Open Access Article
Open Access ArticleExcited state dipole moments and lifetimes of 2-cyanoindole from rotationally resolved electronic Stark spectroscopy†
Marie-Luise
Hebestreit
 *a,
Hilda
Lartian
a,
Christian
Henrichs
*a,
Hilda
Lartian
a,
Christian
Henrichs
 a,
Ralf
Kühnemuth
b,
W. Leo
Meerts
a,
Ralf
Kühnemuth
b,
W. Leo
Meerts
 c and
Michael
Schmitt
c and
Michael
Schmitt
 a
a
aHeinrich-Heine-Universität, Institut für Physikalische Chemie I, D-40225 Düsseldorf, Germany. E-mail: Marie-Luise.Hebestreit@uni-duesseldorf.de; Tel: +49 211 81 13219
bHeinrich-Heine-Universität, Lehrstuhl für Molekulare Physikalische Chemie, D-40225 Düsseldorf, Germany
cRadboud University, Institute for Molecules and Materials, Felix Laboratory, Toernooiveld 7c, 6525 ED Nijmegen, The Netherlands
First published on 19th March 2021
Abstract
The permanent dipole moments of 2-cyanoindole (cyanoindole = CNI) in its ground and lowest excited singlet states have been determined from rotationally resolved electronic Stark spectroscopy under jet-cooled conditions. From the orientation of the transition dipole moment and the geometry changes upon electronic excitation the lowest excited singlet state could be shown to be of Lb-symmetry. The general statement, that the La-state has the larger permanent dipole moment of the two lowest excited singlet states, will be challenged in this contribution. On the basis of the different electronic nature of the first excited singlet state the behavior of 2-, 3-, 4- and 5-CNI is discussed. The excited state lifetime of isolated 2-CNI in the gas phase has been determined to be 9.4 ns. This value is compared to the excited state lifetime in ethyl acetate solution of 2.6 ns, which was quantified with a Strickler–Berg analysis. Using water as solvent shortens the 2-CNI lifetime to <40 ps. The reason for this drastic shortening is discussed in detail. Additionally, the rotationally resolved electronic spectrum of 2-CNI(1-d1) has been measured and analyzed.
1 Introduction
Of the three natural fluorescing amino acids, tryptophan, tyrosine, and phenylalanine, the indole chromophore derived tryptophan has the most prominent position, due to its large oscillator strength that makes it an ideal candidate for in vivo spectroscopic and microscopic fluorescence techniques.1–4 Distance dependent fluorescence techniques like Förster resonance energy transfer (FRET) are used to study conformers of proteins and their time-dependent folding mechanisms. Frequently, synthetic fluorescence probes are used which have large oscillator strength and good photochemical stability. These, however, are often linked to the protein via flexible linkers for sampling reasons, causing additional flexibility, which is not present in the native protein. Therefore, the use of intrinsic fluorescence probes, with only slight modifications has found widespread interest. Recently, cyano-substituted tryptophanes have been proposed as ideal FRET and photo-induced electron transfer (PET) chromophores.5 Furthermore, they find increasing interest for fluorescence imaging and microscopy applications.6 The photophysical properties of this new class of modified intrinsic fluorophores can be studied, using the isolated chromophore in the gas-phase, and compared with the results of solution data.A thorough theoretical study of the excited state dynamics of all structural isomers of cyanoindole has been presented by Abou-Hatab and Matsika.7 Their calculations predict that “in absorption the Lb-excited state is lower in energy than the La-state for all positional isomers in the gas phase and in solution”. The La and Lb nomenclature for the close-lying excited singlet ππ*-states goes back to the original proposal by Platt for cata-condensed aromatics8,9 and has later been extended to the case of indole by Weber.10 However, if the C2-axis, present in cata-condensed aromatics is not preserved,‡ the excited states of same multiplicity start to mix and the labeling becomes increasingly difficult. Nonetheless, it seems useful to maintain this nomenclature, because it is widely used in the fluorescence spectroscopy community and can be connected to observables like excited state dipole moments, transition dipole moment orientation, and oscillator strengths. In general, La-states have the larger oscillator strengths than the respective Lb-states. This can be traced back to the fact that Lb-states are composed of two orbital contributions (LUMO ← HOMO−1 and LUMO+1 ← HOMO), which are out-of-phase and approximately cancel each other out, while the La-state is characterized by a more or less pure LUMO ← HOMO excitation. The fluorescence lifetime of indole and substituted indoles depends on the energy gap between the La- and Lb-state. The smaller the gap, the shorter the lifetime.11 It is widely believed that the La-state has the larger permanent dipole moment of the two lowest excited singlet states, but we will challenge this general statement in the present contribution.
Gas phase spectroscopy can add important contributions to the photo-physical properties of the CNIs, since it is free from solute–solvent interactions, except for the cases in which definite cluster sizes are used to study the subtle interaction between the chromophore and the first solvating molecule(s). This has up to now been performed for the 3-CNI–water12–14 and the 5-CNI–water15 clusters, respectively.
In the present study we analyze the changes of geometry and dipole moments upon electronic excitation of 2-CNI and its N-deuterated isotopologue, using a combination of rotationally resolved fluorescence (Stark) spectroscopy, time-correlated single photon counting (TCSPC) and ab initio calculations on the coupled-cluster level of theory.
