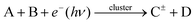Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pickup and reactions of molecules on clusters relevant for atmospheric and interstellar processes
Michal
Fárník
 *a,
Juraj
Fedor
*a,
Juraj
Fedor
 a,
Jaroslav
Kočišek
a,
Jaroslav
Kočišek
 a,
Jozef
Lengyel
a,
Jozef
Lengyel
 b,
Eva
Pluhařová
b,
Eva
Pluhařová
 a,
Viktoriya
Poterya
a and
Andriy
Pysanenko
a
a,
Viktoriya
Poterya
a and
Andriy
Pysanenko
a
aJ. Heyrovský Institute of Physical Chemistry, v.v.i., The Czech Academy of Sciences, Dolejškova 2155/3, 182 23 Prague, Czech Republic. E-mail: michal.farnik@jh-inst.cas.cz
bChair of Physical Chemistry, Department of Chemistry, Technical University of Munich, 85748 Garching, Germany
First published on 19th January 2021
Abstract
In this perspective, we review experiments with molecules picked up on large clusters in molecular beams with the focus on the processes in atmospheric and interstellar chemistry. First, we concentrate on the pickup itself, and we discuss the pickup cross sections. We measure the uptake of different atmospheric molecules on mixed nitric acid–water clusters and determine the accommodation coefficients relevant for aerosol formation in the Earth's atmosphere. Then the coagulation of the adsorbed molecules on the clusters is investigated. In the second part of this perspective, we review examples of different processes triggered by UV-photons or electrons in the clusters with embedded molecules. We start with the photodissociation of hydrogen halides and Freon CF2Cl2 on ice nanoparticles in connection with the polar stratospheric ozone depletion. Next, we mention reactions following the excitation and ionization of the molecules adsorbed on clusters. The first ionization-triggered reaction observed between two different molecules picked up on the cluster was the proton transfer between methanol and formic acid deposited on large argon clusters. Finally, negative ion reactions after slow electron attachment are illustrated by two examples: mixed nitric acid–water clusters, and hydrogen peroxide deposited on large ArN and (H2O)N clusters. The selected examples are discussed from the perspective of the atmospheric and interstellar chemistry, and several future directions are proposed.
1 Introduction
Clusters of atoms and molecules have been investigated in molecular beams since the conception of the molecular beam technique and became a very broad field covered in many reviews and textbooks.1–8 One of the original motivations to study clusters stems from the aspiration to understand bulk materials based on the properties of the individual atoms and molecules, since the clusters can bridge the gas and condensed phases.5,6 From another perspective, clusters can provide insight into the solvent effects in chemistry and physics at a molecular level.9,10 In addition, clusters have their own intrinsic properties, different from the gas and condensed phases, that give rise to the cluster-specific physics and chemistry,4,6,11 which find many applications, e.g., in nanocatalysis.12,13 The modern cluster research spans different disciplines from physics and chemistry, to biology, materials science and astronomy.14The clusters in molecular beams can be doped by other molecules and used as nanomatrices or nanoreactors for molecular spectroscopy and chemical reactions. In this respect, large helium clusters known as He-nanodroplets have been largely exploited and represent a rather special case. They can efficiently capture molecules and provide an extremely cold (0.37 K)15 and superfluid environment for molecular spectroscopy and reactions, as outlined in many studies and recent reviews.16–21
The pickup of molecules and their reactions especially on large argon clusters were implemented to investigate the solvent effect on reaction dynamics, and the pioneering technique was termed Cluster Isolated Chemical Reactions (CICR) and reviewed by Mestdagh et al. more than 20 years ago.22 For the product detection, most of the early CICR experiments considered chemiluminescence, namely the prototypical reaction of the barium atom with N2O yielding the chemiluminescent product BaO.23 Further reactions of Ba with CH4, Cl2, O2, CO2 and SF6 were studied.24–29 The CICR technique has been further exploited for investigations of reactions,30 spectroscopy,31 photoinduced Ca + HBr and Ca + CH3F reactions32,33 or more recent investigation of solvation dynamics.34,35 The rare gas clusters were also implemented in the investigation of the solvent effect on the photodissociation dynamics in Buck's group, where the molecules were deposited in the clusters and dissociated by UV lasers.36–38 Our present work, in a sense, follows up on the CICR methodology, and extends it towards applications in atmospheric and interstellar chemistry.
From the point of view of astrochemistry, ice and dust grains offer surfaces for chemical reactions in the environment of interstellar medium, where collisions between molecules are infrequent and inefficient for reactions to proceed.39–41 The processes on interstellar ice and dust nanoparticles can be mimicked by reactions of molecules on clusters in molecular beam experiments. From the perspective of atmospheric chemistry, the cluster research can provide detailed insight into aerosol formation and reactions.42,43 Despite huge experimental and theoretical efforts, aerosol particles still belong to the least known players in atmospheric chemistry.42,44–47 Nevertheless, their importance is unquestionable; among many other effects, they provide surfaces for heterogeneous chemistry and photochemistry.44,48,49 Even small pure water clusters were found to mediate chemistry in the atmosphere.50 Clusters in molecular beams can represent proxies for atmospheric and interstellar aerosols in laboratory experiments. Early studies by Castleman's group investigated atmospherically relevant phenomena, such as nitric acid solvation and dissolution in water clusters,51,52 and further cluster ion reactions.53,54 Many recent examples exist in the literature exploiting mass spectrometry,43,55–57 spectroscopy,58–61 photodissociation62,63 and other molecular beam experiments with atmospherically and astrochemically relevant clusters.43,64
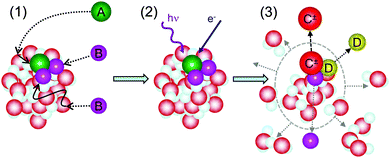
Here, we focus on the special experiments with clusters, where different molecules are deposited on the clusters in pickup processes, and subsequently, reactions between the molecules on/in the clusters can be triggered by photons or electrons, as illustrated schematically in Fig. 1. We review some of our work and the related experiments of other groups in the broader context of atmospheric and interstellar chemistry. In the spirit of the perspective article, we also report some unpublished results, and suggest future directions in this field.
The article is organized as follows: Section 2 briefly describes our cluster beam apparatus. The pickup technique is introduced in Section 3, and exploited for cluster characterization (Section 3.1), and for mimicking the processes of atmospheric aerosol nucleation (Section 3.2) using pure ice nanoparticles (Section 3.2.1) and nitric acid–water clusters (Section 3.2.2). In Section 3.3, pickup on clusters relevant for interstellar chemistry is briefly discussed. The molecules adsorbed on large clusters can coagulate, which is discussed in Section 3.4. Reactions on the clusters are discussed in Section 4, starting with photodissociation (Section 4.1). Further, we show examples of reactions on the clusters between the adsorbed molecules after their ionization or excitation by photons or electrons (Section 4.2). Finally, we review some reactions initiated by slow electron attachment (Section 4.3). We close by summarizing the general observations and possible future directions.
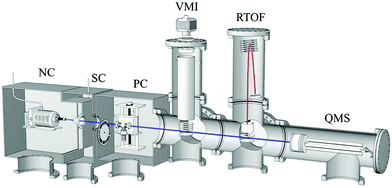
2 CLUB experiment
The cluster pickup experiments reviewed here were mostly performed using the cluster beam apparatus (CLUB) in Prague (Fig. 2).43 The CLUB is based on the apparatus used originally for photodissociation experiments in the group of U. Buck,36 which was extended in our laboratory as a versatile tool for various cluster experiments. The different experiments can be done with the same cluster beam probing the same species and thus the results can provide information about different properties of the same clusters.The clusters are produced in the nozzle chamber (NC) by supersonic continuous expansion through a conical nozzle (typically 50–130 μm in diameter, full opening angle of 30 deg, length 2 mm). The nozzle can be attached to different cluster sources. A simple rare gas cluster source consists essentially of a tube kept at a controlled temperature via external heating and cooling. More sophisticated sources are used for evaporation of liquid and solid samples such as polycyclic hydrocarbons and simple biomolecules. A special case is the recently developed source of microsolvated molecules described in ref. 65. Fig. 2 shows the CLUB with the sketch of a water cluster source in the NC. The continuous expansion allows achieving stable and well reproducible cluster generation, and the conical nozzle shape ensures efficient clustering and sufficient beam densities along the entire cluster beam path of about 2 m.
