Open Access Article
Open Access ArticlePulse sequence and sample formulation optimization for dipolar order mediated 1H→13C cross-polarization†
Stuart J.
Elliott
 *a,
Olivier
Cala
a,
Quentin
Stern
a,
Samuel F.
Cousin
ab,
Dmitry
Eshchenko
c,
Roberto
Melzi
d,
James G.
Kempf
e and
Sami
Jannin
a
*a,
Olivier
Cala
a,
Quentin
Stern
a,
Samuel F.
Cousin
ab,
Dmitry
Eshchenko
c,
Roberto
Melzi
d,
James G.
Kempf
e and
Sami
Jannin
a
aCentre de Résonance Magnétique Nucléaire à Très Hauts Champs - FRE 2034 Université de Lyon/CNRS/Université Claude Bernard Lyon 1/ENS de Lyon, 5 Rue de la Doua, 69100 Villeurbanne, France. E-mail: stuart-james.elliott@univ-lyon1.fr
bInstitut de Chimie Radicalaire - UMR 7273, Saint-Jérôme Campus, Av. Esc. Normandie Niemen, Aix-Marseille Université/CNRS, 13397 Marseille, Cedex 20, France
cBruker Switzerland, Industriestrasse 26, 8117 Fällanden, Switzerland
dBruker Italia Srl, Viale Vincenzo Lancetti, 43, 20158 Milan, Italy
eBruker Biospin, 15 Fortune Drive Billerica, Massachusetts, USA
First published on 1st April 2021
Abstract
We have recently demonstrated the use of contactless radiofrequency pulse sequences under dissolution-dynamic nuclear polarization conditions as an attractive way of transferring polarization from sensitive 1H spins to insensitive 13C spins with low peak radiofrequency pulse powers and energies via a reservoir of dipolar order. However, many factors remain to be investigated and optimized to enable the full potential of this polarization transfer process. We demonstrate herein the optimization of several key factors by: (i) implementing more efficient shaped radiofrequency pulses; (ii) adapting 13C spin labelling; and (iii) avoiding methyl group relaxation sinks. Experimental demonstrations are presented for the case of [1-13C]sodium acetate and other relevant molecular candidates. By employing the range of approaches set out above, polarization transfer using the dipolar order mediated cross-polarization radiofrequency pulse sequence is improved by factors approaching ∼1.65 compared with previous results. Dipolar order mediated 1H→13C polarization transfer efficiencies reaching ∼76% were achieved using significantly reduced peak radiofrequency pulse powers relative to the performance of highly sophisticated state-of-the-art cross-polarization methods, indicating 13C nuclear spin polarization levels on the order of ∼32.1% after 10 minutes of 1H DNP. The approach does not require extensive pulse sequence optimization procedures and can easily accommodate high concentrations of 13C-labelled molecules.
1. Introduction
Conventional nuclear magnetic resonance (NMR) techniques are limited by an intrinsic lack of sensitivity and generate weak signals. Dissolution-dynamic nuclear polarization (dDNP) has proven to be a widely applicable hyperpolarization method to intensify detectable NMR signals from a number of small molecules and materials by up to four orders of magnitude,1 with far-reaching applications in clinical research.2–4 Adequate dDNP of dilute insensitive nuclear spins such as 13C at low temperature is particularly slow, with low polarizations typically accrued over extensive timescales of hours or more.5 Shortened polarization build-up times are engendered by employing radiofrequency (rf) pulse sequences which efficiently transfer polarization from faster polarizing nuclear spins such as 1H.6Cross-polarization (CP) boosts 13C polarizations and shortens build-up time constants (by a factor of up to 40) under dDNP conditions (typ. at temperatures of ∼1.0–1.6 K in superfluid helium),7–15 with a CP-DNP combination proving to be pivotal in the transportation of highly polarized metabolites.16 The CP method, however, is required to adhere to the strict Hartmann–Hahn matching condition,7i.e. intense B1-field matching (typ. >15 kHz) of concurrent 1H and 13C spin-locking rf-irradiation during an optimized polarization transfer time (typ. >1 ms). This often leads to high rf-pulse powers for low-gamma nuclear spins and poor rf-pulse coverage of the NMR line in the case of the strongly polarized nuclear spins, i.e. the spin-locking performance of the high-gamma nuclear spins is strictly limited. The requirement of high power and energy rf-pulses risks detrimental arcing in the superfluid helium bath. This limitation has so far prevented the use of CP for sample volumes exceeding 500 μL, which is essential for the scaling-up of dDNP to sample volumes required for parallel hyperpolarization17 and human imaging.2–4 A minor side-effect is the heat introduced from the high-power rf-pulses, which in turn raises the temperature of the helium bath, and thus affects the DNP efficiency.
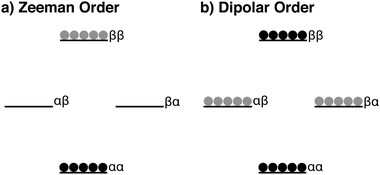
Polarization transfer between heteronuclear spins in the solid-state on static samples can alternatively be mediated in a “contactless” manner by a reservoir of dipolar order (see Fig. 1 for a representation of the dipolar order reservoir).18–28 Although rf-techniques for generating, evolving and reconverting dipolar order are demonstrated in the literature, little use has been documented since transfer efficiencies are dramatically affected in the case of magic angle spinning (MAS) NMR experiments‡. However, there is no such limitation under the conditions of static DNP experiments at low temperatures. Furthermore, these alternative rf-methods advantageously often require lower power, and could therefore be more applicable for hyperpolarizing larger sample volumes.