2 Experimental section
2.1 Experimental procedures
2.2 Quantum chemical calculations
Structure optimizations were performed employing a Dunning's correlation-consistent polarized valence triple zeta (cc-pVTZ) basis set from the Turbomole library.22,23 The equilibrium geometries of the electronic ground and the lowest excited singlet states were optimized using the approximate coupled cluster singles and doubles model (CC2) employing the resolution-of-the-identity (RI) approximation.24–26 For the structure optimizations spin-component scaling (SCS) modifications to CC2 were taken into account.27 Vibrational frequencies and zero-point corrections to the adiabatic excitation energies were obtained from numerical second derivatives using the NumForce script.282.3 Fits of the rovibronic spectra using evolutionary algorithms
Evolutionary strategies are perfect instruments to automatically fit rotationally resolved electronic spectra, even of large molecules with congested spectra.29–32 They are powerful tools to handle complex multi-parameter optimizations and to find the global optimum and are inspired by evolutionary processes in nature, which are based on reproduction, mutation and selection. For the analysis of high resolution spectra in the present work, we apply the covariance matrix adaption evolution strategy (CMA-ES), which is described in detail elsewhere.31,33,343 Results
3.1 Computational results
The optimization of the geometry of 2-CNI yields a planar structure in the ground and the lowest excited singlet state both with genuine CC2 as well as with SCS-CC2. The molecular parameters (rotational constants A, B, and C in both electronic states, the inertial defects ΔI, the angle θ of the transition dipole moment with the inertial a-axis, the angle θD of the permanent dipole moment with the inertial a-axis, and the zero-point corrected origin frequency ν0) are compiled in Table 1 and are compared to the experimental results, which are described in detail in Section 3.2. Contrary to the case of 5-CNI, both generic CC2 and its spin-component scaled variant SCS-CC2 reveal that the lowest excited singlet state is the Lb-state.35 However, the Lb- and La-states are considerably stronger mixed in the CC2 calculations than in the spin-component scaled variant.| Theory SCS-CC2 | Theory CC2 | Experiment | |||
|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | ||
| A′′/MHz | 3850.97 | 3853.67 | 3852.31(3) | ||
| B′′/MHz | 695.28 | 697.25 | 698.76(1) | ||
| C′′/MHz | 588.95 | 590.42 | 591.57(1) | ||
| ΔI′′/amu Å2 | 0.00 | 0.01 | −0.14(2) | ||
| μ a′′/D | 3.23 | 3.25 | ±3.57(1) | ||
| μ b′′/D | 1.50 | 1.50 | ±1.00(1) | ||
| μ′′/D | 3.56 | 3.58 | 3.71(1) | ||
| θ D′′/° | −24.94 | −24.79 | ±15.7 | ||
| A′/MHz | 3736.30 | 3792.22 | 3777.31 | 3759.26 | 3746.10(4) |
| B′/MHz | 687.69 | 687.32 | 686.76 | 691.10 | 691.84(2) |
| C′/MHz | 580.79 | 581.86 | 581.11 | 583.78 | 584.14(2) |
| ΔI′/amu Å2 | 0.00 | 0.00 | 0.00 | 0.00 | −0.22(3) |
| μ a′/D | 4.52 | 3.18 | 5.01 | 3.22 | ±5.16(1) |
| μ b′/D | 2.01 | 1.33 | 2.12 | 1.34 | ±0.71(1) |
| μ′/D | 4.95 | 3.44 | 5.44 | 3.49 | 5.21(1) |
| θ D′/° | −24.01 | −22.65 | −22.94 | −22.66 | ±7.8 |
| ΔA/MHz | −114.67 | −58.75 | −76.36 | −94.41 | −106.21(1) |
| ΔB/MHz | −7.59 | −7.96 | −10.49 | −6.51 | −6.91(1) |
| ΔC/MHz | −8.16 | −7.09 | −9.31 | −6.64 | −7.43(1) |
| ΔνLorentz/MHz | — | — | — | — | 16.94(1) |
| τ/ns | — | — | — | — | 9.40(1) |
| θ/° | +36.4 | −15.3 | +48.1 | −9.4 | ±45.9(20) |
| ν 0/cm−1 | 34229.35 | 37812.63 | 34123.46 | 37476.13 | 33422.63(5) |
A clear indication for the Lb-state as lowest excited singlet state is the orientation of the calculated transition dipole moment with the inertial a-axis (Table 1 and Fig. 1), which is +36.4° (+48.1°) at the SCS-CC2 (CC2) level of theory and which points away from the nitrogen atom. To make a better comparison to indole, the transition dipole moment (TDM) is rotated to the inertial axis-frame of indole. An angle of +13° with the inertial a-axis of indole is obtained.