The cluster beam passes through a skimmer into a differentially pumped scattering chamber (SC), which is used as the first pickup chamber in the present pickup experiments. In the next vacuum chamber (PC), further two pickup cells can be inserted in the cluster beam. In Fig. 2, only one pickup cell is drawn, which has recently been developed for the pickup of hydrogen peroxide.66 A pseudorandom chopper is located in this vacuum chamber as well, which allows for accurate velocity measurements of the clusters, exploited in the cross section measurements discussed below.67–69
The following chamber is devoted to the photodissociation experiments using velocity map imaging (VMI). Our VMI setup and its first implementation in the CLUB are described elsewhere.62,70 Various UV lasers are available in the laboratory for the photolysis and resonance enhanced multiphoton ionization (REMPI) of the photodissociation fragments, and IR lasers can be also used for IR-UV experiments.71
The reflectron time-of-flight mass spectrometer (RTOF) in the next chamber was first described in ref. 72 and 73. The clusters can be ionized by different methods: electron ionization introduced in the first studies,72,73 multiphoton processes using UV lasers,74,75 or by the special method of Na-doping and subsequent electron photodetachment.72,76,77 We use also electron attachment and negative cluster ion mass spectrometry.78 These different ionization methods can produce quite different mass spectra from the same clusters, and information about the original neutral clusters can be recovered by their combination. An example represents the investigation of the mixed nitric oxide–water clusters by electron ionization, Na-doping, and electron attachment.79
Some of our mass spectrometry experiments have recently been reviewed.43 The power of the combination of VMI with high-resolution RTOF mass spectrometry has been illustrated for a few examples investigating the photodissociation dynamics of molecules in clusters, where the complex information about the nature of the clusters could not be revealed by VMI or mass spectrometry experiments alone.80,81
A quadrupole mass spectrometer (QMS) with continuous electron ionization is mounted in the last chamber. It can be used to monitor the molecular beam, or to measure the beam velocity by the time-of-flight in connection with the chopper in the PC chamber.67,68
3 Uptake of molecules on clusters
The pickup technique has been used since the 1980s82 for doping clusters with different molecules as the cluster beam passes through a diluted gas and the gas molecules are embedded in the clusters upon sticking collisions. It has been exploited especially in experiments with He-nanodroplets, where numerous examples exist, e.g., for spectroscopy,15,18,19,83,84 reactions,20,21,85–90 and generation of clusters91 in the cold superfluid environment of He-nanodroplets. Interesting applications have recently been developed, such as the decoration and visualization of superfluid vorticies with atoms in He-nanodroplets.92,93Here we focus on large water clusters (H2O)N (ice nanoparticles) and other molecular species such as, e.g., nitric acid–water clusters. We often perform analogous pickup experiments also with the ArN clusters for comparison of the molecular cluster with the inert environment of the rare-gas cluster. The pickup of molecules on ArN was reviewed in the context of the CICR method.22 Huisken and Stemmler used ArN for infrared spectroscopy of adsorbed methanol clusters.94 The pickup of hydrogen halide molecules on large rare gas clusters was implemented in Buck's group for the photodissociation studies.36–38 Among molecular clusters, especially water clusters were used for uptake of different molecules.95–98 A rather special example is the pickup of Na atoms,99,100 which was exploited for mass spectrometry and size resolved investigations of large water, ammonia, and other clusters.101–104
An interesting implementation of the pickup technique was exploited to develop the Exchange Metal Cluster source (EXMEC):105 argon clusters were first generated by supersonic expansion, which then entered the pickup cell with metal vapor or other atoms or molecules; the picked up species eventually replaced the Ar atoms in the cluster completely, and clusters composed purely of metal atoms or other species then left the source. This method was used to produce small neutral clusters of several different elements and compounds, especially alkali halide clusters.106–109 Water clusters were used in these experiments as the primary clusters to investigate the solvation effects on alkali-halides.110,111
3.1 Pickup cross section
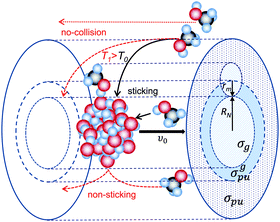
In a pickup experiment, the cluster flies through a very diluted gas and collides with the gas molecules, which can stick to the cluster. This process can be characterized by the cluster pickup cross section. The pickup cross section depends on the cluster size, i.e., on its geometrical cross section, and on the size of the molecule (see Fig. 3). The geometrical pickup cross section can be written as σgpu = π·(RN + rm)2, where RN and rm are the radius of the cluster and the molecule, respectively. However, the actual pickup cross section can be significantly larger than the geometrical one, since the molecules can be attracted to the cluster due to the long-range interactions between the cluster and the molecules even from distances outside of the area corresponding to σgpu. On the other hand, the pickup cross section includes also the sticking probability of the molecule to the cluster, which can be smaller than one. In addition, the collision and sticking probabilities depend on the collision energy. Thus the pickup is a complex process and some of these points were investigated in the references reviewed in this section and will be discussed below.One of the early implementations of the pickup technique was to determine the size of the large neutral clusters.112 The measurement of the cluster size in a molecular beam is difficult. Mass spectrometry involves ionization, which can cause severe cluster fragmentation.43,76 Even the threshold photoionization, which can be relatively soft, can cause fragmentation in some cases.113,114 For large clusters, other methods were used, e.g. high energy electron diffraction,115–119 helium atom diffraction,120 and cluster beam scattering by a buffer gas.121 Some special methods were developed, such as elastic scattering122–124 and sodium doping with subsequent photodetachment of electrons,101,102 the latter of which was later proposed as the sizer for neutral weakly bound ultrafine aerosol particles.103,104 Clusters can be also deflected according to their sizes in strong inhomogeneous electric fields due to their size-dependent dipole moments and polarizability,125–127 which is, however, limited to small complexes. None of the above methods is universal. Elastic scattering is fairly elaborate and applicable only for small clusters, and Na-doping worked nicely with water and ammonia,101 and some other clusters,103,104,113 yet, it turned out not to be applicable for clusters containing molecules reacting with the solvated electron.72,77,79,128
Alternatively, the cluster size can be determined by the methods based on the pickup. Historically, there have been essentially two approaches: in the first one, sticking collisions of molecules with clusters lead to momentum transfer, and thus by measuring the variations of the average cluster beam velocity with the number of collisions (the pickup gas pressure) the mean cluster size could be determined.112 The second approach relies on measuring the Poisson distributions of the adsorbed molecules.129 We have compared and combined these two methods for the pickup of different molecules on ArN clusters, and established a method to determine the pickup cross sections.67
The original methods112,129 required certain knowledge about the pickup cross section σpu in order to evaluate the cluster mean size ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) . The simplest assumption was to put the pickup cross section of a large cluster equal to its geometrical pickup cross section σgpu, which could be simply determined from the hard sphere geometrical cross section σg assuming a spherical cluster shape and close packing of the molecules in the cluster. This assumption reduced the unknown parameters to only the cluster size
. The simplest assumption was to put the pickup cross section of a large cluster equal to its geometrical pickup cross section σgpu, which could be simply determined from the hard sphere geometrical cross section σg assuming a spherical cluster shape and close packing of the molecules in the cluster. This assumption reduced the unknown parameters to only the cluster size ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) , and thus
, and thus ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) could be determined from the experiments. However σpu could be significantly different from σgpu, as discussed below. Already in their original paper, Cuvellier et al.112 used collision dynamics simulations to account for a more realistic attractive interaction between the molecule and the ArN cluster yielding a correction to σgpu. Another theoretical study of the role of long-range forces in the cluster–molecule collisions for ArN resulted also in the capture cross sections larger than the geometrical ones.130 In our experiments,67 we worked with the ArN clusters, for which the mean size
could be determined from the experiments. However σpu could be significantly different from σgpu, as discussed below. Already in their original paper, Cuvellier et al.112 used collision dynamics simulations to account for a more realistic attractive interaction between the molecule and the ArN cluster yielding a correction to σgpu. Another theoretical study of the role of long-range forces in the cluster–molecule collisions for ArN resulted also in the capture cross sections larger than the geometrical ones.130 In our experiments,67 we worked with the ArN clusters, for which the mean size ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) was known from the expansion conditions based on semiempirical Hagena's scaling laws,131–133 confirmed experimentally by helium atom diffraction.120 The independent determination of
was known from the expansion conditions based on semiempirical Hagena's scaling laws,131–133 confirmed experimentally by helium atom diffraction.120 The independent determination of ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) for ArN clusters allowed the evaluation of the pickup cross sections σpu from our velocity measurements, and we have confirmed our measured cross sections with molecular dynamics (MD) and Monte-Carlo simulations.67
for ArN clusters allowed the evaluation of the pickup cross sections σpu from our velocity measurements, and we have confirmed our measured cross sections with molecular dynamics (MD) and Monte-Carlo simulations.67
The water (H2O)N clusters represent another example, for which the mean cluster size ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) was determined by independent experiments.101 Thus, we could measure the pickup cross sections for uptake of different molecules on large water clusters.68 The pickup cross sections were measured for ArN and (H2O)N in the size range of
was determined by independent experiments.101 Thus, we could measure the pickup cross sections for uptake of different molecules on large water clusters.68 The pickup cross sections were measured for ArN and (H2O)N in the size range of ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 50–600 and compared to MD simulations as well as to an analytical model.69 The pickup cross sections for both ArN and (H2O)N were larger than the corresponding geometrical pickup cross sections; however, there was also a difference in the cross section size dependence. While the pickup cross sections for ArN were consistent with their assumed spherical shapes, the cross sections of (H2O)N departed from the spherical cluster model for
≈ 50–600 and compared to MD simulations as well as to an analytical model.69 The pickup cross sections for both ArN and (H2O)N were larger than the corresponding geometrical pickup cross sections; however, there was also a difference in the cross section size dependence. While the pickup cross sections for ArN were consistent with their assumed spherical shapes, the cross sections of (H2O)N departed from the spherical cluster model for ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≥ 300 towards larger values. The larger cross sections could be justified by assuming irregular shapes. These clusters occur in supersonic expansions under the conditions where large clusters are generated by smaller cluster coagulation rather than by the addition of the individual molecules. Thus the pickup experiments delivered not only the cluster pickup cross sections σpu but also indirect information about the cluster shape.69
≥ 300 towards larger values. The larger cross sections could be justified by assuming irregular shapes. These clusters occur in supersonic expansions under the conditions where large clusters are generated by smaller cluster coagulation rather than by the addition of the individual molecules. Thus the pickup experiments delivered not only the cluster pickup cross sections σpu but also indirect information about the cluster shape.69
The velocity measurements for cluster size determination were implemented also by Kresin et al.134 They used the momentum transfer in a single sticking collision, which was proved by the mass spectrometry of isotopically labeled species, to determine the neutral (H2O)N and (D2O)N cluster sizes of smaller clusters (N ≤ 50). These results confirmed the extensive water cluster fragmentation after electron ionization observed previously,76 emphasizing the conclusion that the simple mass spectrometry might be misleading for cluster size determination.