In a previous Paper,29 we described the dynamics of a dipolar-order-mediated cross-polarization (dCP) rf-pulse sequence which relied on a contactless transfer of polarization from 1H to 13C nuclear spins hypothesized to be governed by a dipolar order reservoir under dDNP conditions. Polarization transfer via a bath of dipolar order was shown to perform well with respect to the rf-pulse powers employed, but the efficiency of the 1H→13C polarization transfer process was found to be reduced with respect to a conventional CP approach for standard DNP-compatible sample formulations.
In the current Paper, we present an optimization of the dCP rf-pulse sequence for 1H to 13C polarization transfer in the context of dDNP experiments. We outline the following improvements: (i) extensions to shaped rf-pulses; and (ii) exploration of diversified spin systems with favourable molecular properties, such as stronger 1H–13C dipolar couplings and absent CH3 group relaxation sinks. We report experimental observations for [1-13C]sodium acetate and other suitable molecules. We show improvements in the dCP transfer efficiency approaching factors of up to ∼1.65 compared with previously reported results.291H→13C polarization transfer efficiencies of the dCP rf-pulse sequence on the order of ∼76% were realized with respect to a conventional and optimized CP experiment, revealing 13C polarizations in the region of ∼32.1% in the frozen solid after 10 minutes of 1H DNP.
2. Methods
2.1. Molecular systems
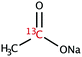
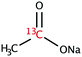
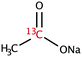
The range of 13C-labelled molecular candidates under investigation in this study are shown in Table 1. Molecule I is used as a “benchmark” molecular system for which all rf-pulse sequences will be optimized. Molecules II and III are employed in order to verify the following hypotheses: (i) relocating the 13C spin label to a carbon site adjacent to the methyl group protons will result in larger 1H–13C dipolar couplings which should strongly influence the 1H→13C polarization transfer efficiency; and (ii) the absence of CH3 group relaxation sinks is expected to improve the dCP transfer efficiency, respectively.2.2. Sample preparation and freezing
Solutions of 3 M [1-13C]sodium acetate (I), [2-13C]sodium acetate (II) and [1-13C]sodium formate (III) in the glass-forming mixture H2O/D2O/glycerol-d8 (1/3/6 v/v/v) were doped with 50 mM TEMPOL radical (all compounds purchased from Sigma Aldrich) and sonicated for ∼10 minutes. Paramagnetic TEMPOL radicals (nitroxides) were chosen to most efficiently polarize 1H spins under our dDNP conditions. 100 μL volumes of each sample were separately pipetted into a Kel-F sample cup and inserted into a 7.05 T prototype Bruker Biospin polarizer equipped with a specialized dDNP probe and running TopSpin 3.5 software. The sample temperature was reduced to 1.2 K by submerging the sample in liquid helium and reducing the pressure of the variable temperature insert (VTI) towards ∼0.7 mbar.2.3. Dynamic nuclear polarization and microwave gating
The samples were polarized by applying microwave irradiation at fμw = 197.568 GHz (positive lobe of the microwave spectrum) with triangular frequency modulation of amplitude Δfμw = ±120 MHz30 and rate fmod = 0.5 kHz at a power of ca. Pμw = 125 mW at the output of the microwave source and ca. Pμw = 30 mW reaching the DNP cavity, which were optimized by using sample I prior to commencing experiments to achieve the best possible level of 1H polarization. Microwave gating was employed shortly before and during dDNP polarization transfer experiments to allow the electron spin ensemble to return to a highly polarized state, which happens on the timescale of the longitudinal electron relaxation time (typ. T1e = 100 ms with Pe = 99.93% under our conditions).31 Microwave gating hence provides a way to strongly attenuate paramagnetic relaxation, and consequently the 1H and 13C T1ρ relaxation time constants in the presence of spin-locking rf-fields are extended by orders of magnitude. This allows the spin-locking rf-pulses used to be much longer, which significantly increases the efficiency of nuclear polarization transfer.2.4. RF-Pulse sequences
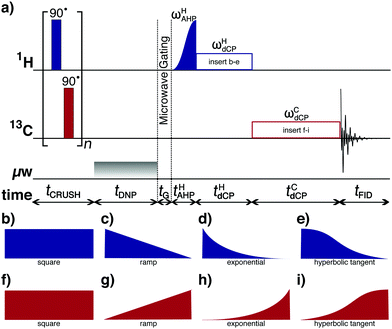
In the current study, we used variants of the adiabatic demagnetization/remagnetization in the rotating frame (ADRF/ARRF) rf-pulse sequence.20–28 The ADRF/ARRF rf-pulse sequence has been shown to possess improved dipolar order preparation and conversion efficiencies versus the non-Zeeman polarization transfer (NZPT) experiment proposed by Vieth and Yannoni.19 In our work, we will generally refer here to such polarization transfer schemes as dCP for dipolar-order-mediated cross-polarization. Fig. 2a shows this sequence adapted for our dDNP experiments.The dCP rf-pulse sequence operates as follows:
(i) A crusher sequence of 90° rf-pulses with alternating phases separated by a short delay (typ. 11 ms) repeated n times (typ. n = 50) kills residual magnetization on both rf-channels;
(ii) The microwave source becomes active for a time tDNP during which 1H DNP builds-up;
(iii) The microwave source is deactivated and a delay of duration tG = 0.5 s occurs before the next step, thus permitting the electron spins to relax to their highly polarized thermal equilibrium state;31
(iv) A preparatory 1H adiabatic half-passage (AHP) rf-pulse creates transverse magnetization;
(v) A 1H dCP rf-pulse of amplitude ωHdCP and length tHdCP prepares 1H–1H dipolar order;
(vi) A 13C dCP rf-pulse of amplitude ωCdCP and length tCdCP presumably converts the 1H–1H dipolar order into 13C magnetization;
(vii) The induced 13C NMR signal is detected.