Another indication for the Lb-state are the main contributions to the excitation to the lowest excited singlet state at the SCS-CC2 level of theory: 70.3% LUMO ← HOMO−1 + 12.2% LUMO+1 ← HOMO and 8.8% LUMO ← HOMO excitation. The adiabatically second state in contrast is an almost pure LUMO ← HOMO transition (80%), what is typical for an La-state. The results are shown in Fig. 2. As mentioned above, the results for the CC2 calculations are much less clear: 47.4% LUMO ← HOMO−1 + 5.4% LUMO+1 ← HOMO and 38.4% LUMO ← HOMO excitation for the S1-state, pointing to a considerable amount of mixing of the lowest excited singlet states.
 | ||
| Fig. 2 Molecular orbitals of 2-CNI with the percentage of the S1 (Lb) and S2 (La) excitations according to SCS-CC2/cc-pVTZ calculations. | ||
The zero-point corrected adiabatic excitation energy to the lowest excited singlet state amounts to 34229.35 cm−1 (34123.46 cm−1) at the SCS-CC2 (CC2) level of theory.
The value of the permanent dipole moment is 3.23 D (3.25 D) in the ground state and 4.95 D (5.44 D) in the lowest excited singlet state at the SCS-CC2 (CC2) level of theory with larger amounts of the a-component of the dipole vector. The orientation of the calculated permanent dipole moments with the inertial a-axis stay nearly constant.
3.2 Experimental results
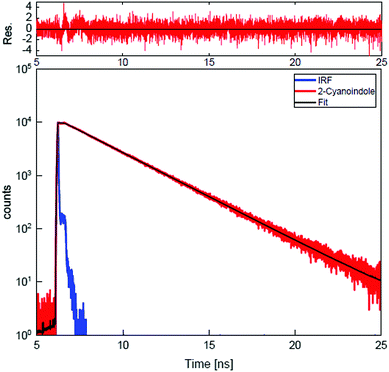
Trace (a) of Fig. 3 shows the rotationally resolved electronic spectrum of the electronic origin of 2-CNI at 33422.63(5) cm−1, which is set to 0 on the scale of the figure. In trace (b) a zoomed part of the spectrum, along with the best fit at zero field is shown. Trace (c) presents the same spectral range at a field strength of 638.90 V/cm. Experimental spectra are shown as black traces, simulations, using the best fit parameters, as red traces. The selection rules ΔM = 0 for the Stark spectrum are held, because the electric field in the chosen set-up is parallel to the polarization of the plane of the exciting light. The spectrum was fit using a CMA-ES and the molecular parameters are compiled in Table 1 and are compared to the results of the SCS-CC2/ccpVTZ calculations. The analysis of the ab-hybrid origin band yields an angle of ±45.9° with the inertial a-axis. By comparison to the ab initio computed value of +36.4° (+48.1°) at the SCS-CC2 (CC2) level of theory the indeterminacy of the TDM orientation can be resolved. A Lorentzian linewidth of 16.94 ± 0.01 MHz to the Voigt line profile using a fixed Gaussian contribution of 18.70 MHz corresponds to an excited state lifetime in the gas phase of 9.40 ± 0.01 ns. The Gaussian contribution was determined from a Voigt fit to a few single rovibronic lines with the boundary condition of equal Gaussian contribution to each line. The lifetime increases to 10.38 ± 0.08 ns upon N-deuteration. A very good agreement between the experimental and the simulated spectrum, using the best fit parameters has been obtained.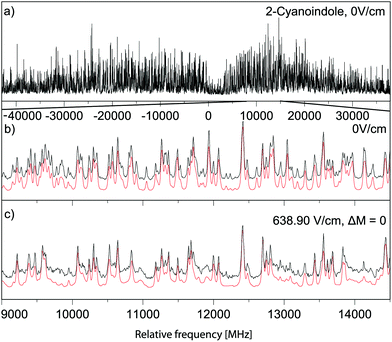 | ||
| Fig. 3 Rotationally resolved electronic spectrum of the electronic origin of 2-CNI, along with a simulation with the best CMA-ES fit parameters. | ||
The rotationally resolved spectrum of the electronic origin of the deuterated isotopologue 2-CNI(1-d1) at 33426.61(1) cm−1 is shown in Fig. S1 of the ESI† the molecular parameters, obtained from the CMA-ES fit, are compiled in Table S1 of the ESI.† The angle θ of the TDM in 2-CNI(1-d1) is slightly smaller than in 2-CNI.
Furthermore, lifetime measurements using TCSPC in ethyl acetate and water as solvents were performed. A single exponential decay with an excited state lifetime of 2.60 ± 0.03 ns is found for ethyl acetate as solvent. The decay curve along with the best fit of a single exponential decay and the residue for 2-CNI in ethyl acetate is shown in Fig. 4. The fluorescence decay in water as solvent is considerably faster (<40 ps). For the deuterated isotopologue the excited state lifetime in ethyl acetate as solvent is also 2.60 ± 0.03 ns and in D2O <40 ps.