3.2 Mimicking atmospheric aerosol nucleation
![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 260 for various atmospherically relevant molecules (H2O, HCl, HBr, NO, NO2, CH3OH, CH3CH2OH, CH4).68 The pickup cross sections for the uptake of H2O molecules on (H2O)N are useful for modeling of the uptake of water molecules on aqueous particles, although the homogeneous nucleation of water is unlikely to occur in the Earth's atmosphere. The spherical cluster radius corresponds to RN = r0·N1/3, where r0 ≈ 2 Å could be obtained from the theoretical calculations.135 The corresponding geometrical pickup cross section was σgpu = 685 Å2; however, the experimentally measured cross section was significantly larger, σpu = 1020 ± 100 Å2. This was due to the attractive interaction between the water molecule and the cluster as shown by our MD simulations using realistic interaction potentials for water, which yielded a cross section of 950 Å2.68
≈ 260 for various atmospherically relevant molecules (H2O, HCl, HBr, NO, NO2, CH3OH, CH3CH2OH, CH4).68 The pickup cross sections for the uptake of H2O molecules on (H2O)N are useful for modeling of the uptake of water molecules on aqueous particles, although the homogeneous nucleation of water is unlikely to occur in the Earth's atmosphere. The spherical cluster radius corresponds to RN = r0·N1/3, where r0 ≈ 2 Å could be obtained from the theoretical calculations.135 The corresponding geometrical pickup cross section was σgpu = 685 Å2; however, the experimentally measured cross section was significantly larger, σpu = 1020 ± 100 Å2. This was due to the attractive interaction between the water molecule and the cluster as shown by our MD simulations using realistic interaction potentials for water, which yielded a cross section of 950 Å2.68
The pickup cross section depends also on the molecular mass and collision velocity. Our measured pickup cross section for the uptake of H2O molecules on ice nanoparticles was larger than the simple geometrical cross section by at least a factor of 1.5. However, it can be further enhanced under atmospheric conditions by lower collision speed (∼500 ms−1) compared to our cluster beam experiment (v0 = 1450 ms−1). This could enhance the pickup cross section by another factor of ≈1.4.68 The fact that the real pickup cross sections can be larger than the geometrical ones by a factor of more than 2 can have a pronounced effect in atmospheric aerosol modeling, where the geometrical cross sections are often considered. Finally, it should be noted that the evaluation of the pickup cross section required the assumption of sticking collisions and the total momentum transfer. These assumptions were justified for H2O molecules by our MD simulations; however, similar simulations would be needed for the other molecules picked up by the ice nanoparticles.
In the context of the aerosol nucleation, it is interesting to compare our pickup cross sections with the recent work of Signorell's group.136 Their Laval-nozzle experiments allowed determination of the monomer association rates k1,N for H2O molecules with water clusters of different sizes N. In their work,136k1,N for the clusters above the dimer up to N = 30 were compared to the free molecular collision rates obtained assuming the geometrical cross section of spherical clusters. The measured association rates were 4–5 times larger than the collision rates. This was attributed to the long range forces, e.g., dipole–dipole interactions and dispersion forces. Although we cannot provide a direct quantitative comparison between the association rates and our pickup cross sections, there is a clear correlation between them, and the larger measured association rates qualitatively agree with our pickup cross sections being larger than the geometrical ones.
From another point of view, the uptake of water molecules on mass selected protonated water clusters was measured by Zamith et al.137–140 The mass-spectrometric experiments with the protonated clusters provided the advantage of the known single cluster size, and the sticking collisions could be determined from the mass spectra. On the other hand, they were limited to smaller clusters and somewhat higher collision energies. For their upper size limit of N = 250, the measured pickup cross section was ≈800 Å2. Comparison with our neutral (H2O)N pickup cross section suggests that the charge on the clusters does not seem to increase the pickup cross section, which calls for theoretical simulations.
Despite an extensive effort in the atmospheric aerosol research, there are still many open questions concerning the new particle formation and aerosol growth. Nucleation theories rely on macroscopic properties and gas kinetic arguments.141–143 Classical nucleation theory (CNT), which represents the current benchmark,143 can provide reasonable water vapor nucleation rates in some cases;143–146 nevertheless, empirical corrections and modifications along with refined theories are implemented in some cases to account for the differences between the experimental results and CNT predictions.136,147,148 From the perspective of the molecular-level approach, the nucleation starts with the individual molecules colliding and sticking to small clusters. Typically, hard sphere collision rates and geometrical cross sections are considered in the modeling.46,149,150 The experiments mentioned above demonstrate that the actual pickup cross sections can be significantly larger, and more realistic values should be utilized.
In the experiments with the large (H2O)N clusters above, many collisions in the pickup cell led to multiple pickup and momentum transfer processes, and the dependence of the cluster velocity on the pickup pressure was measured. Here, the experimental method was modified. Relatively small (HNO3)M·(H2O)N clusters with an average size of ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) ≈ 2
≈ 2 ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 6 passed through the pickup gas at a fixed pressure, which was carefully controlled so that the clusters of mean size underwent only a few (about 3) collisions in the pickup cell. Under these conditions, we could distinguish two mass peak series in the mass spectra measured after the pickup of a molecule X: one corresponding to the pure protonated cluster fragments (HNO3)m·(H2O)nH+, and the other one corresponding to the fragments with the adsorbed molecules X·(HNO3)m·(H2O)nH+. Both series had almost identical character and differed only by their intensities. This is illustrated for our test case of methanol, X = CH3OH, in Fig. 4. The top panel of Fig. 4a shows the spectrum of the pure (HNO3)M·(H2O)N clusters analyzed elsewhere.51,72 The middle panel of Fig. 4b shows the spectrum after the pickup of methanol. Clearly, new weaker series are observed in Fig. 4b as indicated, which are analogous to (HNO3)m·(H2O)nH+ fragment ions and shifted by the mass of methanol m/z = 32. By velocity measurements performed with the QMS tuned to different fragment ions, we could prove that (HNO3)m·(H2O)nH+ fragments corresponded to the clusters which passed through the pickup cell without undergoing sticking collisions with CH3OH. The velocities measured for the CH3OH·(HNO3)m·(H2O)nH+ fragments were significantly lower than those of (HNO3)m·(H2O)nH+ fragments. Clearly, the former fragments originated from the clusters which picked up the CH3OH molecule and were slowed down by the momentum transfer. The later velocities of (HNO3)m·(H2O)nH+ fragments were shifted only very little to lower values with respect to the velocities measured without any methanol gas in the pickup cell. This small shift was attributed to non-sticking collisions of a grazing character. Therefore, we could assign the CH3OH·(HNO3)m·(H2O)nH+ and (HNO3)m·(H2O)nH+ fragments to the clusters which underwent sticking and non-sticking collisions, respectively, and the pickup probability could be determined from the abundance ratio of the corresponding fragment series. The method was demonstrated for methanol pickup on (HNO3)m·(H2O)nH+ clusters,158 and several other molecules X discussed below showed qualitatively the same behavior.159 These experiments represented the proof of concept for measuring the pickup probabilities, which can be useful for atmospheric aerosol modeling.
≈ 6 passed through the pickup gas at a fixed pressure, which was carefully controlled so that the clusters of mean size underwent only a few (about 3) collisions in the pickup cell. Under these conditions, we could distinguish two mass peak series in the mass spectra measured after the pickup of a molecule X: one corresponding to the pure protonated cluster fragments (HNO3)m·(H2O)nH+, and the other one corresponding to the fragments with the adsorbed molecules X·(HNO3)m·(H2O)nH+. Both series had almost identical character and differed only by their intensities. This is illustrated for our test case of methanol, X = CH3OH, in Fig. 4. The top panel of Fig. 4a shows the spectrum of the pure (HNO3)M·(H2O)N clusters analyzed elsewhere.51,72 The middle panel of Fig. 4b shows the spectrum after the pickup of methanol. Clearly, new weaker series are observed in Fig. 4b as indicated, which are analogous to (HNO3)m·(H2O)nH+ fragment ions and shifted by the mass of methanol m/z = 32. By velocity measurements performed with the QMS tuned to different fragment ions, we could prove that (HNO3)m·(H2O)nH+ fragments corresponded to the clusters which passed through the pickup cell without undergoing sticking collisions with CH3OH. The velocities measured for the CH3OH·(HNO3)m·(H2O)nH+ fragments were significantly lower than those of (HNO3)m·(H2O)nH+ fragments. Clearly, the former fragments originated from the clusters which picked up the CH3OH molecule and were slowed down by the momentum transfer. The later velocities of (HNO3)m·(H2O)nH+ fragments were shifted only very little to lower values with respect to the velocities measured without any methanol gas in the pickup cell. This small shift was attributed to non-sticking collisions of a grazing character. Therefore, we could assign the CH3OH·(HNO3)m·(H2O)nH+ and (HNO3)m·(H2O)nH+ fragments to the clusters which underwent sticking and non-sticking collisions, respectively, and the pickup probability could be determined from the abundance ratio of the corresponding fragment series. The method was demonstrated for methanol pickup on (HNO3)m·(H2O)nH+ clusters,158 and several other molecules X discussed below showed qualitatively the same behavior.159 These experiments represented the proof of concept for measuring the pickup probabilities, which can be useful for atmospheric aerosol modeling.