Further details regarding dCP rf-pulse sequence operation are given elsewhere.29
Fig. 2(b-i) indicates the 1H and 13C dCP rf-pulse shapes trialled to improve the performance efficiency of the dCP rf-pulse sequence. The amplitude ωdCP and duration tdCP of the 1H and 13C dCP rf-pulse shapes were carefully optimized29 by detecting the induced NMR signal on the 13C rf-channel in an iterative process until the intensity of the resulting 13C NMR signals could not be improved further prior to each heteronuclear polarization transfer experiment.
3. Results
3.1 Cross-polarization
The performance efficiency of the dCP rf-pulse sequence was compared with a traditional CP experiment,7–15 which is described in the ESI,† along with an rf-pulse sequence diagram, all optimized parameters and the 13C polarization level PCP(13C) achieved for each sample after a single CP contact. Experiments employed either 5 or 600 s of direct 1H DNP at 1.2 K prior to polarization transfer to the 13C heteronuclear spins. 13C polarization levels in excess of 60% are anticipated by using a multiple CP contact approach.9–15 The 13C polarization level PdCP(13C) obtained as a result of using the dCP rf-pulse sequence is calculated by scaling PCP(13C) by a factor of IdCP/ICP, where IdCP and ICP are the integrals of the optimized dCP and CP 13C NMR signal maxima, respectively. IdCP/ICP is referred to as the dCP rf-pulse sequence 1H→13C nuclear polarization transfer efficiency.3.2. Shaped RF-pulses
For all the samples employed in this study, it was found that the best performing 1H and 13C dCP rf-pulses (i.e. the most efficient rf-pulses for preparing and converting 1H–1H dipolar order) were a ramp which linearly decreases in amplitude (Fig. 2c) and an increasing amplitude hyperbolic tangent function (Fig. 2i), respectively. The amplitude ωdCP and duration tdCP of the aforementioned 1H and 13C dCP rf-pulse shapes for the samples used in this investigation are shown in Table 2.The experimental 13C NMR signal intensities resulting from the above described shaped rf-pulse parameter optimization protocol for sample I are detailed in Fig. 3. For the 1H dCP rf-pulse it was found that the linearly decreasing amplitude ramp was a factor of ∼1.25 more efficient in generating 1H–1H dipolar order than the practically simpler spin-locking 1H dCP rf-pulse (see Fig. 3a). In the case of the 13C dCP rf-pulse, the hyperbolic tangent shaped rf-pulse was only a factor of ∼1.05 more efficient in supposedly transferring 1H–1H dipolar order to the 13C heteronuclear spin (see Fig. 3b). It was also found that for all samples the effect of including a preparatory 1H AHP rf-pulse within the dCP rf-pulse sequence (in place of a hard 90° rf-pulse) was to maximize the acquired 1H NMR signal intensity, by a factor of ∼1.13 (Fig. 3a) in the case of sample I, compared with the dCP rf-pulse sequence of Vieth and Yannoni.19,29 This leads to cumulative effect whereby the preparation efficiency of 1H–1H dipolar order is improved by a factor of ∼1.41.
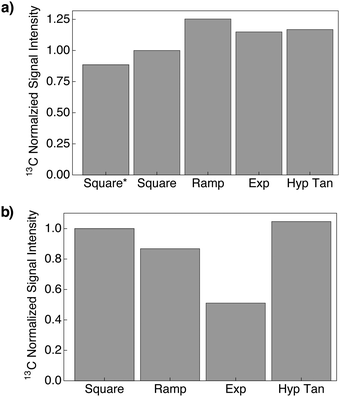 | ||
| Fig. 3 Normalized 13C NMR signal intensities of I as a function of the optimized (a) 1H and (b) 13C dCP rf-pulse shapes acquired at 7.05 T (1H nuclear Larmor frequency = 300.13 MHz, 13C nuclear Larmor frequency = 75.47 MHz) and 1.2 K with 1 transient after 5 s of 1H DNP. *AHP → hard 90° rf-pulse (bandwidth ≃ 40 kHz). Exp = Exponential; Hyp Tan = Hyperbolic Tangent. The 13C NMR signal intensities in (a) were acquired by using the dCP rf-pulse sequence (Fig. 2a) with an increasing amplitude hyperbolic tangent 13C dCP rf-pulse (Fig. 2i) of maximum amplitude ωCdCP and duration tCdCP (Table 2). The 13C NMR signal intensities in (b) were acquired by using the dCP rf-pulse sequence (Fig. 2a) with a linearly decreasing amplitude ramp 1H dCP rf-pulse (Fig. 2c) of maximum amplitude ωHdCP and duration tHdCP (Table 2). | ||
In comparison with a sophisticated CP approach (see Section 3.1 and the ESI†) the integral of the optimized dCP 13C NMR signal maximum for sample I is scaled by a factor of ∼0.46 relative to the result achieved by using a high-power CP approach (see Table 3). This indicates a solid-state 13C polarization of PdCP(13C) ≃ 19.0%. This is consistent with previous results reported in the literature.5,15 These results demonstrate that a dCP rf-pulse sequence containing shaped rf-pules has an increased efficiency for creating and transferring 1H–1H dipolar order.