4 Discussion
4.1 Excited state structure
The planarity in both electronic states is shown by the small negative inertial defects of 2-CNI in the electronic ground state with a value of −0.14 amu Å2 and in the lowest electronically excited state with a value of −0.22 amu Å2, what is slightly more negative than in the ground state. A comparison of the inertial defect to those of other cyanoindoles shows a close agreement to that of 5-CNI (Table S2 of the ESI†). Equivalent to the increase of the moment of inertia, all rotational constants decreases upon electronic excitation (Table 1).The bond lengths changes upon electronic excitation are quite symmetric, with respect to the molecular plane and along the a-axis of the molecule. This is a clear indication for an Lb-state. In contrast to the symmetric changes upon excitation to the Lb-state are the much more irregular changes upon the excitation to the La-state. These results were obtained from ref. 36, where the properties of the three lowest singlet electronic states (ground, La-and Lb-state) of the chromophore indole have been calculated with second-order approximate coupled-cluster theory (CC2) within the resolution-of-the-identity approximation. The difference in the structural changes upon electronic excitation for the La-and the Lb-state and also for the geometry changes upon electronic excitation obtained from the SCS-CC2/cc-pVTZ optimized structure can be seen in Fig. 5. A Kraitchman analysis37 has been performed for a determination of the H-atom position of the pyrrolic NH group using the rotational constants of the ND-deuterated isotopologue in both electronic states. The results, which are described in detail in the ESI,† support the interpretation of the Lb-state as electronically excited state.
 | ||
| Fig. 5 Changes of the bond lengths upon electronic excitation from SCS-CC2/cc-pVTZ calculations. (a) Schematic geometry changes of the parent chromophore indole upon excitation to the La-state, adapted from ref. 36. (b) Schematic geometry changes of the parent chromophore indole upon excitation to the Lb-state, adapted from ref. 36. (c) Geometry changes of 2-CNI in pm upon electronic excitation to the lowest excited singlet state, obtained from the SCS-CC2/cc-pVTZ optimized structures. Comparison to trace (a) and (b) show that this must be the Lb-state. For more details see text. | ||
4.2 Excited state lifetime
The excited state lifetime of isolated 2-CNI in the gas phase (9.40 ± 0.01 ns) is shorter than that of the parent indole (16.4 ns).38–41 Compared to the other cyanoindoles, which were investigated in our group using HRLIF (3-, 4-, 5-CNI), the excited state lifetimes in the gas phase and in solution are the shortest for 2-CNI.14,21,35For the N-deuterated isotopologue of 2-CNI, the excited state lifetime is nearly 1 ns (10.38 ± 0.08 ns) longer than for the undeuterated isotopologue. In most cases, deuteration at the chromophore leads to an increase of the excited state lifetime, like for indole. In indole, the excited state lifetime increases slightly from 16.4 ns to 17.2 ns upon N-deuteration.40 Another example is isolated 3-CNI, in which an increase from 9.8 ns to 14.8 ns is observed.14 In contrast to these results, the experimentally determined excited state lifetime for isolated 4-CNI (11.0 ns) is nearly twice as large compared to the three investigated deuterated species, where the lifetime was remarkably independent on the position of deuteration.21
The lifetimes of 2-, 3-, 4-, and 5-CNI in solution are in general much shorter than in the gas phase. A single exponential decay with a lifetime of 2.60 ± 0.03 ns is found in the lifetime measurement of 2-CNI in ethyl acetate solution, which shortens to <40 ps in water as solvent.
The pure radiative lifetime of an isolated molecule in the gas phase can be approximated by the following expression:21,42
 | (1) |
| f = fυ′υ′′/qυ′υ′′ | (2) |
Using eqn (1) and the SCS-CC2/cc-pVTZ calculated oscillator strength and transition wavelength, and the FC factors from eqn (2), we compute a pure radiative lifetime τr of 33 ns for isolated 2-CNI after excitation of the vibrationless origin. This value can be converted into an excited state fluorescence lifetime τf, if the fluorescence quantum yield Θf is known. Unfortunately, the fluorescence quantum yield of 2-CNI in the gas phase is not known, and cannot be determined easily experimentally from molecular beam experiments. From the quotient of the experimental fluorescence lifetime τexpf (9.4 ns) and the calculated radiative lifetime τr (33 ns), the fluorescence quantum yield Θf of 2-CNI in the gas phase can be approximated to 0.3.
