The role of surface stoichiometry in NO2 gas sensing using single and multiple nanobelts of tin oxide†
Abstract
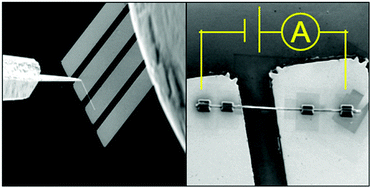
Typically used semiconducting metal oxides (SMOs) consist of several varying factors that affect gas sensor response, including film thickness, grain size, and notably the grain–grain junctions within the active device volume, which complicates the analysis and optimisation of sensor response. In comparison, devices containing a single nanostructured element do not present grain–grain junctions, and therefore present an excellent platform to comprehend the correlation between nanostructure surface stoichiometry and sensor response to the depletion layer (Debye length, LD) variation after the analyte gas adsorption/chemisorption. In this work, nanofabricated devices containing SnO2 and Sn3O4 individual nanobelts of different thicknesses were used to estimate their LD after NO2 exposure. In the presence of 40 ppm of NO2 at 150 °C, LD of 12 nm and 8 nm were obtained for SnO2 and Sn3O4, respectively. These values were associated to the sensor signals measured using multiple nanobelts onto interdigitated electrodes, outlining that the higher sensor signal of the Sn4+ surface (up to 708 for 100 ppm NO2 at 150°) in comparison with the Sn2+ (up to 185) can be explained based on a less depleted initial state and a lower surface electron affinity caused by the Lewis acid/base interactions with oxygen species from the baseline gas. To support the proposed mechanisms, we investigated the gas sensor response of SnO2 nanobelts with a higher quantity of oxygen vacancies and correlated the results to the SnO system.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...