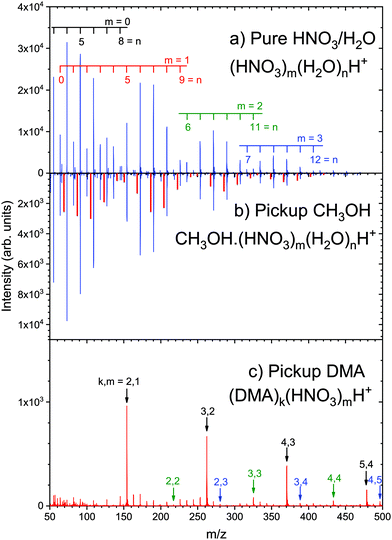 | ||
| Fig. 4 Mass spectra showing the pickup of methanol (CH3OH) and dimethylamine (CH3)2NH (DMA) on the mixed nitric acid–water clusters: (a) pure (HNO3)M·(H2O)N clusters without the pickup; (HNO3)m·(H2O)nH+ series with different m, n are labeled.51,72 (b) The pickup of methanol at a pickup pressure of about 4 × 10−4 mbar results in the additional analogous CH3OH·(HNO3)m·(H2O)nH+ series highlighted in red.158 The spectrum is plotted upside down for better comparison with the spectrum (a). (c) The spectrum after the DMA pickup at a pickup pressure of about 2 × 10−3 mbar. The black arrows indicate the major series DMA·(DMA·HNO3)mH+. Further, less pronounced series (DMA·HNO3)mH+ and (DMA·HNO3)mHNO3H+ are indicated by green and blue arrows, respectively. | ||
In atmospheric chemistry, bulk surface properties such as uptake coefficient γ and surface (or mass) accommodation coefficient αS are generally used in the modeling of heterogeneous processes.160–163 These properties are usually determined in the experiments with macroscopic bulk surfaces. In such experiments, adsorption and desorption of the molecules from the surface, diffusion of the molecules from the surface into the bulk and their solubility in the bulk, and also chemical reactions have to be taken into account. In addition, the involved rates often change with time as the gas concentration gradient near the surface, as well as the concentration gradient in the bulk, changes. On the other hand, in our experiments, the clusters move through the pickup gas with extremely diluted concentration under high vacuum (10−4 mbar) undergoing just a few collisions within the pickup chamber. Thus, we can neglect any diffusion in the gas due to the gas concentration gradient near the cluster surface. No chemical reactions take place for the molecules discussed below on (HNO3)M·(H2O)N clusters, and the adsorbed molecules do not dissolve nor diffuse into the cluster interior due to the small size of the clusters. We are able to determine the fraction of the clusters to which the molecules stick upon collisions; therefore our pickup probability is purely a kinetic parameter disentangling the molecule pickup from its evaporation. Under these conditions the pickup probability corresponds to the surface accommodation coefficient αS. Thus our experiments provide a unique and direct way to determine experimentally αS for the investigated molecules on the proxies of ultrafine aerosol particles.
Now, with the method established,158 we can investigate the uptake of other atmospheric molecules. The volatile organic compounds (VOCs) belong to the key components in the aerosol generation.164–168 Therefore we have investigated the pickup of different VOCs and their oxidation products on (HNO3)M·(H2O)N clusters.159 The pickup of isoprene, α-pinene, 2-methyl-3-buten-2-ol, 3-methyl-3-buten-1-ol and verbenone was studied. The experiments delivered the αS coefficients, which can be used for atmospheric aerosol modeling, and demonstrated that the oxidation increases the surface accommodation of VOCs by more than an order of magnitude. Accompanying theoretical calculations justified the experimental findings by forming of hydrogen bonds between the oxidized compounds and the clusters, whereas the interactions of the parent VOCs with the clusters were weaker and nonspecific.159
Bases, e.g., ammonia and amines, contribute strongly to the aerosol nucleation as well.46,142,169 Therefore we picked up dimethylamine, (CH3)2NH (DMA), on the hydrated acid clusters. However, a completely different mass spectrum was encountered (Fig. 4c). It is strongly dominated by a single series, which could be assigned to the DMA·(DMA·HNO3)mH+ ions. This suggests the acid–base reaction between HNO3 and DMA leading to proton transfer and generating the ion pair NO3−⋯DMA·H+. The energy released in the acid–base reactions leads to water evaporation from the clusters, since the anhydrous DMA·(DMA·HNO3)mH+ ions dominate the mass spectrum. The water evaporation is in line with recent investigation of nanoparticle formation and growth from dimethylamine and nitric acid,170 where relatively low binding energies of water molecules in these neutral clusters were calculated. There are also weaker mass peaks corresponding to the (DMA·HNO3)mH+ and (DMA·HNO3)mHNO3H+ series in Fig. 4c indicated by the green and blue arrows, respectively. These series are also consistent with the assumption of the acid–base reaction and (DMAH)+⋯NO3− ion pair generation.
It should be noted that the above assignment is not unambiguous due to the mass coincidences between various (DMA)k·(HNO3)m·(H2O)nH+ fragments with different number of DMA, HNO3 and H2O molecules. In principle, this could be resolved by high-resolution measurements. We have demonstrated previously72 that the resolution of M/ΔM ≈ 4 × 103 could be achieved with our RTOF allowing a clear separation of protonated water heptamer (H2O)7H+ and nitric acid dimer (HNO3)2H+ peaks in the mass spectra. However, the separation of, e.g., protonated water hexamer (H2O)6H+ from nitric acid-DMA complex HNO3·DMA·H+ would require even much higher mass resolution. Nevertheless, the present assignment is the most straightforward one, based on the acid–base reaction. There are still many open questions, for example: Does the acid–base reaction and subsequent water evaporation happen in the neutral cluster, or only after the ionization process? The presence of mainly anhydrous clusters in the spectrum supports the former scenario, since complete water evaporation is not observed upon ionization of the pure (HNO3)M·(H2O)N clusters (Fig. 4a). However, such questions can be addressed and clarified by theoretical calculations and further experiments which are currently performed.
Similar experiments might be useful for the interpretation of the mass spectra measured in aerosol chambers, such as the Cosmics Leaving Outdoor Droplets (CLOUD) experiment.47 A significant increase of the aerosol nucleation was observed in the presence of ammonia169,171 and DMA172 in the CLOUD experiments. In those experiments, all aerosol nucleation precursors are added into the system (acid, base, water, ionizing radiation, etc.), and the mass spectra are recorded varying the conditions. In the CLUB experiments, we start with relatively well-defined hydrated acid clusters, on which the amine molecules are picked up. Thus, we can investigate the processes step by step providing complementary information to the CLOUD experiments, where the final results of more complex processes are monitored. For example, we can clearly see the water evaporation upon DMA and HNO3 reactions in our cluster experiments.
3.3 Clusters for interstellar chemistry
Despite the seemingly hostile conditions for chemistry in space, more than 200 molecules have been identified in the interstellar medium (ISM), among them some quite large and complex ones such as amino acids and polycyclic aromatic hydrocarbons (PAHs).173 How are these molecules formed in the ISM? Low densities of molecules, which in the densest regions of the giant molecular clouds hardly exceed 106 cm−3 (some 13 orders of magnitude less than the air in the Earth's atmosphere), imply infrequent collisions. Low temperatures, typically ∼10–100 K, often do not allow to surmount reaction barriers. However, there are other chemical pathways prevailing in the ISM, e.g., surface-catalyzed reactions or radical and ion–molecule reactions, which are essentially barrier-less.39–41,174,175 The ISM contains dust/ice grains acting as “sponges”, where the molecules can be adsorbed and stored for a long time, and eventually interact and react spontaneously or the reactions can be triggered by radiation. To simulate the dust/ice catalyzed chemistry, usually bulk surfaces are employed in the laboratory studies.176–178The clusters and nanoparticles in molecular beams can provide an alternative approach. They can mimic the dust/ice grains and offer some advantages for understanding the surface catalyzed chemistry. For example, the reaction intermediates and even the final products are sometimes difficult to detect in the bulk system.177 In the case of clusters, they often leave the finite-size cluster and can be detected and characterized by mass spectrometry or optical spectroscopy. Thus the clusters can offer a detailed insight into the individual steps of complex reactions on dust/ice grains.