The fluorescence lifetime for emission from the lowest singlet state solutions of ethylacetate (EA) and water are much shorter than for the isolated molecule. A Strickler–Berg (SB) analysis43 has been chosen for quantification of the solution lifetime. The pure radiative lifetime of a fluorescing molecule in a solvent of index of refraction n is approximated by:
 | (3) |
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) f−3〉−1 is the inverse of the expectation value of
f−3〉−1 is the inverse of the expectation value of ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) f−3. The integral
f−3. The integral 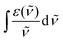 has been approximated from a fit of the steady-state absorption spectrum, which was scaled in order to bring it into agreement with discrete values of the molar extinction coefficient ε published in ref. 44. The integral of the lowest energy Gaussian has been used to evaluate the integral in eqn (3). The inverse of the expectation value of
has been approximated from a fit of the steady-state absorption spectrum, which was scaled in order to bring it into agreement with discrete values of the molar extinction coefficient ε published in ref. 44. The integral of the lowest energy Gaussian has been used to evaluate the integral in eqn (3). The inverse of the expectation value of ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) f−3 is approximated by the cube of the wavenumber of maximum fluorescence emission, as proposed by Cohen et al.:45
f−3 is approximated by the cube of the wavenumber of maximum fluorescence emission, as proposed by Cohen et al.:45 | (4) |
The steady state fluorescence spectra of 2-CNI in water are too weak to successfully apply the SB analysis. From the TCSPC experiment however, we have the value for the lifetime of <40 ps in water. What makes the lifetime of 2-CNI in water so short? For the isolated binary 3-CNI–water cluster we found a reduction of the lifetime from 9.8 ns to 3.6 ns, already for the first water molecule attached to the chromophore.14 Of course we tried to measure the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water cluster using rotationally resolved LIF spectroscopy. Although the origin of the cluster was found at 32984.5 cm−1, i.e. red-shifted by 438.1 cm−1 relative to the monomer band in a low resolution LIF spectrum in a free jet (laser bandwidth 0.2 cm−1, Doppler width ≈0.01 cm−1 ≡ 300 MHz) and showed strong fluorescence (cf. Fig. S5 of the ESI†), we failed to record a rovibronically resolved spectrum (laser bandwidth 800 kHz, Doppler width 18.7 MHz). Instead of a well-resolved spectrum like for the monomer, a very broad onset of fluorescence is observed. The respective trace, along with the iodine spectrum and the vibronically resolved spectrum is shown in Fig. S5 of the ESI.† The only reason for this broadening (often encountered before) is a large Lorentz contribution to the line profile from a short lifetime. This additional broadening brings the peak intensity of individual rovibronic transitions below the noise level of our experimental set-up. From a convolution of the experimental spectrum with Lorentz profiles with varying bandwidths, we found this limit to be at 500 MHz equivalent to a lifetime of 300 ps. We suggest that this is the upper limit for the lifetime of the 1
1 water cluster using rotationally resolved LIF spectroscopy. Although the origin of the cluster was found at 32984.5 cm−1, i.e. red-shifted by 438.1 cm−1 relative to the monomer band in a low resolution LIF spectrum in a free jet (laser bandwidth 0.2 cm−1, Doppler width ≈0.01 cm−1 ≡ 300 MHz) and showed strong fluorescence (cf. Fig. S5 of the ESI†), we failed to record a rovibronically resolved spectrum (laser bandwidth 800 kHz, Doppler width 18.7 MHz). Instead of a well-resolved spectrum like for the monomer, a very broad onset of fluorescence is observed. The respective trace, along with the iodine spectrum and the vibronically resolved spectrum is shown in Fig. S5 of the ESI.† The only reason for this broadening (often encountered before) is a large Lorentz contribution to the line profile from a short lifetime. This additional broadening brings the peak intensity of individual rovibronic transitions below the noise level of our experimental set-up. From a convolution of the experimental spectrum with Lorentz profiles with varying bandwidths, we found this limit to be at 500 MHz equivalent to a lifetime of 300 ps. We suggest that this is the upper limit for the lifetime of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water cluster of 2-CNI. It thus seems that the short fluorescence lifetime of 2-CNI in water solution is caused already by the first water molecule attached to the chromophore. The effect is similar to the one observed in 3-CNI–water, however it is considerably larger.
1 water cluster of 2-CNI. It thus seems that the short fluorescence lifetime of 2-CNI in water solution is caused already by the first water molecule attached to the chromophore. The effect is similar to the one observed in 3-CNI–water, however it is considerably larger.
The fact that the lifetime of 2-CNI increases upon deuteration at the pyrrolic NH-site, points to a tunneling dominated process, which is responsible for the lifetime. Sobolewski et al.46 have shown for indole and phenol that a conical intersection between the primarily excited ππ*-state and the repulsive πσ*-state induces a barrier at the S1 surface, which is the reason for the strong isotope effect in phenol. The smaller isotope effect in indole and the cyanoindoles is caused by a higher barrier towards dissociation through the ππ*–πσ* conical intersection compared to phenol. Water complexation at the phenolic OH group has the effect of shifting the πσ*-state up in energy and removing the second conical intersection with the ground state. For indole however, the energy πσ*-state decreases considerably along the azine NH coordinate, already for weak electric fields as exerted by a nearby water molecule, resulting in shorter lifetimes for the water cluster.47 Our results for the cyanoindoles imply that the relative position of ππ* and πσ*-state is strongly modulated by the position of the cyano group leading to the very different lifetimes of the isolated water clusters as well as the ultrashort lifetimes in water as solvent.
4.3 Dipole moments
For 3-CNI, a decrease from 5.90 D to 5.35 D upon electronic excitation was observed for the permanent dipole moment.14 For 4-CNI the experimentally determined dipole moment increases from 6.31 D to 8.92 D21 and for 5-CNI from 7.14 D to 8.17 D48 upon electronic excitation.
It is commonly believed that the change of the permanent dipole moment upon excitation of the singlet state can be used as a sensitive indicator of the electronic nature of this excited state. La-states tend to have larger dipole moments than Lb-states. This would imply that the excited state of 2-CNI is of La-character, since the experimentally determined dipole moment agrees well with the one calculated for the S1, which is larger than for the S2 state. This has been confirmed both for SCS-CC2 and for CC2 calculations. However, geometry changes, TDM orientation, and orbital contributions clearly indicate that the experimentally observed state is the Lb-state. With special regard to the experimentally determined transition dipole moment orientation, the angle of the reorientation of the inertial axis system upon excitation and the leading contributions to the excitation to the lowest excited singlet state, the lowest excited singlet state for 3-CNI was specified as Lb-, and for 4- and 5-CNI as La-character. In these examples, an increase of the permanent dipole moment was observed for excitation to La-states, while for the Lb-states the permanent dipole moment in general was equal or even smaller than in the electronic ground state.
Regarding the 5-CNI case, there is a discrepancy between the analysis of Abou-Hatab and Matsika7 and our results,35 which shows that an assignment using the original La and Lb notation becomes meaningless, when the states mix considerably.§ The assumption of the observed state being Lb is supported by the orbital character of the natural transition orbitals7 and the leading contributions of the configurations to the excited state from the SCS-CC2 wavefunctions.35 On the other hand, the orientation of the transition dipole moment, is more “La-like”, and the large increase of the permanent dipole moment in 5-CNI upon excitation (more than 1 D) also points to an La-state, like the geometry changes from a Franck–Condon analysis.49
As it is well known, the sign of the angle θ between the TDM and the a-axis cannot be determined directly from the experimentally recorded rotationally resolved spectra. From the intensities of the rovibronic transitions of the spectrum it is only possible to obtain the projection of the TDM onto the main inertial axes.
To obtain the sign of this angle in the method of Hougen and Watson the wave functions of the excited state are rotated into the coordinate system of the ground state after diagonalization of the respective Hamiltonian. For planar molecules it is possible to determine the angle of the reorientation of the inertial axis system upon electronic excitation θT:50
 | (5) |
| In | 2-CNI | 3-CNI | 4-CNI | 5-CNI | |
|---|---|---|---|---|---|
| a Ref. 40. b Ref. 14. c Ref. 21. d Ref. 35. e Ref. 44. f Excitation of indole in ethyl acetate (EA) at 284 nm. g Excitation of 3-CNI in EA at 283 nm. h Excitation of 4-CNI in EA at 302 nm. i Excitation of 5-CNI in EA at 295 nm. | |||||
| θ/° | +38a | +13 | +35b | −15c | −12d |
| τ/ns | 16.4a | 9.4 | 9.8b | 11.0c | 12d |
| τ(EA)solv/ns | 3.1cf | 2.6 | 2.6cg | 4.7ch | 3.6ci |
| τ(H2O)solv/ns | 4.5e | <0.04 | <0.05e | 9.2c | 0.3e |
5 Conclusions
It is commonly believed that La-states of substituted indoles tend to have larger dipole moments than the respective Lb-states.51,52 Apart from the change of the permanent dipole moment, the transition dipole moment orientation, the angle of the reorientation of the inertial axis system upon excitation, and the leading contributions to the excitation to the lowest excited singlet state are used for identification of these states. With regard to these factors the lowest excited singlet state for 3-CNI was specified as being of Lb-character, and for 4- and 5-CNI as La-character. The present investigation of 2-CNI concludes that the change of the permanent dipole moment upon excitation of the singlet state can not be used as a sensitive indicator of this excited state in this case, because the results of the experimentally determined dipole moments and the calculated ones would imply that the excited state of 2-CNI has mainly La-character. The conclusion that the lowest excited singlet state of 2-CNI is the Lb-state, has been made from the experimentally determined orientation of the transition dipole moment as well as the main molecular orbital contributions to the excitation to the lowest excited singlet state. Also the geometry changes upon electronic excitation could be identified as an Lb-like distortion of 2-CNI. The assignment to the Lb-state as lowest excited singlet state is the same as for 3-CNI, but contrary to 4- and 5-CNI. A confirmation of the conclusion that substitution in the pyrrole ring leads to the Lb-state as lowest excited singlet state is possible, when the missing 6- and 7-CNI will be investigated. Compared to the theoretical analysis of Abou-Hatab and Matsika7 there is only discrepancy regarding the 5-CNI case, which shows that an assignment using the original La and Lb notation becomes meaningless, when the states mix considerably. Their optimizations of the lowest excited singlet state in the gas phase converged to the Lb minimum for all structural isomers, except for 4-CNI which converged to the La. This result is in accordance with our conclusion for the lowest excited singlet state of 4-CNI.21 In the 5-CNI case, where their calculations predict an Lb-state as lowest excited singlet state, our experimental data point to an La-state: the orientation of the transition dipole moment, which is more “La-like”, the large increase of the permanent dipole moment upon excitation and the geometry changes from a Franck–Condon analysis.49 In the 2-CNI case there is no discrepancy. Moreover, the fluorescence lifetime could be extracted from the Lorentzian contribution to the Voigt line profile using a fixed Gaussian contribution. The increasing lifetime upon deuteration points to a tunneling process along the NH coordinate. The results from the gas phase were compared to the excited state lifetimes using TCSPC. For quantification of the solution lifetime in ethyl acetate a Strickler–Berg analysis has been chosen. A drastic reduction of the excited state lifetime upon solvation in water has been found, where the steady state spectra of 2-CNI were to weak to apply the Strickler–Berg analysis. The short fluorescence lifetime of 2-CNI in water solution is caused already by the first water molecule attached to the chromophore what is in accord with the broad onset of fluorescence signal instead of a well-resolved spectrum by using high resolution laser induced fluorescence spectroscopy for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water cluster of 2-CNI. The πσ*-state shifts to lower energies along the azine NH coordinate by the electric field exerted by the first water molecule, resulting in shorter lifetimes for the water cluster.
1 water cluster of 2-CNI. The πσ*-state shifts to lower energies along the azine NH coordinate by the electric field exerted by the first water molecule, resulting in shorter lifetimes for the water cluster.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Peter Gilch for helpful discussions and advice about limitations of the Strickler–Berg relationship. Financial support of the Deutsche Forschungsgemeinschaft via grant SCHM1043/14-1 is gratefully acknowledged. Computational support and infrastructure was provided by the “Center for Information and Media Technology” (ZIM) at the Heinrich-Heine-University Düsseldorf (Germany).References
- P. Talukder, S. Chen, B. Roy, P. Yakovchuk, M. M. Spiering, M. Alam, M. M. Madathil, C. Bhattacharya, S. J. Benkovic and S. M. Hecht, Biochemistry, 2015, 54, 7457–7469 CrossRef CAS PubMed.
- B. N. Markiewicz, D. Mukherjee, T. Troxler and F. Gai, J. Phys. Chem. B, 2016, 210, 936–944 CrossRef PubMed.
- M. R. Hilaire, I. A. Ahmed, C.-W. Lin, H. Jo, W. F. DeGrado and F. Gai, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6005–6009 CrossRef CAS PubMed.
- D. Mukherjee, L. I. O. Rodriguez, M. R. Hilaire, T. Troxler and F. Gai, Phys. Chem. Chem. Phys., 2016, 20, 2527–2535 RSC.
- I. A. Ahmed, J. M. Rodgers, C. Eng, T. Troxler and F. Gai, Phys. Chem. Chem. Phys., 2019, 21, 12843–12849 RSC.
- A. Acharyyaa, I. A. Ahmed and F. Gai, Methods Enzymol., 2020, 639, 191–215 Search PubMed.
- S. Abou-Hatab and S. Matsika, J. Phys. Chem. B, 2019, 123, 7424–7435 CrossRef CAS PubMed.
- J. R. Platt, J. Chem. Phys., 1949, 17, 484–495 CrossRef CAS.
- J. N. Murrell, The Theory of the Electronic Spectra of Organic Molecules, Chapman and Hall, London, 1971 Search PubMed.
- G. Weber, Biochem. J., 1960, 75, 335–345 CrossRef CAS PubMed.
- A. A. Sukhodola, J. Appl. Spectrosc., 2008, 75, 527–531 CrossRef CAS.
- A. Ahn, A. Min, C. J. Moon, J. H. Lee and M. Y. Choi, Chem. Phys. Lett., 2014, 616–617, 55–60 CrossRef CAS.
- A. Min, A. Ahn, C. J. Moon, J. H. Lee, M. Y. Choi and S. K. Kim, Chem. Phys. Lett., 2014, 614, 263–268 CrossRef CAS.
- M. Schneider, M.-L. Hebestreit, M. M. Lindic, H. Parsian, A. Y. Torres-Boy, L. Alvarez-Valtierra, W. L. Meerts, R. Kühnemuth and M. Schmitt, Phys. Chem. Chem. Phys., 2018, 20, 23441–23452 RSC.
- A. Min, C. J. Moon, A. Ahn, J. H. Lee, S. K. Kim and M. Y. Choi, Chem. Phys. Lett., 2016, 658, 63–70 CrossRef CAS.
- S. Gerstenkorn and P. Luc, Atlas du spectre d'absorption de la molécule d'iode 14800-20000 cm−1, CNRS, Paris, 1986 Search PubMed.
- M. Schmitt, Habilitation, Heinrich-Heine-Universität, Math. Nat. Fakultät, Düsseldorf, 2000 Search PubMed.
- M. Schmitt, J. Küpper, D. Spangenberg and A. Westphal, Chem. Phys., 2000, 254, 349–361 CrossRef CAS.
- J. Wilke, M. Wilke, W. L. Meerts and M. Schmitt, J. Chem. Phys., 2016, 144, 044201 CrossRef PubMed.
- K. Wohlfart, M. Schnell, J. U. Grabow and J. Küpper, J. Mol. Spectrosc., 2014, 247, 119–121 CrossRef.
- M.-L. Hebestreit, H. Lartian, M. Schneider, R. Kühnemuth, A. Y. Torres-Boy, S. Romero-Servin, J. A. Ruiz-Santoyo, L. Alvarez-Valtierra, W. L. Meerts and M. Schmitt, J. Mol. Struct., 2020, 1210, 127992 CrossRef CAS.
- R. Ahlrichs, M. Bär, M. Häser, H. Horn and C. Kölmel, Chem. Phys. Lett., 1989, 162, 165–169 CrossRef CAS.
- J. T. H. Dunning, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef.
- C. Hättig and F. Weigend, J. Chem. Phys., 2000, 113, 5154–5161 CrossRef.
- C. Hättig and A. Köhn, J. Chem. Phys., 2002, 117, 6939–6951 CrossRef.
- C. Hättig, J. Chem. Phys., 2002, 118, 7751–7761 CrossRef.
- A. Hellweg, S. Grün and C. Hättig, Phys. Chem. Chem. Phys., 2008, 10, 1159–1169 RSC.
- P. Deglmann, F. Furche and R. Ahlrichs, Chem. Phys. Lett., 2002, 362, 511–518 CrossRef CAS.
- W. L. Meerts, M. Schmitt and G. Groenenboom, Can. J. Chem., 2004, 82, 804–819 CrossRef CAS.
- W. L. Meerts and M. Schmitt, Phys. Scr., 2005, 73, C47–C52 CrossRef.
- W. L. Meerts and M. Schmitt, Int. Rev. Phys. Chem., 2006, 25, 353–406 Search PubMed.
- M. Schmitt and W. L. Meerts, in Handbook of High Resolution Spectroscopy, ed. M. Quack and F. Merkt, John Wiley and Sons, 2011 Search PubMed.
- A. Ostenmeier, A. Gawelcyk and N. Hansen, in Parallel Problem Solving from Nature, PPSN III, ed. Y. Davidor, H.-P. Schwefel and R. Männer, Springer, Berlin/Heidelberg, 1994 Search PubMed.
- N. Hansen and A. Ostermeier, Evol. Comput., 2001, 9, 159–195 CrossRef CAS PubMed.
- O. Oeltermann, C. Brand, B. Engels, J. Tatchen and M. Schmitt, Phys. Chem. Chem. Phys., 2012, 14, 10266–10270 RSC.
- C. Brand, J. Küpper, D. W. Pratt, W. L. Meerts, D. Krügler, J. Tatchen and M. Schmitt, Phys. Chem. Chem. Phys., 2010, 12, 4968–4997 RSC.
- J. Kraitchman, Am. J. Phys., 1953, 21, 17 CrossRef CAS.
- G. Berden, W. L. Meerts and E. Jalviste, J. Chem. Phys., 1995, 103, 9596–9606 CrossRef CAS.
- J. Küpper, D. W. Pratt, W. L. Meerts, C. Brand, J. Tatchen and M. Schmitt, Phys. Chem. Chem. Phys., 2010, 12, 4980–4988 RSC.
- G. A. Bickel, D. R. Demmer, E. A. Outhouse and S. C. Wallace, J. Chem. Phys., 1989, 91, 6013 CrossRef CAS.
- J. W. Hager and S. C. Wallace, J. Phys. Chem., 1983, 87, 2121 CrossRef CAS.
- R. S. F. Peter and W. Atkins, Molecular Quantum Mechanics, Oxford University Press, New York, 2011 Search PubMed.
- S. J. Strickler and R. A. Berg, J. Chem. Phys., 1962, 37, 814–822 CrossRef CAS.
- M. R. Hilaire, D. Mukherjee, T. Troxler and F. Gai, Chem. Phys. Lett., 2017, 685, 133–138 CrossRef CAS PubMed.
- B. Cohen, C. E. Crespo-Hernández and B. Kohler, Faraday Discuss., 2004, 127, 137–147 RSC.
- A. L. Sobolewski, W. Domcke, C. Dedonder-Lardeux and C. Jouvet, Phys. Chem. Chem. Phys., 2002, 4, 1093–1100 RSC.
- C. Dedonder-Lardeux, C. Jouvet, S. Perun and A. Sobolewski, Phys. Chem. Chem. Phys., 2003, 5, 5118 RSC.
- J. Wilke, M. Wilke, C. Brand, W. L. Meerts and M. Schmitt, ChemPhysChem, 2016, 17, 2736–2743 CrossRef CAS PubMed.
- B. Stuhlmann, A. Gräßle and M. Schmitt, Phys. Chem. Chem. Phys., 2014, 16, 899–905 RSC.
- J. T. Hougen and J. K. G. Watson, Can. J. Phys., 1965, 43, 298–320 CrossRef CAS.
- E. H. Strickland, J. Horwitz, E. Kay, L. M. Shannon, M. Wilchek and C. Billups, Biochem. J., 1970, 9, 4914–4921 CrossRef CAS PubMed.
- D. Brisker-Klaiman and A. Dreuw, ChemPhysChem, 2015, 16, 1695–1702 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cp00097g |
| ‡ In indole a pseudo-C2-axis can be defined, which runs through C(2) and bisects the C(5)–C(6) bond. This pseudo-symmetry axis nearly coincides with the inertial a-axis of indole, with the deviation to be attributed to the mass difference of N(1)–H and C(3)–H. |
| § The leading contributions of the configurations to the excited states shows that the adiabatically lowest S1 state is characterized by 0.56 (LUMO ← HOMO) − 0.51 (LUMO ← HOMO−1) + 0.44 (LUMO+1 ← HOMO−1) + 0.42 (LUMO+1 ← HOMO) excitation. The S2-state is comprised of 0.77 (LUMO ← HOMO) + 0.44 (LUMO ← HOMO−1) − 0.29 (LUMO+1 ← HOMO) − 0.26 (LUMO ← HOMO−2). A pure La-state would show as pure HOMO–LUMO excitation. |
| This journal is © the Owner Societies 2021 |