3.3. Molecular modifications
Table 3 displays the 1H polarization levels P(1H), 13C nuclear polarizations obtained after a single 1H–1H dipolar order to 13C magnetization conversion step PdCP(13C) and 1H→13C nuclear polarization transfer efficiencies IdCP(13C)/ICP(13C) for each molecular candidate in our series of experiments. The value of IdCP(13C)/ICP(13C) was determined using just 5 s of 1H DNP.All samples show a similar level of 1H polarization after 5 s of 1H DNP, however, the 13C polarizations can significantly differ. The 1H→13C nuclear polarization transfer efficiencies follow a similar trend. It is worth noting that, after 10 minutes of 1H DNP and a sole dCP nuclear polarization transfer step, sample II achieves an impressive 13C polarization of ∼32.1% (with a conversion efficiency of ∼76% for the case of tDNP = 5 s). These results indicate that although the protons within each sample polarize to a similar extent, the 13C polarization of each sample is dominated by the efficiency of the nuclear polarization transfer stages in the dCP rf-pulse sequence. This trend also appears to correlate with the nature and number of bonded 1H nuclear spins.
Table 4 indicates the proton molarities C(1H), 13C dCP rf-pulse build-up rate constants (RCdCP= 1/τCdCP) and effective dCP build-up rate constants 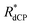 for the samples employed in our experiments under dDNP conditions, where the effective dCP build-up rate constant
for the samples employed in our experiments under dDNP conditions, where the effective dCP build-up rate constant 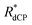 is defined as:
is defined as:
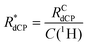 | (1) |
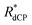 for the samples investigated during this work (see text for details)
for the samples investigated during this work (see text for details)
The 13C dCP rf-pulse build-up rate constants RCdCP were extracted by following the presumed conversion of 1H–1H dipolar order to 13C magnetization using the dCP rf-pulse sequence described in Fig. 2, and the associated text, with tDNP = 5 s. The experimental 13C polarization build-up curves (shown in the ESI†) are well fitted with a stretched exponential function using a build-up rate constant denoted 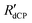 . Stretched exponential build-up function: A(1-exp{−(
. Stretched exponential build-up function: A(1-exp{−(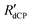 t)β}), where A is a constant,
t)β}), where A is a constant, 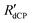 is the 13C dCP rf-pulse build-up rate constant extracted from the above fitting procedure and β is the breadth of the distribution of 13C dCP rf-pulse build-up rate constants. The average 13C dCP rf-pulse build-up rate constants RCdCP are calculated as follows: RCdCP =
is the 13C dCP rf-pulse build-up rate constant extracted from the above fitting procedure and β is the breadth of the distribution of 13C dCP rf-pulse build-up rate constants. The average 13C dCP rf-pulse build-up rate constants RCdCP are calculated as follows: RCdCP = 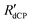 β/Γ(1/β), where Γ(1/β) is the gamma function.
β/Γ(1/β), where Γ(1/β) is the gamma function.
The 13C dCP rf-pulse build-up rate constant RCdCP for samples without methyl groups, i.e. sample III, was found to be reduced. However, the effective dCP build-up rate constants (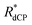 ) for all samples used in this study are in approximate agreement.
) for all samples used in this study are in approximate agreement.
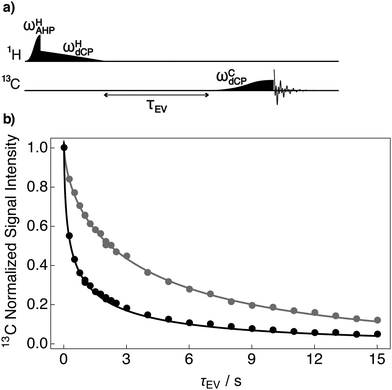
The lifetime of dipolar order for samples I and III was measured by using the variant of the dCP rf-pulse sequence depicted in Fig. 4a. The dCP rf-pulse sequence is repeated with incremented values of the variable evolution delay (τEV) to monitor the decay of dipolar order. By fitting the integrated 13C NMR signal decay as a function of the variable evolution time (τEV) the dipolar order lifetime can be estimated.
Experimental decay curves showing the relaxation of dipolar order are presented in Fig. 4b. The decay of dipolar order was found to have a stretched exponential behaviour in both cases. The experimental decays are well fitted with a stretched exponential decay function (solid lines) using a relaxation time constant denoted 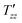 . Stretched exponential decay function: A
. Stretched exponential decay function: A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−(t/
exp{−(t/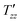 )β}, where A is a constant,
)β}, where A is a constant, 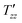 is the dipolar order relaxation time constant extracted from the above fitting procedure and β is the breadth of the distribution of dipolar order relaxation time constants. The mean dipolar order relaxation time constants Tzz are calculated as follows: Tzz =
is the dipolar order relaxation time constant extracted from the above fitting procedure and β is the breadth of the distribution of dipolar order relaxation time constants. The mean dipolar order relaxation time constants Tzz are calculated as follows: Tzz = 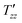 Γ(1/β)/β, where Γ(1/β) is the gamma function. In these cases, the measured average lifetimes of dipolar order Tzz are: Sample I: Tzz = 2.3 ± 0.2 s; and Sample III: Tzz = 6.0 ± 0.1 s.
Γ(1/β)/β, where Γ(1/β) is the gamma function. In these cases, the measured average lifetimes of dipolar order Tzz are: Sample I: Tzz = 2.3 ± 0.2 s; and Sample III: Tzz = 6.0 ± 0.1 s.
4. Discussion
4.1. Shaped RF-pulses
In prior work,29 we investigated the spin dynamics of 1H→13C nuclear polarization transfer by employing a dCP rf-pulse sequence which uses sequential spin-locking 1H and 13C dCP rf-pulses. The polarization transfer efficiency of the dCP rf-pulse sequence was found to be reasonable with respect to the conversion performance of a sophisticated and high-power cross-polarization experiment given the dCP rf-pulse powers employed. We postulated above that the dCP rf-pulse sequence performance efficiency could be increased further by the implementation and optimization of shaped rf-pulses.A number of non-constant amplitude rf-pulses have previously been reported in the literature in the context of heteronuclear polarization transfer via a reservoir of 1H–1H dipolar order, and examples include the ADRF/ARRF rf-pulse sequence.20–28 Such rf-pulse sequences are known to be efficient with respect to the transfer of polarization between heteronuclear spins but are more difficult to implement technically compared with the more conventional dCP rf-pulse sequence.19,29
Non-constant amplitude 1H dCP rf-pulses slowly transform transverse 1H magnetization into 1H–1H dipolar order, and often require longer rf-pulse durations compared with spin-locking dCP rf-pulses. Shaped rf-pulses also allow finer control over the amplitude ωHdCP, and span a number of different B1-field strengths, which appears more beneficial for the preparation of 1H–1H dipolar order. As a result of the improved dCP rf-pulse sequence adiabaticity, it is possible that proton dipolar order is generated in larger quantities. The results demonstrated in Fig. 3 confirm this phenomenon (1H dCP rf-pulse durations, after optimization, are approximately one order of magnitude longer for shaped rf-pulses compared with spin-locking rf-pulses29) and indicate that, in suitable circumstances, the polarization transfer performance of the entire dCP rf-pulse sequence is most likely to be governed by the preparation efficiency of 1H–1H dipolar order (see Fig. 3a), since the improved shape of the 13C dCP rf-pulse has relatively little influence on the resulting 13C NMR signal integral (see Fig. 3b).
Furthermore, the inclusion of an adiabatic half-passage (AHP) additionally improved the transfer efficiency of the dCP rf-pulse sequence. AHP rf-pulses are well-known to: (i) achieve broadband excitation which is insensitive to spatially inhomogeneous magnetic fields (such as those present in our dDNP apparatus);32 and (ii) prepare non-negligible quantities of dipolar order.33 This improvement in efficiency could be due to either the additional broadband character of the 1H AHP rf-pulse excitation profile or the creation of surplus 1H–1H dipolar order. We did not investigate the effects of generating 1H–1H dipolar order by using 1H AHP rf-pulses further.
4.2. 13C-Labelling strategies
Sample I is a typical molecule of choice for dDNP experiments, due its biochemical importance and long spin–lattice relaxation time constants in solution. Sample II has recently been suggested as a novel lineshape polarimeter,34 and the location of 13C spin labels has previously been explored with respect to dDNP performances.35There is a major improvement in the observed dCP sequence transfer efficiency and final 13C polarization for sample II compared with sample I (see Table 3). This is likely associated with the fact that sample II has three directly-bound 1H nuclear spins, whereas the 1H nuclear spins in sample I are not directly bonded. The larger 1H–13C dipolar coupling constants likely lead to an improved dCP rf-pulse sequence performance efficiency, given the similar initial 1H polarizations (see Table 3).
During the 13C dCP rf-pulse, the 13C polarization build-up rate constants RCdCP for the samples employed in this study can also be used to infer information regarding the key factors of the supposed 1H–1H dipolar order to 13C magnetization conversion process. It is worth noting that in the cases of samples I and II, the value of RCdCP appears to be approximately independent of the molecular labelling position of the 13C site. This is an unexpected result, given the increased 1H–13C dipolar coupling constants in sample II. The 13C dCP rf-pulse build-up rate constant for sample II is therefore longer compared with our expectations, implying a slower than anticipated 1H–1H dipolar order to 13C polarization transfer.
At present, we do not have a clear explanation of the above described results. The proximity of the 13C-labelled nuclear site of interest to the molecularly bound 1H nuclear spins, and any resulting deleterious relaxation phenomena, may be responsible, and as such; this result could be related to the presence of the methyl group in samples I and II. These influences will be described in the next section.
It is of interest to compare the 13C polarization accrued per unit time for the dCP method with direct 13C DNP. After ∼30 minutes of directly polarizing 13C nuclear spins, 13C polarizations on the order of ca. ∼10–12% are typically achieved.10,12 This gives a 13C polarization per unit time of ∼0.33–0.4% min−1. For the dCP approach, the 13C polarization per unit time for the case of sample II is ∼3.2% min−1. This corresponds to an improvement in the level of 13C polarization by a factor of ∼8.0–9.7 coupled with a three-fold acceleration of the 13C polarization build-up rate constant, compared with the case of direct 13C DNP. As shown in the ESI,† improved results may also be achieved by implementing state-of-the-art and optimized CP rf-pulse sequences. Future dDNP experiments should take advantage of the described dCP and CP methodologies, and their multiple-step variants,12,31 if the experimental apparatus permits the implementation of such rf-pulse techniques.