The ice nanoparticles investigated in our experiments can represent a model system for the water-ice covered grains in the ISM. Quite common in the ISM are various carbonaceous dust particles.179 Also PAHs and their clusters have been suggested to be implemented in the ISM molecule synthesis.180,181 The PAH clusters have already been investigated experimentally by different methods, e.g., IR and UV spectroscopy182 mass spectrometry,183,184 collisions with energetic ions,185–187 photoelectron spectroscopy,188,189 and in He-nanodroplets.190 We have started to investigate the pickup of molecules by the carbonaceous and PAH clusters.
The mixed clusters of PAHs with other molecules were produced by other than pickup techniques in numerous studies. For example benzene–water191,192 and naphthalene–water193,194 cluster cations were generated by solvation of PAH cations in collision with water. The naphthalene–water clusters were also produced by co-expansion of naphthalene with water vapor in argon carrier gas, and subsequently ionized by vacuum ultraviolet photoionization.195 In connection with our experiments on adamantane (AD) discussed below, we should mention also the spectroscopy of the ions formed by electron ionization of adamantane,196 and the adamantane–water cluster cation.197
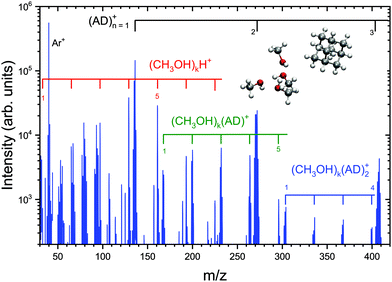
Here, we present one of our first investigations, the pickup of methanol, which can be quite abundant in the ISM, on adamantane clusters (AD)N as a prerequisite for the future investigations of chemical reactions of molecules adsorbed on such carbonaceous clusters. Fig. 5 shows the corresponding mass spectrum. The dominating cluster ion series (AD)n+ is indicated together with clear methanol containing series (CH3OH)kH+ and (CH3OH)k(AD)n+, n = 1, 2. Each mass peak is accompanied by satellites with different number of H atoms; nevertheless, the major observations can be summarized: adamantane clusters are generated, and several methanol molecules can be adsorbed on (AD)N in the pickup cell and coagulate to (CH3OH)K clusters with K ≥ 7. An interesting observation is that the protonated (CH3OH)kH+ fragments are ejected from the clusters after the ionization. The protonated cluster fragments are typical for the hydrogen bonded clusters such as methanol or water in the gas phase. On the other hand, if the methanol clusters stick to one or two AD molecules after the ionization, the major peaks correspond to (CH3OH)k(AD)n+ ions, i.e., non-protonated clusters. Theoretical investigation can help to investigate the structure of these clusters.
3.4 Coagulation of molecules on clusters
So far, we have concentrated on the uptake process of the molecule by the cluster. The next logical question is: What happens with the molecules adsorbed on the cluster? Do they migrate on the cluster surface or remain bound to a spot? Do they stay on the surface or submerge into the cluster interior? These questions were addressed previously in some cases.22 For example, the surface mobility of barium atoms and various molecules and generation of their clusters within large argon clusters was observed.24,198 In another spectroscopic experiment, methanol clusters were coagulated from individually adsorbed molecules on argon clusters.94 In helium nanodroplets, complexes of picked up molecules were generated.16–18,21,83,88,89,91,199 However, rare gas clusters, and especially helium, represent rather special cases, and here we concentrate on the molecular clusters such as the ice nanoparticles.We addressed the questions of migration and coagulation of molecules on/in clusters200 picking up different atmospherically relevant molecules X (X = HCl, CH4, CH3Cl, CH3CH2CH2Cl, chlorobenzene, and benzene) on ArN and (H2O)N clusters with the mean sizes ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 330 and 430, respectively, corresponding to approximately the same geometrical cross sections. The clusters underwent multiple pickup collisions with the molecules. In the case of ArN, the electron ionization mass spectra exhibited Xk+ ion fragments up to k ≈ 10 for most molecules. For methane, which did not stick to ArN easily, and is not prone to clustering, CH4 complexes up to the trimer were observed.
≈ 330 and 430, respectively, corresponding to approximately the same geometrical cross sections. The clusters underwent multiple pickup collisions with the molecules. In the case of ArN, the electron ionization mass spectra exhibited Xk+ ion fragments up to k ≈ 10 for most molecules. For methane, which did not stick to ArN easily, and is not prone to clustering, CH4 complexes up to the trimer were observed.
On the other hand, there was no evidence for cluster formation on (H2O)N for the above molecules. This was not caused by the lack of adsorption of the molecules on (H2O)N. The nanoparticles were slowed down by the momentum transfer, and individual molecules X could be seen in the mass spectra arriving with the ice nanoparticles into the mass spectrometer. In some cases, also the photodissociation experiments (discussed in Section 4.1) proved the presence of the molecules on (H2O)N.62 Thus we could conclude that the above molecules coagulated to XK clusters on ArN, while they remained isolated on (H2O)N during the flight time of about 1 ms from the pickup cell to the RTOF.
Accompanying theoretical MD simulations justified our findings: the molecules coagulated on ArN while they mostly remained bound where they landed on ice nanoparticles. The simulations also suggested that the molecules partly submerged in the cluster surface on ArN, despite the low temperature of the cluster of about 30–40 K and thus its solid-like nature.115 In the case of the ice nanoparticles the molecules remained bound to the surface. In another experiment, we adsorbed Freon CF2Cl2 molecules on ArN and ice nanoparticles,63 and we observed the same pattern: coagulation to (CF2Cl2)K clusters on ArN and pickup but no coagulation on (H2O)N.
Yet another interesting example of the difference between the coagulation of molecules picked up on ArN and (H2O)N can be found in our recent study of the hydrogen peroxide (H2O2) pickup and reactions on these clusters.66 The top panel of Fig. 6a shows the electron ionization mass spectrum of the clusters generated after the pickup on ArN, ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 160. The spectrum exhibits several fragment ion series containing H2O2 clusters. We label the major series corresponding to the protonated hydrogen peroxide (H2O2)kH+ (further series and their discussion can be found in our recent publication66). Fragments with up to k = 20 H2O2 molecules could be identified in the spectrum upon detailed analysis.
≈ 160. The spectrum exhibits several fragment ion series containing H2O2 clusters. We label the major series corresponding to the protonated hydrogen peroxide (H2O2)kH+ (further series and their discussion can be found in our recent publication66). Fragments with up to k = 20 H2O2 molecules could be identified in the spectrum upon detailed analysis.
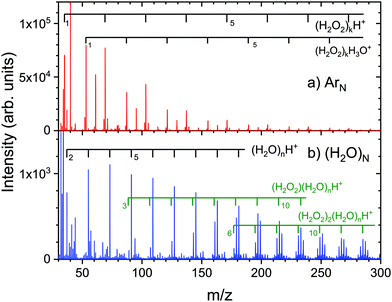 | ||
Fig. 6 Mass spectra of H2O2 molecules picked up on (a) ArN, ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 160, and (b) (H2O)N, ≈ 160, and (b) (H2O)N, ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 120. The major fragment ion series are indicated and discussed in the text. ≈ 120. The major fragment ion series are indicated and discussed in the text. | ||
Fig. 6b shows the spectrum after the pickup of H2O2 on (H2O)N, ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 120. Here, the spectrum is dominated by the protonated water (H2O)nH+; nevertheless, it contains also the series with H2O2 molecules, (H2O2)k·(H2O)nH+, with k up to 8 (k = 1 and 2 are labeled in Fig. 6b). Clearly, multiple H2O2 molecules could be adsorbed on the ice nanoparticles; however, there are no anhydrous cluster ion fragments (H2O2)kH+. For the (H2O2)k·(H2O)nH+ series, we find that each k number of H2O2 molecules is accompanied by n > k water molecules, and the maximum of each series corresponds to the fragment composition with many more water molecules than H2O2 molecules, n ≫ k. This suggests that the H2O2 molecules do not coagulate to (H2O2)K clusters on (H2O)N, just that the electron ionization generates the protonated water fragments and some H2O2 molecules remain attached. However, this hypothesis needs further experimental and theoretical verification.
≈ 120. Here, the spectrum is dominated by the protonated water (H2O)nH+; nevertheless, it contains also the series with H2O2 molecules, (H2O2)k·(H2O)nH+, with k up to 8 (k = 1 and 2 are labeled in Fig. 6b). Clearly, multiple H2O2 molecules could be adsorbed on the ice nanoparticles; however, there are no anhydrous cluster ion fragments (H2O2)kH+. For the (H2O2)k·(H2O)nH+ series, we find that each k number of H2O2 molecules is accompanied by n > k water molecules, and the maximum of each series corresponds to the fragment composition with many more water molecules than H2O2 molecules, n ≫ k. This suggests that the H2O2 molecules do not coagulate to (H2O2)K clusters on (H2O)N, just that the electron ionization generates the protonated water fragments and some H2O2 molecules remain attached. However, this hypothesis needs further experimental and theoretical verification.
Apparently, all the picked up molecules, discussed so far, coagulated on ArN, but were immobilized on (H2O)N. Is this a general pattern? It turns out that we had evidence for molecules coagulating on the ice nanoparticles as well. The first example was represented by 2-hydroxypyridine (HP).74 These molecules generated large clusters on ArN, but we found the evidence for generation of (HP)K clusters with K up to 8 also on (H2O)N. It is worth noting that these molecules showed a strong propensity for dimer formation on (H2O)N. Accompanying calculations showed that the dimers exhibited hydrogen bonds analogous to the base pairing in DNA. Thus this result can contribute to the discussion of the biomolecule synthesis on the ice grains in the space.40,174,201–203 Similarly, we observed coagulation of uracil and 5-bromouracil on (H2O)N.204 Thus the observed propensity for aggregation on ice nanoparticles seems to be a more general trend for molecules forming strong hydrogen bonds among themselves, e.g., biomolecular analogues. Recently, we have observed also pyruvic and valeric acid coagulating on (H2O)N.205,206Table 1 provides the overview of molecules picked up on different clusters within our investigations, and indicates whether the molecules coagulated to clusters or not by labels c or x, respectively. Theoretical investigations could elucidate the interplay of forces acting between the molecules on nanoparticles, which lead to their mobility and coagulation.
| Molecules/cluster | ArN | (H2O)N | (HNO3)M·(H2O)N | (NH3)N | (AD)N | Ref. |
|---|---|---|---|---|---|---|
| Ar | σ pu | — | — | — | — | 67 |
| H2O | σ pu | σ pu | — | — | — | 67 and 68 |
| HCl, HBr | σ pu, c, hν | σ pu, x, hν | — | — | — | 62, 67, 68 and 207 |
| NO, NO2 | — | σ pu | — | — | — | 68 |
| Methanol (CH3OH) | σ pu, c | σ pu | σ pu, αS | c, i+ | c, i+ | 67, 68, 158, 159, 208 and 209 |
| Methane (CH4) | c | σ pu, x | — | — | — | 68 and 200 |
| Ethanol (CH3CH2OH) | — | σ pu | — | — | c, i+ | 68 |
| CH3Cl, CH3CH2CH2Cl, C6H5Cl, C6H6, CF2Cl2 | c | x | — | — | — | 63 and 200 |
| Isoprene, α-pinene, verbenone, 2-methyl-3-buten-2-ol, 3-methyl-3-buten-1-ol | — | — | α S | — | — | 159 |
| Hydrogen peroxide (H2O2) | c, i+, e− | x, i+, e− | — | — | — | 66 |
| Formic acid (HCOOH) | c, i+, hν | — | — | — | — | 209 |
Amylene (2-methyl-2-butene) (H3C–C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3) CHCH3) |
c, i+, hν | — | — | — | — | 75 |
Pyruvic acid (H3C–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–(C O)–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–OH) O)–OH) |
— | c, i+, hν | — | — | — | 205 |
Valeric acid (H3C–(CH2)3–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–OH) O)–OH) |
c, i+, hν | c, i+, hν | — | — | — | 206 |
| 2-Hydroxypyridine | c, i+, hν | c, i+, hν | — | — | — | 74 |
| Uracil, 5-Br–uracil | — | c, i+ | — | — | — | 204 |
| Dimethylamine ((CH3)2NH) | — | — | c, i+ | — | — | — |
| Methylamine (CH3NH) | — | — | — | — | c, i+ | — |
4 Reactions triggered by photons and electrons
So far, we have focused on the pickup of molecules by the clusters. Now, we will discuss reactions between the adsorbed molecules. The reactions between neutral ground-state molecules were observed in some special cases previously, e.g., the reactions of Ba atoms with different molecules mentioned in the introduction.23–29 We provide some evidence for the acid–base reaction between HNO3 and DMA in Section 3.2.2. However, in this section, we concentrate on the reactions triggered by photons or electrons. In Table 1 we indicate for all the picked up molecules whether we have observed reactions after the electron ionization i+, or photon excitation-ionization hν, or slow electron attachment e−.4.1 Photodissociation of molecules on clusters
The photodissociation of a molecule is sometimes referred to as the half-reaction, and in that sense it can be regarded as one of the simplest chemical reactions.210,211 The effect of a solvent on the photodissociation is of prime importance for understanding the chemistry in bulk systems irradiated by UV photons.212,213 With this motivation the photodissociation of molecules in rare gas clusters was investigated.22,36,37,214Although the rare gas clusters represented a benchmark system emulating a weakly interacting environment, let us concentrate on the atmospherically more relevant systems such as the large water clusters (H2O)N. They can mimic the ice particles in the polar stratospheric clouds (PSCs). Type II PSCs are composed essentially of pure water ice.155 The PSC particles represent the key players in the chemistry leading to the Antarctic ozone hole.155,215–218 The heterogeneous chemistry on their surfaces converts so called reservoir species (HCl, ClONO2) to the active ones (Cl2, HOCl), which can be readily photolyzed by the sun radiation yielding the ozone destroying Cl˙ and other radicals. The reservoir species originate from the photolysis of the Cl-containing molecules in the stratosphere such as the infamous Freon CF2Cl2, and subsequent Cl˙ reactions with other atmospheric molecules, e.g., reactions with CH4 produce HCl.
We have investigated the photodissociation of hydrogen halides HX (X = Cl, Br) on (H2O)N implementing also ArN for comparison.62 The molecules adsorbed on the ArN clusters formed (HX)K clusters, as revealed above, and the H fragments underwent caging after the photodissociation yielding the fragments with essentially zero kinetic energy. The heavy Cl˙ fragments were also caged; however, some of them escaped the cluster without losing their kinetic energy. On (H2O)N clusters, the HX molecules did not coagulate and remained isolated, and their (photo)chemistry was quite different. First, the acidic dissociation occurred on (H2O)N already in the ground state generating the H3O+⋯X− ion pair. This ion pair was then excited by the UV photon yielding a neutral hydronium radical H3O˙.219–223 H3O˙ was metastable and quickly decayed yielding the observed H˙ fragments with low kinetic energy. This reaction pathway was observed in previous photodissociation studies,207,224,225 and also confirmed theoretically.219,220,226 The corresponding Cl˙ fragment was trapped in the ice nanoparticles. Possible relevance of such photodissociation of HCl on the PSC particles was discussed.62,224 The acidic dissociation enhances the HCl photolysis rate in the 200–300 nm region by about four orders of magnitude.224 On the other hand, this enhancement of the Cl˙ radical yield from HCl on the ice could be counterbalanced by the trapping of the Cl˙ in the particles.62
The photodissociation of Freon CF2Cl2 was investigated in pure (CF2Cl2)N clusters and also when adsorbed on ArN and (H2O)N clusters,63 as well as a single CF2Cl2 molecule embedded inside Ar and Xe clusters.227 The general feature of the photodissociation process was the caging of Cl˙ observed in all kinds of clusters. However, the Cl˙ fragments from ArN clusters were detected, which meant that the fragments were slowed down to near-zero kinetic energies, but they still exit the cluster or the cluster decayed upon the photodissociation process. On the other hand, no Cl˙ fragments from (H2O)N were detected, despite the clear evidence for the uptake of CF2Cl2 on (H2O)N. The loss of Cl˙ can be atmospherically important. The Cl˙ fragments trapped in the ice nanoparticle after the photodissociation can be washed out from the atmosphere. The accompanying theoretical calculations rationalized this observation by the formation of halogen bonds, where the Cl atoms of CF2Cl2 pointed to the oxygen of the water molecules in the nanoparticle. MD simulations of the dissociation process showed that the Cl˙ entered the cluster after the photodissociation, and remained trapped.63
4.2 Reactions after excitation and ionization
The clusters represent a very special environment for reactions of molecules in the excited or ionized states, and the reaction pathways can differ from the reactions in both the gas and the condensed phases. In clusters, the reactants are in direct contact already before they are excited or ionized. Thus the collision process, preceding a reaction in the gas phase, is absent in the cluster, and the reaction can readily proceed at a kinetic rate. The interaction potentials can be altered by the cluster environment, and reaction pathways different from the gas phase can be followed. The cluster can lead to the product caging as discussed above for the photodissociation processes. The cluster can serve as an efficient heat bath with many closely spaced degrees of freedom, where excited states can be efficiently quenched. On the other hand, in comparison with the condensed phase, the clusters provide a much simpler environment allowing molecular-level understanding of the investigated reactions. The energy released in the reaction leads to the cluster decay and the reaction products, which are usually trapped in the bulk, can be released from the cluster and detected. Thus, reaction intermediates can be observed, such as activated complexes and radicals, which usually react immediately in the bulk. All these aspects are well known and have been discussed in numerous studies.3,4,22,43,53,55,104,113,228We have investigated quite a few systems where the molecules were picked up on different clusters and reactions were subsequently triggered by interaction with electrons (typically of 70 eV) and/or photons (usually at 193 nm, 6.4 eV). For example, the reactions of amylene (2-methyl-2-butene, H3C–C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH3) molecules picked up on ArN have been observed recently.75 The mass spectra after the electron ionization and photoionization exhibited striking differences pointing to the excited state reactions, which occurred only during the photoionization process. From the perspective of atmospheric chemistry, small carboxylic acids play an important role in the secondary organic aerosol formation.46,229–231 We have investigated valeric acid (H3C–(CH2)3–(C
CH–CH3) molecules picked up on ArN have been observed recently.75 The mass spectra after the electron ionization and photoionization exhibited striking differences pointing to the excited state reactions, which occurred only during the photoionization process. From the perspective of atmospheric chemistry, small carboxylic acids play an important role in the secondary organic aerosol formation.46,229–231 We have investigated valeric acid (H3C–(CH2)3–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–OH) and pyruvic acid (H3C–(C
O)–OH) and pyruvic acid (H3C–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–(C
O)–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–OH) in clusters and picked up on (H2O)N and ArN clusters.205,206 From the perspective of the interstellar chemistry, ammonia and methanol together with water represent the primary hydrogen-bonding molecules detected in the interstellar clouds, ices and comets in high abundance.232–236 We have studied the uptake and ionization of methanol on large ammonia (NH3)N,
O)–OH) in clusters and picked up on (H2O)N and ArN clusters.205,206 From the perspective of the interstellar chemistry, ammonia and methanol together with water represent the primary hydrogen-bonding molecules detected in the interstellar clouds, ices and comets in high abundance.232–236 We have studied the uptake and ionization of methanol on large ammonia (NH3)N, ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 230, clusters.208 However, we are not discussing these experiments in more detail and the interested reader is referred to the above references.
≈ 230, clusters.208 However, we are not discussing these experiments in more detail and the interested reader is referred to the above references.
Our ultimate goal was to observe a reaction between two different molecules adsorbed on a cluster. This has recently been achieved for methanol (CH3OH) and formic acid (HCOOH) adsorbed on ArN nanoparticles.209 The ArN nanoparticles picked up the molecules in two sequential pickup cells in SC and PC chambers (see Fig. 2). We probed the clusters by photon and electron ionization yielding very similar spectra in this case. When only one kind of molecules was adsorbed on ArN, the spectra revealed protonated clusters of either (CH3OH)mH+ or (HCOOH)mH+. When both molecules were adsorbed on the nanoparticles, mixed clusters appeared as well. We used isotopically labeled molecules to prove the reactions between CH3OH and HCOOH. Perdeuterated methanol (CD3OD) demonstrated the deuteron transfer reactions to formic acid resulting in (HCOOH)mD+ cluster ions, as well as the proton transfer from formic acid resulting in (CD3OD)mH+. The latter process was expected for the acidic molecule, while the former reaction was, perhaps, less intuitive. The experiments with the partially deuterated methanol CD3OH and CH3OD further revealed the proton transfer from both methyl and hydroxyl groups. These findings were supported by ab initio calculations.209 Both molecules CH3OH and HCOOH are abundant in the ISM, and the methoxy radical CH3O˙ and the hydromethoxy radical CH2OH˙ were implemented in the formation of complex organic molecules in astrochemistry.237–240 Our study shows that such radicals might be generated in the proton transfer processes on the grains and released from them. More realistic proxies of interstellar grains will be used in future experiments, e.g., the carbonaceous clusters discussed in Section 3.3.
4.3 Electron attachment
Another area of reactions on free clusters is the electron attachment and negative ion reactions. The dissociative electron attachment (DEA) of isolated molecules is important in many areas of physics and chemistry, as reviewed recently by Fabrikant et al.241 Indeed, the question, how does a more complex environment of clusters influence the DEA process, has been ever-present already in the early studies.242,243 A recent review covered several fundamental effects of the environment on the DEA process in clusters, which can lead to an enhancement as well as to a suppression of the electron attachment cross section.244In relevance to the atmospheric aerosols discussed above, we have concentrated on the electron attachment to the mixed acid–water complexes, namely HNO3/H2O156,157 and H2SO4/H2O.57 The former system was a representative example of the role of the environment in the DEA process, where the solvent molecules influence the reaction pathways and change the products. The DEA of an isolated HNO3 molecule yielded almost exclusively NO2− ions (96.5%) and the minor product OH− (3.4%). The nitrate anion NO3− had an abundance of less than 0.1%. This picture changed completely in the mixed (HNO3)M(H2O)N clusters, where the negatively charged cluster ion fragments with NO3− dominated the mass spectra with 57% abundance. It was interpreted by the acidic dissociation of HNO3 molecules in the water containing clusters, and by cascade intracluster ion–molecule reactions terminated by NO3−. The cluster ions containing HNO3− were also detected in significant amounts (8%). This ion was not formed in the gas phase DEA, and its generation in the clusters was attributed to the stabilization of HNO3− by caging. The measured energy dependence of the DEA157 revealed that the degree to which the major gas phase product NO2− is converted to NO3− in the clusters depends strongly on the electron energy: namely NO2− prevails at low electron energies below approximately 3.5 eV, while NO3− is the major product at higher energies.
The atmospheric relevance of these experiments is underlined by the fact that NO2− and NO3− are among the most abundant anions in the atmosphere having a strong influence on the aerosol generation and other processes.245–247 In addition, the proposed negative ion reactions in (HNO3)M(H2O)N clusters yield the OH˙ radical and HONO as the neutral products, which can be evaporated from the particles to the atmosphere. They represent the key species in atmospheric chemistry, namely the hydroxyl radical is the major oxidation agent.45 The photolysis of nitrous acid serves as the source of OH˙ in urban areas; yet the detailed mechanism of its formation is still discussed.
The experiments in M. Beyer's group, in which the negatively charged water clusters (H2O)n− are generated and subsequently react with molecules,56,248–252 can represent a complementary approach to our investigations of the electron attachment in water clusters. The (H2O)n− clusters represent the solvated electron eaq− in the gas phase.56,252 This way, the reaction of CF2Cl2 with the solvated electron was investigated.250 The reaction yielded the (H2O)nCl− product, and the reaction enthalpy was determined. These results demonstrated that CF2Cl2 can undergo the dissociative electron transfer in condensed aqueous environments if thermalized hydrated electrons are present. It can be relevant to the atmospheric chemistry of ozone depletion, since the earlier studies253,254 revealed huge enhancement of the bond cleavage in CF2Cl2 and other chloro-fluoro-carbon compounds due to the dissociative electron transfer on ice surfaces. Later on, this process was proposed as the mechanism enhancing the Cl˙ radical yield in PSCs;255 however, the actual contribution of this process to ozone depletion remains questionable.256–260 The study revealed the details and energetics of the dissociative electron transfer process at the molecular level, but its relevance for the stratospheric ozone depletion could not be established by laboratory experiments.250
Hydrogen peroxide, H2O2, plays an important role in many areas of atmospheric and interstellar chemistry as a source of HOx radicals261,262 and oxidation agent contributing to the aerosol nucleation.45,46,151,153 We have investigated the electron attachment to hydrogen peroxide (H2O2) adsorbed on ArN and ice (H2O)N nanoparticles.66 The hydrogen peroxide coagulates to (H2O2)M clusters on ArN, as illustrated by the mass spectra in Fig. 6. The electron attachment to the (H2O2)M clusters on ArN yielded (H2O2)mO2− ions as the major products, as shown in Fig. 7a. There are further weaker O2− containing series, and also HO2− and other ions (see the inset of Fig. 7a and ref. 66 for discussion). The presence of O2− ions was surprising, since they could not be generated in the DEA of the isolated H2O2 molecule. Nandi et al.263 showed that the DEA of H2O2 yielded only OH− (75%) and O− (25%). We have proposed possible reaction pathways leading from the nascent OH− and O− ions to the observed O2−, HO2− and the other ions in the spectra in ref. 66.
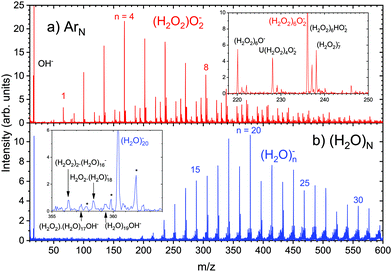 | ||
Fig. 7 Negative ion mass spectra of H2O2 molecules picked up on (a) ArN, ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 160, and (b) (H2O)N, ≈ 160, and (b) (H2O)N, ![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ≈ 120. The spectra recorded at the electron energies between 0 and 5 eV were integrated. The spectrum (a) is dominated by the (H2O2)mO2− ion series as indicated, and further ion series are shown in the inset. All ions have been discussed in ref. 66. The spectrum (b) is dominated by the water (H2O)n− series, and further minor ions containing H2O2 are shown in the inset (the star indicates metastable species). ≈ 120. The spectra recorded at the electron energies between 0 and 5 eV were integrated. The spectrum (a) is dominated by the (H2O2)mO2− ion series as indicated, and further ion series are shown in the inset. All ions have been discussed in ref. 66. The spectrum (b) is dominated by the water (H2O)n− series, and further minor ions containing H2O2 are shown in the inset (the star indicates metastable species). | ||
On the other hand, O2− is a well known product of the reaction of solvated electron with H2O2 in aqueous solutions in radiation chemistry.264–266 Therefore, we have investigated also H2O2 adsorbed on the (H2O)N clusters, where the electron attachment yields the solvated electron (H2O)n−. The uptake of H2O2 molecules on (H2O)N was proved by the positive spectrum in Fig. 6b. Nevertheless, the negative ion spectrum is dominated by (H2O)n− and no O2− containing products were detected, as shown in Fig. 7b. The (H2O)n− series was also observed in the electron attachment to the pure water clusters.267 There are other negative ion series of minor abundance, pointing to the presence of H2O2, illustrated in the inset of Fig. 7b. It is worth noting that a relatively strong OH− peak at m/z = 17 is observed in both spectra in Fig. 6. The DEA of H2O2 yields OH−, which leaves the clusters. Although the analysis of the spectrum in Fig. 7b might be still tentative, it is clear that none of the strong peaks could be assigned to O2− containing ions. Thus, the reaction of eaq− with H2O2 yielding O2− was not observed in the case of (H2O)N clusters. The cluster environment changed the chemistry in a way, which could not be predicted based on the free DEA in the gas phase, nor based on the known chemistry of H2O2 in the bulk aqueous solutions and the reactions with the solvated electrons. It was even different from the chemistry of the electron attachment to the H2O2 clusters on ArN.
5 Conclusions, challenges and perspectives of the pickup experiments
We have reviewed a few examples of the experiments with the pickup and reactions of molecules on clusters from the perspective of atmospheric and interstellar chemistry. The uptake cross sections of the ice nanoparticles for various molecules were determined. The cross section for the pickup of water molecules turned out to be substantially larger than the geometrical cross section, which is important for the nucleation of water aerosols.The pickup experiments with the smaller hydrated nitric acid clusters enabled determination of purely kinetic surface accommodation coefficient for the proxies of ultrafine aerosol particles. We have established a promising method providing important parameters for atmospheric aerosol modeling; nevertheless, the method is not universal. When the character of the mass spectra with and without pickup is different, the pickup probability cannot be evaluated as the ratio of the integrated spectra. Such was the case for the uptake of molecules on hydrated sulfuric acid clusters. The investigation of sulfuric acid clusters is thus challenging, yet highly desirable, and will be followed in the future.
Some questions were touched in our pickup studies; yet future work can answer them more comprehensively. For example, the coagulation of molecules on nanoparticles could be modeled in more detail to understand their mobility and interplay of the bonds. Also the question of molecules submerging into the cluster or remaining on its surface should be addressed in more detail.
A clear perspective to follow in the future was shown by the experiments with the pickup of dimethylamine on the hydrated nitric acid clusters, which pointed to the acid–base reaction in the neutral clusters. From a purely fundamental point of view, it can provide molecular-level insight into the basic chemistry. In the atmosphere, such systems play the key role in the new particle formation.
This will be one of our major directions in future studies. On our way towards the clusters of atmospheric relevance, we have moved from rare gas model systems to water clusters, and recently to mixed binary systems such as nitric acid–water clusters. Evidence from aerosol field measurements and model simulations suggests that the new particle formation in the atmosphere is most likely dominated by ternary nucleation of H2SO4–H2O–NH3 and subsequent condensation of semivolatile organic compounds (SVOCs).268 Clusters mimicking the composition of the real atmospheric aerosols should be generated. The present experiments with HNO3–H2O clusters and DMA pickup represent the first steps in this direction in our experiments. We will continue generating clusters of hydrated inorganic acids (nitric acid, sulfuric acid, methanesulfonic acid) with bases (ammonia, amines). The pickup of other atmospheric molecules, such as SVOCs, by these clusters will be studied, providing uptake coefficients for atmospheric modeling. Another challenging project represents incorporating a radical source into the pickup region of our CLUB apparatus and embedding radicals, such as OH˙, into the clusters. This will open the doorway to radical chemistry investigations in these clusters. The reactions of radicals, positive and negative ions and excited species in the clusters can contribute to the understanding of the atmospheric aerosol chemistry.
In relevance to the polar stratospheric clouds and ozone depletion we have reviewed the photodissociation of hydrogen halides and CF2Cl2 on the ice nanoparticles. In the case of HX, our experiments revealed their acidic dissociation to the H3O+⋯X− ion pair in the ground state, analogous to the above acid–base reaction of DMA. However, it is interesting to note that the acid–base reaction in the DMA case was revealed by mass spectrometry, while in the HX case a completely different photochemistry experiment in synergy with theory provided the evidence. Further extension of this work to the photodissociation of molecules on the mixed nitric acid–water clusters and to the clusters with sulfuric acid is planned also with other molecules involved in the ozone depletion, e.g., ClONO2, CH3Cl, Cl2, HOCl.
We have discussed reactions between molecules deposited on the nanoparticles triggered by electrons or photons. The first reactions observed between two different molecules on ArN were the proton transfer processes between methanol and formic acid. Here, a vast field is opened for simulating atmospheric and interstellar chemistry, e.g., reactions of different molecules on carbonaceous and PAH clusters, and ice nanoparticles including also other than water nanoices.
Finally, reactions triggered by slow electron attachment in molecules deposited on clusters were revealed. Generally, the reactions of negatively charged ions have been investigated less extensively compared to the positive ion chemistry, and thus the negatively charged clusters represent a large relatively unexplored field.
In terms of the experiment, the following challenges may be defined: (1) new ways of cluster generation with improved control of cluster composition and size; (2) implementing the action spectroscopy to provide information about the bonding motifs and structure of the fragments; (3) detect and analyze the neutral fragments in addition to the charged ones.
Author contributions
MF: conceptualization, methodology, writing-original draft, review and editing, visualization, supervision, funding acquisition, project administration; JF: methodology, writing-review and editing; JK: investigation, writing-review and editing; JL: investigation, writing-review and editing; EP: software, writing-review and editing; VP: investigation, writing-review and editing; AP: investigation, formal analysis, visualization.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the support of Praemium Academiae of the Czech Academy of Sciences.References
- A. W. Castleman and R. G. Keesee, Science, 1988, 241, 36–42 CrossRef CAS.
- A. W. Castleman, Int. J. Mass Spectrom. Ion Processes, 1992, 118–119, 167–189 CrossRef CAS.
- B. Brutschy, Chem. Rev., 1992, 92, 1567–1587 CrossRef CAS.
- A. W. Castleman and S. Wei, Annu. Rev. Phys. Chem., 1996, 45, 685–719 CrossRef.
- T. D. Märk, Linking the gaseous and condensed phases of matter, Plenum Press, New York, 1994, p. 155 Search PubMed.
- A. W. Castleman and K. H. Bowen, J. Phys. Chem., 1996, 100, 12900–12944 CrossRef.
- H. Haberland, Clusters of Atoms and Molecules, Springer, Berlin, 1994 Search PubMed.
- H. Pauly, Atom, Molecule and Cluster Beams II: Cluster Beams, Fast and Slow Beams, Accessory Equipment and Applications, Springer, Berlin, 2000, pp. 71–136 Search PubMed.
- T. S. Zwier, Annu. Rev. Phys. Chem., 1996, 47, 205–241 CrossRef CAS.
- C. Dedonder-Lardeux, G. Grégoire, C. Jouvet, S. Martrenchard and D. Solgadi, Chem. Rev., 2000, 100, 4023–4037 CrossRef CAS.
- P. Jena and A. W. Castleman, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10560 CrossRef CAS.
- Y. D. Kim, Int. J. Mass Spectrom., 2004, 238, 17–31 CrossRef CAS.
- U. Heiz and U. Landman, Nanocatalysis, Springer-Verlag, Berlin Heidelberg, 2007 Search PubMed.
- P. Jena and A. W. Castleman, Nanoclusters: A Bridge Across Disciplines, Elsevier, Amsterdam, 2010 Search PubMed.
- M. Hartmann, R. E. Miller, J. P. Toennies and A. F. Vilesov, Phys. Rev. Lett., 1995, 75, 1566 CrossRef CAS.
- J. P. Toennies, A. F. Vilesov and K. B. Whaley, Phys. Today, 2001, 2, 31–37 CrossRef.
- J. P. Toennies and A. F. Vilesov, Angew. Chem., Int. Ed., 2004, 43, 2622–2648 CrossRef CAS.
- M. Y. Choi, G. E. Douberly, T. M. Falconer, W. K. Lewis, C. M. Lindsay, J. M. Merritt, P. L. Stiles and R. E. Miller, Int. Rev. Phys. Chem., 2006, 25, 15 Search PubMed.
- F. Stienkemeier and K. K. Lehmann, J. Phys. B, 2006, 39, R127–R166 CrossRef CAS.
- M. Mudrich and F. Stienkemeier, Int. Rev. Phys. Chem., 2014, 33, 301–339 Search PubMed.
- A. Mauracher, O. Echt, A. M. Ellis, S. Yang, D. K. Bohme, J. Postler, A. Kaiser, S. Denifl and P. Scheier, Phys. Rep., 2018, 751, 1–90 CrossRef CAS.
- J. M. Mestdagh, M. A. Gaveau, C. Gée, O. Sublemontier and J. P. Visticot, Int. Rev. Phys. Chem., 1997, 16, 215 Search PubMed.
- A. Lallement, J. M. Mestdagh, P. Meynadier, P. de Pujo, O. Sublemontier, J.-P. Visticot, J. Berlande, X. Biquard, J. Cuvellier and C. G. Hickman, J. Chem. Phys., 1993, 99, 8705 CrossRef CAS.
- A. Lallement, O. Sublemontier, J.-P. Visticot, A. J. Bell, J. Berlande, J. C. J. M. Mestdagh and P. Meynadier, Chem. Phys. Lett., 1993, 204, 440–444 CrossRef CAS.
- X. Biquard, O. Sublemontier, J. Berlande, M. A. Gaveau, J. M. Mestdagh, B. Schilling and J. P. Visticot, J. Chim. Phys., 1995, 92, 264–282 CrossRef CAS.
- X. Biquard, O. Sublemontier, J. Berlande, M. A. Gaveau, J. M. Mestdagh and J. P. