition, if the formed nitrate is not regularly washed off, photocatalysis of the adsorbed nitrate can lead to renoxification and to additional O3 formation.14,22 Finally, since the expected average NOx reductions when applying photocatalytic surfaces in typical urban street canyons will be limited to a few percent,23,24 photocatalysis should be taken only as one measure among others to improve urban air quality.
Modeling of typical average NOx reductions by photocatalysis under urban conditions is highly uncertain since photocatalytic degradation of NOx strongly depends on many variables, like concentration, humidity, UV irradiance and surface temperature. While the first three variables have been often tested in former laboratory studies, the influence of temperature on the uptake of NO and NO2 and on the product formation were investigated only in a few controversial studies. However, detailed knowledge about the temperature dependence is of paramount importance, since urban surfaces (streets, walls, roofs etc.) can significantly heat up when irradiated by sunlight, especially during summertime.25
A positive temperature dependence of the photocatalytic oxidation of NO was observed for concrete surfaces.10 Very recently, similar results were observed for photocatalytic roofing granules and aluminum plates coated with a pure TiO2 photocatalyst (P25). Here, more efficient NO abatement was observed at 60 °C compared to room temperature.25 Both studies are in contradiction with decreasing NO removal rates observed on photocatalytic mortar when the temperature was increased from 21 °C to 30 °C.26 Also on a photocatalytic cementitious coating the reaction of NO showed a negative temperature dependence from 30 °C to 40 °C at low humidity (20% RH).27 Moreover, in an outdoor study decreasing NOx degradation on concrete was observed with increasing temperature.8
A negative temperature dependence was also observed for the uptake of NO2 on aluminum plates coated with a pure TiO2 photocatalyst (P25) when the surface temperature was increased from 25° to 60 °C.25 In contrast, for the same catalyst (P25) an increasing uptake of NO2 and decreasing HONO yields were observed with increasing temperature in a low pressure flow tube under dry conditions (buffer gas He).28,29 A qualitatively similar temperature dependence of the NO2 uptake was observed on an indoor photocatalytic paint, while the HONO yields were found to increase with temperature in this environmentally more realistic study.30 However, the absolute water vapor pressure was kept constant, leading to decreasing relative humidity on the surface with increasing surface temperature. Since it is well known that the NO2 uptake is controlled by the amount of adsorbed water on the photocatalyst, which is a function of the relative humidity,6 the observed temperature dependence30 may be controlled by the relative humidity and not by the temperature itself. The missing decoupling of the temperature- and humidity dependencies may also explain the controversial literature results for NO and NO2 described above.
To carefully decouple both effects for the first time, in the present study both, the influence of the relative humidity and the temperature on the uptake of NO, NO2 and HONO and the corresponding product yields were studied on a commercial photocatalytic dispersion paint. The pure temperature dependencies at fixed relative humidity were derived from the parameterizations of the humidity dependencies at different temperatures. Finally, complete descriptions of the temperature and humidity dependencies for NO, NO2 and HONO were derived from the experimental data.
2 Experimental
The used paint (StoPhotosan NOx dispersion paint, white) was already applied in a former study and showed a high photocatalytic activity against NO, NO2 and HONO.6 Details of the paint and its preparation are explained in the ESI,† Section S1.The photocatalytic activity of the sample was studied in a temperature-controlled (10–50 °C) flow photoreactor which is adapted to ISO 22197-1,31 see Fig. S1 (ESI†). However, in contrast to the ISO standard, the geometry of the reactor, the experimental conditions and the data evaluation were significantly improved, as explained in detail elsewhere.32 In contrast to the original ISO method, which considers zero order kinetics,31–33 uptake coefficients of NO, NO2 and HONO were calculated assuming first order kinetics:
 | (1) |
![[v with combining macron]](https://www.rsc.org/images/entities/i_char_0076_0304.gif) the average velocity of the reactants [cm s−1] according to the gas kinetic theory (
the average velocity of the reactants [cm s−1] according to the gas kinetic theory (![[v with combining macron]](https://www.rsc.org/images/entities/i_char_0076_0304.gif) = (8RTπ−1 M−1)0.5·100; with: R = 8.314 J mol−1 K−1, T: absolute temperature [K] and M: molar mass [kg mol−1]).
= (8RTπ−1 M−1)0.5·100; with: R = 8.314 J mol−1 K−1, T: absolute temperature [K] and M: molar mass [kg mol−1]).
The samples were irradiated by a calibrated diode array (QUMA-Elektonik & Analytik, Wuppertal), allowing variable UVA irradiance levels between 1–16 W m−2, see ESI,† Section S2.
Nitrogen oxides (NOx = NO + NO2) were measured by chemiluminescence detection of NO using a molybdenum converter for NO2 conversion (Eco-Physics CLD 899Y, detection limit: 25 ppt). HONO was measured by a sensitive and selective LOPAP instrument (Long Path Absorption Photometer), which is explained in detail elsewhere.34,35 The temperature of the paint surface was measured by a calibrated thermocouple (Omega Engineering GmbH, Type K, 0.5 mm diameter, accuracy ±0.5 K), while the relative humidity and the temperature of the reaction mixture behind the reactor were measured by a calibrated humidity sensor (HYTELOG-USB, Hygrosens Instruments GmbH, accuracy ±2% RH). Further details of the instrumentation used are provided in the ESI,† Section S3.
Photocatalytic conversion of NOx was studied in the flow photoreactor using mixtures of NO and NO2 diluted in synthetic air, which were humidified by passing through a temperature-controlled stripping coil operated with Millipore water. The flow rate used for the experiments of ∼2.2 L min−1 was controlled by calibrated flow controllers. After passing the flow reactor the gas mixture was analyzed for NOx and HONO, while the humidity was determined in the excess vent (see Fig. S2, ESI†).
After the sample was placed inside the reactor, it was thermally equilibrated for ca. one hour. Then its blank emissions in the dark and under irradiation were determined by passing pure humidified synthetic air through the reactor. Thereafter, NO or NO2 were mixed to the synthetic air. Concentrations were determined, first at the inlet of the reactor (bypass), second at the outlet of the reactor in the dark and finally under irradiation using an UVA irradiance of 2.1 W m−2. The irradiance was lower than the ISO recommended 10 W m−2 because of the very high activity of the photocatalytic paint sample against NO at 10 W m−2, especially at low relative humidity at elevated surface temperatures. For these conditions almost all the NO was photochemically oxidized and small changes in the final NO levels lead to very large uncertainties in the uptake coefficients.
The photocatalytic activity is temperature and relative humidity dependent. Since the surface temperature directly influences the relative humidity, for each temperature, a humidity dependence was studied. The range of the relative humidity was varied from very dry conditions (stripping coil temperature ∼5 °C mixed with dry air) to the highest humidity possible (stripping coil operated at room temperature). High humidity was limited by the condensation of water in the Teflon lines or in the reactor at lower reactor temperatures. Thus, the relative humidity range in the reactor was limited, especially at high surface temperatures.
3 Results
3.1 Photocatalysis of NO
There is some HONO formation on the sample in the dark reactor, which is explained by the heterogeneous conversion of NO2, which was present in the NO mixtures as impurity:
| 2 NO2 + H2O → HONO + HNO3. | (R7) |
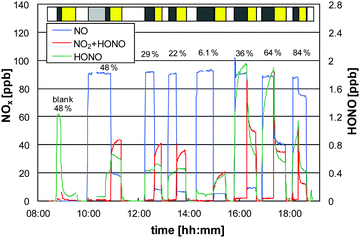
Under irradiation a strong uptake of NO was observed, which increased with decreasing humidity (see Fig. 2), in good agreement with our former study for this paint.6 The negative humidity dependence was parameterized by 3rd order polynomials and is explained by increasing adsorption of water. Adsorbed water blocks active sites for the reaction of NO with photocatalytically formed HO2 radicals, see reaction (R4).6
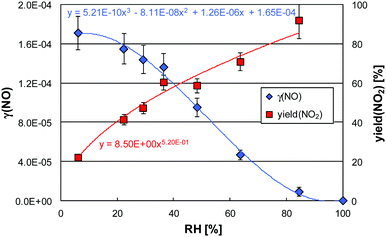 | ||
| Fig. 2 Example of the uptake of NO (γ(NO)) and the NO2 yield (Φ(NO2)) as a function of the relative humidity for the photocatalytic decomposition of NO at 20.6 °C (data from Fig. 1). | ||
To allow fitting of the full humidity range by the 3rd order polynomials and to avoid artificial negative values for high humidity, additional theoretical activities of zero at 100% RH were added to the plots. Missing photocatalytic activity at very high RH is reasonable, since for multilayer adsorption of water no activity of the catalysts against NO is expected.6 This was important, especially when only limited humidity ranges were investigated for the higher temperatures. Here, high humidity was limited by water condensation outside the photoreactor (see Experimental section).
During irradiation significant NO2 formation was observed (see Fig. 1), which is explained by reaction (R4) and its consecutive oxidation by OH radicals, reaction (R6).6 Thus, the intermediate NO2 of the following simplified consecutive sequence:
| NO → NO2 → HNO3, | (R8) |
Increasing NO2 yields were observed for increasing humidity, which was parameterized by a power function (see Fig. 2). For very high humidity the NO2 yield was approaching unity, i.e. NO is quantitatively converted into NO2 without further oxidation to the final end product nitrate, see consecutive sequence (R8).
Reasons for the high NO2 yields at high humidity are (a) the faster kinetic of the NO reaction (R4) compared to the consecutive oxidation of the intermediate NO2 (reaction (R6)) and (b) a stronger humidity dependence of reaction (R6) compared to reaction (R4).
For HONO a more complex behavior was observed under irradiation. While for low humidities HONO concentrations increased with UV lamps on, a significant photocatalytic HONO uptake was observed at higher humidities (s. Fig. 1). This behavior is explained by (a) the increasing NO2 levels under irradiation (precursor of HONO, see reaction (R7)) and (b) the increasing photocatalytic activity of the paint against HONO with increasing humidity.6 Since both effects overlap, the description of HONO formation is more complex in the NO system and its photocatalytic uptake is quantitatively evaluated in the experiments using pure NO2 (see Section 3.2.4).
Since HONO levels under irradiation were lower with NO compared to NO2 (see Section 3.2), the typically proposed photocatalytic HONO formation by the reaction of NO with OH radicals, reaction (R5),2,4,7–10 is considered being of minor importance for the present dispersion paint. This is confirmed by the weak humidity dependence of the HONO levels under irradiation (see Fig. 1). In contrast, if HONO were formed by reaction (R5), HONO levels under irradiation should strongly decrease with humidity, caused by the low values of γ(NO) (see Fig. 2) and the high values of γ(HONO) (see Section 3.2.4) at high humidity.
In conclusion, at typical atmospheric humidities the photocatalytic paint shows a high activity against NO and HONO. The HONO yields under irradiation (Φ(HONO) = ΔHONO/ΔNO) were low (typically <1%) and below the threshold limit of <5% defined in Ifang et al.32 for all humidities and temperatures investigated. However, for very high humidities i.e. close to 100% RH, the photocatalytic paint will not improve urban air quality. Under these conditions, the reaction kinetics of NO slows down and is only converted into the more harmful intermediate NO2, without final oxidation to the desired end-product nitrate, see reaction sequence (R8).
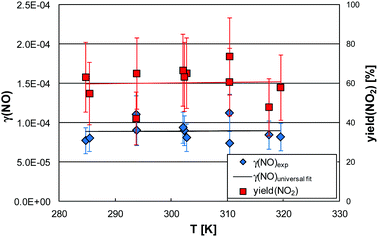 | ||
| Fig. 3 Photocatalytic activity of NO (γ(NO)), its universal parameterization (see Section 3.1.3) and the NO2 yield (Φ(NO2)) as a function of the temperature shown exemplarily for 50% RH. Error bars do not only represent the experimental errors but also result from uncertainties in the parameterizations of the humidity dependencies (see Fig. 2). | ||
It is obvious that both, γ(NO) and Φ(NO2) are independent of the temperature within the experimental uncertainties. This behavior was observed for all humidities. The reason for this behavior may be a missing temperature dependence of the photocatalytic formation of HO2 radicals, reaction (R3), which oxidizes NO to NO2, see reaction (R4). If in addition the rate of reaction (R4) is limited by the HO2 formation and not by the weak adsorption of NO on the catalyst, no temperature dependence of the NO oxidation would be expected.
| γ(NO) = mRH·T + bRH, | (2) |
| mRH = −2.341 × 10−12·(RH)3 + 4.664 × 10−10·(RH)2 − 3.005 × 10−8·RH + 6.448 × 10−7 (K−1), |
| bRH = 1.112 × 10−9·(RH)3 − 1.936 × 10−7·(RH)2 + 8.145 × 10−6·RH + 2.181 × 10−5. |
The missing temperature dependence of the photocatalysis of NO (see Fig. 3) was derived in the present study for constant relative humidities, calculated using the surface temperature of the sample. This is in contrast to the studies by Sikkema et al.10 and Tang et al.25 in which positive temperature dependencies for the NO uptake were determined at fixed absolute humidities. Since the photocatalytic activity is controlled by the adsorption of water on active sites of the photocatalyst, which is a function of the relative humidity (see Fig. 2), the use of constant relative humidity is recommended to quantify the pure temperature dependence of a photocatalytic reaction.
However, the concept used in the other two studies10,25 better describes a typical atmospheric situation, for which the surface temperature is increasing under irradiation leading to decreasing water adsorption for constant absolute humidity. If this concept would be applied in the present study, the photocatalytic activity of the paint against NO would also strongly increase with the surface temperature (see graphical abstract). However, the reason for this result is not the temperature itself, but the decreasing relative humidity on the warmer surface, leading to decreasing levels of adsorbed water. Accordingly, when using eqn (2), e.g. to model the photocatalytic decomposition of NO in the atmosphere, the surface temperature and the water vapor pressure should be known, from which the relative humidity on the surface (used in eqn (2)) can be easily calculated by the Clausius Clapeyron equation.
3.2 Photocatalysis of NO2
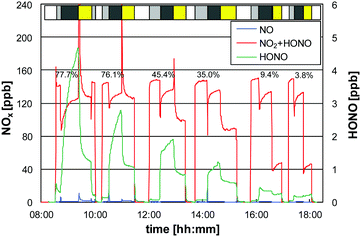
The slightly decreasing NO2 uptake (−15% for γ(NO2)) is explained by the accumulation of the adsorbed reaction product HNO3/nitrate (reaction (R6)). At higher nitrate loading of the surface the increasing formation of NO2 by the photocatalysis of nitrate,14,22 leads to decreasing net NO2 uptake on the surface. However, since higher NO2 levels compared to the atmosphere were used in this experiment (c0 = 140 ppb), the photocatalytic net uptake will decrease much slower in the atmosphere. Assuming a typical high urban NO2 mixing ratio of 20 ppb and an exponential decrease of the NO2 uptake with irradiation time, it would roughly take four completely weeks without rainfall (50% irradiation time) until the NO2 uptake decrease by 30%, which is still acceptable. However, in the future this deactivation should be studied in more detail as a function of the dose of the NO2 uptake or the accumulated nitrate. This data would help to better model net NOx uptake in the atmosphere for extended dry periods when the nitrate is not washed off the surfaces by rain.
In the present study, the uptake was derived for cleaned surfaces which contain only negligible amounts of adsorbed nitrate to get more reproducible results. In addition, from the 26 h experiment mentioned above, any significant reduction of the photocatalytic activity of NO2 by accumulated nitrate can be excluded for the much shorter exposure of the surface to NO2 when studying the humidity dependencies. Assuming an exponential decrease of the photocatalytic NO2 uptake, a reduction of γ(NO2) of only 3.5% by the accumulation of nitrate can be calculated for the NO2 exposure of ∼5.5 h in the experiment shown in Fig. 4, which is significantly smaller than the experimental error.
There is already significant formation of HONO by the dark reaction (R7) (see Fig. 4), which is increasing with humidity (see Fig. 5). Unfortunately, the reaction times applied were often not sufficient to reach steady state levels of HONO (see Fig. 4). The reaction times were chosen here only to reach almost stable NOx levels and to finish each humidity dependency during a single day experiment. Therefore, the final HONO steady state levels were extrapolated from the concentration time profiles, leading to higher uncertainties, especially at high humidity (see error bars in Fig. 5).
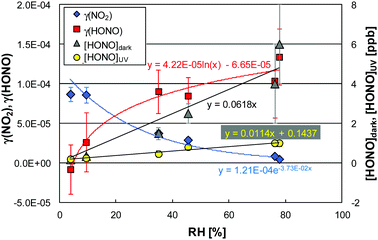 | ||
| Fig. 5 Humidity dependencies of γ(NO2), γ(HONO) and HONO mixing ratios in the dark and under UV irradiation derived from the data shown in Fig. 4 at 12.0 °C. | ||
In our former study, significant HONO levels in the dark were also observed for a photocatalytically non-active similar paint and explained by the porous structure and the high internal surface area.6 For simplicity, the dark levels of HONO were parameterized by linear functions forced through the origin.
No significant NO formation was observed, neither in the dark nor under irradiation (see Fig. 4), which is reasonable with respect to the proposed reaction sequence (R8). The very small NO formation under UV irradiation especially at higher humidity (see Fig. 4) results from the photolysis of adsorbed NO2, but not from any photocatalytic reaction as proposed in other studies.14,36 In addition, gas phase photolysis can be excluded because of the low photolysis frequency of NO2 (J(NO2) = 3.2 × 10−4 s−1) and the very short gas residence time in the photo-reactor (<1 s).
Under UV irradiation the NO2 uptake significantly increased, especially at low relative humidity (see Fig. 4), which was parameterized by an exponential function for simplicity (see Fig. 5). However, it should be pointed out that for higher surface temperatures the humidity dependence of the photocatalytic uptake significantly slows down or even decreases at low relative humidities (<10% RH), i.e. showing a maximum at ∼10% RH. This behavior is explained by reaction (R6), in which OH radicals oxidize NO2. Since OH radicals are formed by the photocatalysis of adsorbed water, reaction (R2),6 some humidity is necessary for the oxidation of NO2. Only at higher humidities (>10% RH) the increasing level of adsorbed water limits the uptake of NO2 (see similar explanation of the humidity dependence of the NO uptake). Thus, the exponential parameterization used in the present study is only valid for typical atmospheric humidities, but overestimates the uptake at <10% RH.
Under UV irradiation HONO levels are typically much lower compared to the dark (see Fig. 4), but still linearly increase with the relative humidity (see Fig. 5). This behavior is explained by a humidity dependent HONO formation in the dark viareaction (R7) and a photocatalytic decomposition of HONO. Caused by the increasing HONO levels in the dark (c0) with increasing humidity, it is reasonable that the remaining HONO after photocatalytic degradation (ct) also increase with humidity – only on a lower level. In contrast to the humidity dependence of the NO2 uptake, the relative photocatalytic decay of HONO increases with humidity (see γ(HONO) in Fig. 5). This behavior is explained by an oxidation of HONO as soluble nitrite in the adsorbed water layer by photocatalytically formed electron holes (hvb+):6
| HONO + H2O ⇆ NO2− + H3O+; | (R9) |
| NO2− + 2hvb+ + H2O → NO3− + 2 H+, |
For very dry conditions the HONO levels slightly increase with the UV lamps switched on (see Fig. 4), which is explained by additional photocatalytic HONO formation:12
| NO2 + O2− + H+ → HONO + O2. | (R10) |
The photocatalytic uptake coefficients for HONO non-linearly increase with humidity, which was parameterized by logarithmic functions (see Fig. 5). The artificial negative uptake coefficient at the lowest humidity shown in Fig. 5, results from the definition of the uptake coefficient (see eqn (1)) and the photocatalytic formation of HONO viareaction (R10). Accordingly, the parameterization of the uptake of HONO derived in the present study should not be applied for RH < 15%.
In conclusion, for typical ambient humidities the paint shows an excellent photocatalytic activity against HONO. Only for unrealistic low humidities HONO is formed from NO2 under UV irradiation. In addition, the HONO yields under irradiation were typically smaller than the recommended threshold of <5% defined by Ifang et al.32 Since HONO formation in the dark was also observed on a similar non-photocatalytic reference paint,6 the use of this photocatalytic paint will clearly improve urban air quality with respect to the harmful pollutant nitrous acid.
| ln(γ(NO2)) = ln(A)RH (−EA/R)RH·T−1, | (3) |
| ln(A)RH = −0.3197·RH − 15.905, |
| (−EA/R)RH = 79.426·RH + 2295.3 (K−1). |
The negative temperature dependence of the NO2 photocatalysis seems to be in contradiction to results by Gandolfo et al.30 who obtained a positive temperature dependence for a photocatalytic indoor paint. However, similar to the explanation of the experiments with NO, the temperature dependence of the NO2 uptake was derived for constant relative humidities in the present study, whereas Gandolfo et al.30 used fixed water vapor pressures. Since the photocatalytic activity against NO2 is also strongly controlled by the adsorption of water on the active sites of the photocatalyst, which is a function of the relative humidity (see Fig. 5), the use of constant relative humidity is recommended to quantify the temperature dependence alone.
If constant absolute humidities were used similar to the concept of Gandolfo et al., the photocatalytic activity of the paint used in the present study would also increase with the surface temperature (see graphical abstract). Accordingly, the negative temperature dependence (see Fig. 6) is overcompensated by the strong negative humidity dependence (see Fig. 5). The relative humidity is strongly decreasing with increasing surface temperature for constant water vapor pressure. Consequently, when using eqn (3), e.g. to model the photocatalytic decomposition of NO2 in the atmosphere, the surface temperature and the water vapor pressure should be known, from which the relative humidity on the surface (see eqn (3)) can be easily calculated.
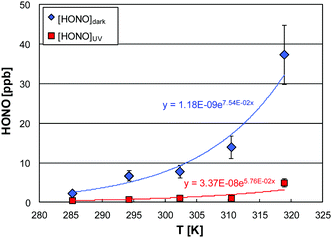 | ||
| Fig. 7 Temperature dependence of the HONO mixing ratio in the dark and under UV irradiation (50% RH as an example). | ||
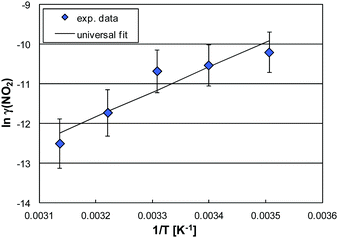
In the dark, the HONO levels exponentially increase with temperature, which can be explained by a positive temperature dependence of reaction (R7). Significant HONO formation under irradiation by reaction (R10) is of minor importance (only at RH < 15%, see above). Thus, it is plausible, that the temperature dependence of the remaining HONO after photocatalytic decomposition follows qualitatively the trend of the dark levels. However, when looking in more detail into the data shown in Fig. 7, the HONO levels under irradiation increase less with temperature compared to the dark levels, which is explained by the faster uptake of HONO with increasing temperature. To confirm this assumption the uptake coefficients of HONO were calculated for similar humidities for the different experimental temperatures applied. When plotting the data according to an Arrhenius expression, an increasing uptake is observed with increasing temperature (see Fig. 8 as an example for 50% RH).
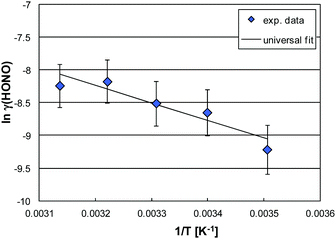 | ||
| Fig. 8 Arrhenius plot of ln(γ(HONO)) vs. T−1 and its universal parameterization as a function of T and RH (50% RH as an example). | ||
The observed positive humidity and temperature dependencies of the photocatalytic uptake of HONO on the dispersion paint agree with the mechanism proposed by Laufs et al.,6 in which HONO is oxidized in its ionic form (NO2−) to nitrate (NO3−) in a layer of adsorbed water by valence band electron holes (hvb+), see reaction (9). For high humidity more HONO gets soluble explaining the positive humidity dependence of the HONO uptake. In addition, for aqueous phase ion chemistry a positive temperature dependence is also expected. In contrast, the photocatalytic oxidation of adsorbed molecular HONO by OH radicals, typically proposed in the literature:2,4,10
| HONO + OH → NO2 + H2O, | (R11) |
The positive humidity (see Fig. 5) and temperature (see Fig. 8) dependencies of the HONO uptake on the dispersion paint are in contrast to a recent study in which negative humidity and temperature dependencies were observed for the photocatalytic uptake of HONO on a pure TiO2 photocatalyst (P25) in a low pressure flow tube.38 It could be speculated that the different experimental conditions (low pressure vs. atmospheric pressure; He vs. synthetic air as buffer gas; different humidity levels; etc.) but also the different acidities of the surfaces used caused the different observations. The pure P25 TiO2 photocatalyst used by El Zein et al.38 shows slightly acidic surfaces properties, favoring adsorbed undissociated HONO as reactant. In contrast, the surface of the present dispersion paint is alkaline (pH 8–8.5). Accordingly, adsorbed HONO will be efficiently converted into nitrite, see equilibrium (R9). Consequently, photocatalysis should be studied not only on pure TiO2 surfaces, but more importantly also on real commercially available photocatalytic substrates such as concrete, paints, self-cleaning glass, etc.
| ln(γ(HONO)) = ln(A)RH(−EA/R)RH·T−1, | (4) |
| ln(A)RH = 506.81·RH−1.556 − 0.85, |
| (−EA/R)RH = −1.292 × 105·(RH)−1.420 − 2170 (K−1). |
When constant water vapor pressure is assumed, γ(HONO) calculated by eqn (4) is almost independent of the surface temperature of the paint (see graphical abstract). Here, the positive temperature dependence of γ(HONO) for constant relative humidity (see Fig. 8) is compensated by a decreasing uptake with decreasing relative humidity (see Fig. 5) at higher temperatures. Accordingly, in the atmosphere, the uptake of HONO is independent of the temperature when photocatalytic surfaces are heated by irradiation with sunlight at constant water vapour pressure.
Since HONO formation and uptake were studied only indirectly in the NO2 experiments, it is desirable to study the humidity and temperature dependencies of the photocatalysis of HONO using a pure HONO source in the future.
4 Conclusions
In the present study humidity and temperature dependencies of the photocatalytic uptake of NO, NO2 and HONO were studied on a commercial dispersion paint in a flow reactor and decoupled for the first time. The obtained humidity dependencies were in good agreement with a former study performed at ambient temperature showing a decreasing uptake for NO and NO2 with increasing humidity, while the opposite has been observed for HONO. The differences were explained by surface adsorption of water blocking active sites for the adsorption of NO and NO2, while HONO is proposed to be oxidized as soluble nitrite in a film of adsorbed water on the alkaline surface of the dispersion paint.In addition to the former study, negligible/negative temperature dependencies are observed for the uptake of NO/NO2 at constant relative humidities, respectively. In contrast, the photocatalytic uptake of HONO increases with temperature. The uptake was parameterized as a function of both, relative humidity and temperature for all species investigated. Since in addition the uptake was found to be first order at low atmospheric relevant NOx levels, the parameterizations can be used to better describe photocatalytic remediation in atmospheric chemistry-transport models under various conditions of c, RH and T.
Since the missing/negative temperature dependencies of the photocatalysis of NO/NO2 are overcompensated by their strong negative humidity dependencies, the atmospheric implication is a better NOx remediation when photocatalytic surfaces are heated by irradiation with sunlight. Reasons for this are not the temperature dependencies, but the strong negative humidity dependencies of the NO and NO2 uptake and the decreasing relative humidity on heated surfaces.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge support by the EU Integrated Infrastructure Initiative EUROCHAMP-2020 (Contract No. 730997).References
- EU Directive 99/30/EC of 22 April, 1999, Journal of the European Communities 29.06.1999. En Series, L163/41.
- J. Ângelo, L. Andrade, L. M. Madeira and A. Mendes, J. Environ. Manage., 2013, 129, 522–539 CrossRef PubMed.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- Y. Boyjoo, H. Sun, J. Liu, V. K. Pareek and S. Wang, Chem. Eng. J., 2017, 310, 537–559 CrossRef CAS.
- K. Hashimoto, K. Wasada, N. Toukai, H. Kominami and Y. Kera, J. Photochem. Photobiol., A, 2000, 136, 103–109 CrossRef CAS.
- S. Laufs, G. Burgeth, W. Duttlinger, R. Kurtenbach, M. Maban, C. Thomas, P. Wiesen and J. Kleffmann, Atmos. Environ., 2010, 44, 2341–2349 CrossRef CAS.
- S. Devahasdin, C. Fan Jr., K. Li and D. H. Chen, J. Photochem. Photobiol., A, 2003, 156, 161–170 CrossRef CAS.
- M. Chen and J.-W. Chu, J. Cleaner Prod., 2011, 19, 1266–1272 CrossRef CAS.
- A. Folli, S. B. Campbell, J. A. Anderson and D. E. Macphee, J. Photochem. Photobiol., A, 2011, 220, 85–93 CrossRef CAS.
- J. K. Sikkema, S. K. Ong and J. E. Alleman, Construct. Build. Mater., 2015, 100, 305–314 CrossRef.
- R. J. Gustafsson, A. Orlov, P. T. Griffiths, R. A. Cox and R. M. Lambert, Chem. Commun., 2006, 3936–3938 RSC.
- M. Ndour, B. D’Anna, C. George, O. Ka, Y. Balkanski, J. Kleffmann, K. Stemmler and M. Ammann, Geophys. Res. Lett., 2008, 35, L05812, DOI:10.1029/2007GL032006.
- S. K. Beaumont, R. J. Gustafsson and R. M. Lambert, ChemPhysChem, 2009, 10, 331–333 CrossRef CAS PubMed.
- M. E. Monge, B. D’Anna and C. George, Phys. Chem. Chem. Phys., 2010, 12, 8991–8998 RSC.
- A. Gandolfo, V. Bartolomei, E. Gomez Alvarez, S. Tlili, S. Gligorovski, J. Kleffmann and H. Wortham, Appl. Catal., B, 2015, 166–167, 84–90 CrossRef CAS.
- T. Salthammer and F. Fuhrmann, Environ. Sci. Technol., 2007, 41, 6573–6578 CrossRef CAS PubMed.
- J. Auvinen and L. Wirtanen, Atmos. Environ., 2008, 42, 4101–4112 CrossRef CAS.
- O. Geiss, C. Cacho, J. Barrero-Moreno and D. Kotzias, Build. Environ., 2012, 48, 107–112 CrossRef.
- F. Mothes, O. Böge and H. Herrmann, Environ. Sci. Pollut. Res., 2016, 23, 15250–15261 CrossRef CAS PubMed.
- C. Toro, B. T. Jobson, L. Haselbach, S. Shen and S. H. Chung, Atmos. Environ., 2016, 139, 37–45 CrossRef CAS.
- A. Gandolfo, S. Marque, B. Temine-Roussel, R. Gemayel, H. Wortham, D. Truffier-Boutry, V. Bartolomei and S. Gligorovski, Environ. Sci. Technol., 2018, 52, 11328–11337 CrossRef CAS PubMed.
- M. E. Monge, C. George, B. D’Anna, J.-F. Doussin, A. Jammoul, J. Wang, G. Eyglunent, G. Solignac, V. Daële and A. Melluki, J. Am. Chem. Soc., 2010, 132, 8234–8235 CrossRef CAS PubMed.
- M. Gallus, R. Ciuraru, F. Mothes, V. Akylas, F. Barmpas, A. Beeldens, F. Bernard, E. Boonen, A. Boréave, M. Cazaunau, N. Charbonnel, H. Chen, V. Daële, Y. Dupart, C. Gaimoz, B. Grosselin, H. Herrmann, S. Ifang, R. Kurtenbach, M. Maille, I. Marjanovic, V. Michoud, A. Mellouki, K. Miet, N. Moussiopoulos, L. Poulain, P. Zapf, C. George, J. F. Doussin and J. Kleffmann, Environ. Sci. Pollut. Res., 2015, 22, 18185–18196 CrossRef CAS PubMed.
- J. Kleffmann, Atmos. Environ., 2016, 129, 95–97 CrossRef CAS.
- X. Tang, L. Ughetta, S. K. Shannon, S. Houzé de l’Aulnoit, S. Chen, R. A. T. Gould, M. L. Russell, J. Zhang, G. Ban-Weiss, R. L. A. Everman, F. W. Klink, R. Levinson and H. Destaillats, Build. Environ., 2019, 160, 106058 CrossRef.
- N. Bengtsson and M. Castellote, J. Adv. Oxid. Technol., 2010, 13, 341–349 CAS.
- C. J. Cros, A. L. Terpeluk, N. E. Crain, M. C. G. Juenger and R. L. Corsi, J. Air Waste Manage. Assoc., 2015, 65, 937–947 CrossRef CAS PubMed.
- A. El Zein and Y. Bedjanian, Atmos. Chem. Phys., 2012, 12, 1013–1020 CrossRef CAS.
- Y. Bedjanian and A. El Zein, J. Phys. Chem. A, 2012, 116, 1758–1764 CrossRef CAS PubMed.
- A. Gandolfo, L. Rouyer, H. Wortham and S. Gligorovski, Appl. Catal., B, 2017, 209, 429–436 CrossRef CAS.
- ISO 22197-1. Reference number ISO 22197-1:2007(E), Switzerland, 2007.
- S. Ifang, M. Gallus, S. Liedtke, R. Kurtenbach, P. Wiesen and J. Kleffmann, Atmos. Environ., 2014, 91, 154–161 CrossRef CAS.
- C. Minero, A. Bedini and M. Minella, Int. J. Chem. React. Eng., 2013, 11(2), 717–732 Search PubMed.
- J. Heland, J. Kleffmann, R. Kurtenbach and P. Wiesen, Environ. Sci. Technol., 2001, 35, 3207–3212 CrossRef CAS PubMed.
- J. Kleffmann, J. Heland, R. Kurtenbach, J. Lörzer and P. Wiesen, Environ. Sci. Pollut. Res., 2002, 9(Special issue 4), 48–54 Search PubMed.
- Y. Ohko, Y. Nakamura, A. Fukuda, S. Matsuzawa and K. Takeuchi, J. Phys. Chem. C, 2008, 112, 10502–10508 CrossRef CAS.
- C. H. Pollema, E. B. Milosavljević, J. L. Hendrix, L. Solujić and J. H. Nelson, Mon. Chem., 1992, 123, 333–339 CrossRef CAS.
- A. El Zein, Y. Bedjanian and M. N. Romanias, Atmos. Environ., 2013, 67, 203–210 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cp01157j |
| This journal is © the Owner Societies 2021 |