4.3. Removal of methyl groups
The rapid rotational motion of methyl group moieties can act as strong relaxation sinks for nearby magnetic nuclear spins. The rotation of CH3 groups might remain active even at liquid helium temperatures.36 As such, it is possible that the methyl group of I can cause significant nuclear spin relaxation of 1H–1H dipolar order. In an effort to combat this detrimental relaxation phenomenon we investigated sample III, which lacks a methyl group moiety. Sample III is a common molecular target in zero and ultra-low field (ZULF) NMR experiments, and the combination of dDNP and ZULF NMR is very promising.The dCP rf-pulse sequence is more efficient for sample III than sample I under the same experimental conditions (see Table 3). This is likely to again be related to both the nature and number of the molecularly bound 1H nuclear spins. Although sample I has three attached protons, these 1H spins are somewhat distant to the 13C-labelled nuclear site, and hence the 1H–13C dipolar couplings are relatively weak. The 1H–13C dipolar coupling in sample III is much stronger due to the direct bonding nature of the spin constituents. The reduced number of directly bound 1H spins in this system also possibly explains the dCP rf-pulse sequence efficiency with respect to that of sample II. These results, and those discussed in Section 4.2, demonstrate that the presence of multiple directly bound 1H spins, and hence the number and strength of 1H–13C dipolar couplings, is advantageous for improving the dCP polarization transfer mechanism.
This result also indicates that the build-up of 1H–1H dipolar order, and presumed subsequent conversion to 13C magnetization, can tolerate (although with a loss of 13C NMR signal intensity) the influence of CH3 group relaxation sinks (possibly due to the sufficiently reduced presence of methyl group rotation at 1.2 K). This statement is qualified by comparing the 13C dCP rf-pulse build-up rate constant RCdCP for samples I and III, which is only a factor of ∼1.73 smaller than for sample III given the type of different molecular moieties near the 13C-labelled sites (see Table 4).
Although the 13C polarization for III was not recorded after 10 minutes of 1H DNP, the relatively favourable efficiency of the dCP rf-pulse sequence for this sample indicates that PdCP(13C) would have exceeded ∼20% under our experimental dDNP conditions (see Table 3).
As demonstrated in Fig. 4b, the mean lifetime of dipolar order Tzz for sample I is shorter than that for sample III. This result is attributed to the presence of the methyl group of [1-13C]sodium acetate, which acts as a relaxation sink for dipolar order established between pairs of proton spins in the sample. However, dipolar order lifetimes can span over many orders of magnitude between different samples.37 This is illustrated in Fig. 4b where dipolar order relaxation slows down by a factor of ∼2.6 upon removal of the methyl moiety in sample I.
Furthermore, the stretched mono-exponential nature of the dipolar order decay is not fully understood but is potentially thought to be related to interactions with nearby 1H spins in the DNP solvent. Such protons are located at a distribution of distances, and hence would explain why a stretched exponential function best fits the experimental data. The presented dipolar order lifetimes are typically shorter by 3 to 4 orders of magnitude than those of 1H polarization (typically more than an hour) under the same experimental conditions.38
4.4. RF-Powers and energies
The rf-power requirements for polarization transfer are dependent upon the rf-pulse sequence used, the molecular spin system of interest, the capabilities of the dDNP probe and potentially the choice of application. Table 2 shows the peak rf-pulse powers PdCP and energies EdCP required for heteronuclear polarization transfer via the dCP rf-pulse sequence under dDNP conditions for each molecular candidate employed in our series of experiments. The dCP rf-pulse sequence powers and energies used were compared to those of an optimized state-of-the-art CP experiment for each sample (see the ESI,† for more details).7–15In general, the peak power for the 13C dCP rf-pulse is up to ∼3.8 times lower than required for CP (see Table 2). The considerably lower peak 13C dCP rf-pulse power is exceedingly beneficial. Not only is there a significantly reduced tendency for detrimental rf-probe arcing incidents within the superfluid liquid helium bath but the decreased peak rf-pulse power requirement also ensures stable rf-pulse amplitudes at adequately long rf-pulse durations, which is highly advantageous in the case that the applied B1-field of the rf-probe is weak or unstable at higher peak rf-pulse powers. Furthermore, since the total rf-energy deposited is reduced, the temperature variation of the liquid helium reservoir is likewise decreased, and the thermal shock applied to the cold and rigid capacitors is much smaller, which extends the lifetime of rf-probe circuitry, and hence rf-probe functionality, in such a far from standard environment.
The longer durations of the shaped 1H dCP rf-pulses allow for the implementation of reduced rf-pulse amplitudes with respect to a conventional spin-locking rf-pulse,29 in suitable cases, and as a result; a comparison with CP provides an immediate benefit in terms of the deposited rf-energies. The peak 1H rf-powers of both rf-pulse sequences are the same order of magnitude, but the duration of the 1H dCP rf-pulse is easily a factor of ∼12.6–60.0 times shorter than utilized for sufficient CP.
Furthermore, since the CP rf-pulse sequence is only operational under the application of simultaneous rf-pulses, and since the peak rf-pulse powers for each rf-pulse sequence are mostly governed by the 13C rf-pulses, the peak rf-pulse power for the entire dCP rf-pulse sequence is significantly lower than that of CP, which is the main rf-pulse sequence characteristic responsible for rf-probe arcing.
Although the deposited rf-energies for shaped 1H dCP rf-pulses are greater (due to extended 1H rf-pulse durations) compared with conventional spin-locking rf-pulses,29 this result is outweighed by the increased generation of 1H–1H dipolar order and hence an improved performance efficiency of the dCP rf-pulse sequence compared to optimized CP.
Consequently, the reduced deposited 1H and 13C peak rf-powers required for a successful implementation of the dCP rf-pulse sequence provide a suitable alternative polarization transfer approach to CP, with the less strict optimization of the dCP rf-pulse sequence a strong benefit for potential applications in a clinical environment.
5. Conclusions
We have demonstrated that it is possible to improve 1H→13C polarization transfer techniques which operate by passing nuclear spin order through a bath of 1H–1H dipolar terms. The increased performance of the dCP rf-pulse sequence was achieved by adhering to the following protocols: (i) implementing shaped rf-pulses; (ii) employing suitable 13C spin labelling modifications; and (iii) utilizing molecular derivatives which lack a methyl group moiety. Enhancements of 13C NMR signal intensities were observed in all cases, with improvements approaching factors of ∼1.65 compared with previously reported results.29 The dCP rf-pulse sequence obtained 1H→13C polarization transfer efficiencies approaching ∼76% compared with the performance of a high-power and energy CP experiment. 13C polarization levels up to ∼32.1% were achieved by using the dCP rf-pulse sequence after 10 minutes of 1H DNP.The results of our experimental study indicate that 13C nuclear spins at high concentrations can be readily polarized by a simple and low power rf-pulse sequence which requires minimal rf-pulse optimization. The findings also suggest that the dCP rf-pulse sequence requires a number of proximal 1H spins in order to operate to a high polarization transfer efficiency. This effect is clearly highlighted for the case of sample II. This phenomenon is explored in more detail in a future publication.39 These observations are encouraging for the future usage of the dCP rf-pulse sequence, permitting hyperpolarization experiments conducive to larger sample volumes such as those compatible with clinical studies performed on human patients.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by ENS-Lyon, the French CNRS, Lyon 1 University, the European Research Council under the European Union's Horizon 2020 research and innovation program (ERC Grant Agreements No. 714519/HP4 all and Marie Skłodowska-Curie Grant Agreement No. 766402/ZULF). The authors gratefully acknowledge Bruker Biospin for providing the prototype dDNP polarizer, and particularly Marc Rossire and Marco Sacher for scientific and technical support. The authors additionally acknowledge Gerd Buntkowsky (Technische Universitat Darmstadt) who kindly communicated data associated with prior publications to us; Burkhard Luy (Karlsruhe Institute of Technology) for enlightening discussions; Catherine Jose and Christophe Pages for use of the ISA Prototype Service; Stéphane Martinez of the UCBL mechanical workshop for machining parts of the experimental apparatus; and the reviewers for their comments which led to the improvement of this article.References
- J. H. Ardenkjær-Larsen, B. Fridlund, A. Gram, G. Hansson, L. Hansson, M. H. Lerche, R. Servin, M. Thaning and K. Golman, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10158–10163 CrossRef PubMed.
- S. E. Day, M. I. Kettunen, F. A. Gallagher, D.-E. Hu, M. Lerche, J. Wolber, K. Golman, J. H. Ardenkjær-Larsen and K. M. Brindle, Nat. Med., 2007, 13, 1382–1387 CrossRef CAS PubMed.
- K. M. Brindle, S. E. Bohndiek, F. A. Gallagher and M. I. Kettunen, Magn. Reson. Med., 2011, 66, 505–519 CrossRef PubMed.
- S. J. Nelson, J. Kurhanewicz, D. B. Vigneron, P. E. Z. Larson, A. L. Harzstark, M. Ferrone, M. van Criekinge, J. W. Chang, R. Bok, I. Park, G. Reed, L. Carvajal, E. J. Small, P. Munster, V. K. Weinberg, J. H. Ardenkjær-Larsen, A. P. Chen, R. E. Hurd, L.-I. Odegardstuen, F. J. Robb, J. Tropp and J. A. Murray, Sci. Transl. Med., 2013, 5, 198 Search PubMed.
- J. M. O. Vinther, V. Zhurbenko, M. M. Albannay and J. H. Ardenkjær-Larsen, Solid State Nucl. Mag., 2019, 102, 12–20 CrossRef CAS PubMed.
- G. Hartmann, D. Hubert, S. Mango, C. C. Morehouse and K. Plog, Nucl. Instrum. Methods Phys. Res., Sect. A, 1973, 106, 9–12 CrossRef CAS.
- S. R. Hartmann and E. L. Hahn, Phys. Rev., 1962, 128, 204–205 CrossRef.
- A. Pines, M. Gibby and J. Waugh, Chem. Phys. Lett., 1972, 15, 373–376 CrossRef CAS.
- S. Jannin, A. Bornet, S. Colombo and G. Bodenhausen, Chem. Phys. Lett., 2011, 517, 234–236 CrossRef CAS.
- A. Bornet, R. Melzi, S. Jannin and G. Bodenhausen, Appl. Magn. Reson., 2012, 43, 107–117 CrossRef CAS.
- M. Batel, M. Krajewski, A. Däpp, A. Hunkeler, B. H. Meier, S. Kozerke and M. Ernst, Chem. Phys. Lett., 2012, 554, 72–76 CrossRef CAS.
- A. Bornet, R. Melzi, A. J. Perez Linde, P. Hautle, B. van den Brandt, S. Jannin and G. Bodenhausen, J. Chem. Phys. Lett., 2013, 4, 111–114 CrossRef CAS PubMed.
- B. Vuichoud, A. Bornet, F. de Nanteuil, J. Milani, E. Canet, X. Ji, P. Miéville, E. Weber, D. Kurzbach, A. Flamm, R. Konrat, A. D. Gossert, S. Jannin and G. Bodenhausen, Chem. – Eur. J., 2016, 22, 14696–14700 CrossRef CAS PubMed.
- M. Cavaillès, A. Bornet, X. Jaurand, B. Vuichoud, D. Baudouin, M. Baudin, L. Veyre, G. Bodenhausen, J.-N. Dumez, S. Jannin, C. Copéret and C. Thieuleux, Angew. Chem., Int. Ed., 2018, 130, 7575–7579 CrossRef.
- A. J. Perez Linde, PhD thesis, University of Nottingham, UK, 2009.
- X. Ji, A. Bornet, B. Vuichoud, J. Milani, D. Gajan, A. J. Rossini, L. Emsley, G. Bodenhausen and S. Jannin, Nat. Commun., 2017, 8, 13975 CrossRef CAS PubMed.
- K. W. Lipsø, S. Bowen, O. Rybalko and J. H. Ardenkjær-Larsen, J. Magn. Reson., 2017, 274, 65–72 CrossRef PubMed.
- J. Jeener and P. Broekaert, Phys. Rev., 1967, 157, 232–240 CrossRef CAS.
- H.-M. Vieth and C. S. Yannoni, Chem. Phys. Lett., 1993, 205, 153–156 CrossRef CAS.
- N. D. Kurur and G. Bodenhausen, J. Magn. Reson., Ser. A, 1995, 114, 163–173 CrossRef CAS.
- S. Emid, J. Konijnendijk, J. Smidt and A. Pines, Physica B + C, 1980, 100, 215–218 CrossRef CAS.
- S. Zhang, E. Stejskal, R. Fornes and X. Wu, J. Magn. Reson., Ser. A, 1993, 104, 177–179 CrossRef CAS.
- A. K. Khitrin, J. Xu and A. Ramamoorthy, J. Magn. Reson., 2011, 212, 95–101 CrossRef CAS PubMed.
- J. Jeener, R. Du Bois and P. Broekaert, Phys. Rev., 1965, 139, A1959–A1961 CrossRef.
- A. G. Redfield, Science, 1969, 164, 1015–1023 CrossRef CAS PubMed.
- D. E. Demco, J. Tegenfeldt and J. S. Waugh, Phys. Rev. B: Condens. Matter Mater. Phys., 1975, 11, 4133–4151 CrossRef CAS.
- M. Kunitomo, H. Hatanaka and T. Hashi, Phys. Lett. A, 1974, 49, 135–136 CrossRef CAS.
- J.-S. Lee and A. K. Khitrin, J. Chem. Phys., 2008, 128, 114504 CrossRef PubMed.
- S. J. Elliott, S. F. Cousin, Q. Chappuis, O. Cala, M. Ceillier, A. Bornet and S. Jannin, Magn. Reson., 2020, 1, 89–96 CrossRef.
- A. Bornet, J. Milani, B. Vuichoud, A. J. Perez Linde, G. Bodenhausen and S. Jannin, Chem. Phys. Lett., 2014, 602, 63–67 CrossRef CAS.
- A. Bornet, A. Pinon, A. Jhajharia, M. Baudin, X. Ji, L. Emsley, G. Bodenhausen, J. H. Ardenkjær-Larsen and S. Jannin, Phys. Chem. Chem. Phys., 2016, 18, 30530–30535 RSC.
- K. J. Harris, A. Lupulescu, B. E. J. Lucier, L. Frydman and R. W. Schurko, J. Magn. Reson., 2012, 224, 38–47 CrossRef CAS PubMed.
- P. Berthault, H. Desvaux, G. Le Goff and M. Pétro, Chem. Phys. Lett., 1999, 314, 52–56 CrossRef CAS.
- S. J. Elliott et al. , Submitted, 2021.
- P. Niedbalski, C. Parish, A. Kiswandhi, Z. Kovacs and L. Lumata, J. Phys. Chem. A, 2017, 121, 3227–3233 CrossRef CAS PubMed.
- L. Latanowicz, Concepts Magn. Reson., Part A, 2005, 27A, 38–53 CrossRef CAS.
- S. F. J. Cox, S. F. J. Read and W. T. Wenckebach, J. Phys. C, 1977, 10, 2917 CrossRef CAS.
- Q. Stern et al. In Press, 2021.
- S. J. Elliott et al. , In Preparation, 2021.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cp00429h |
| ‡ Gerd Buntkowsky, Personal Communication. |
| This journal is © the Owner Societies 2021 |

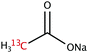
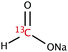
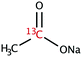
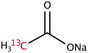
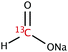
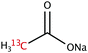
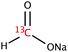
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif)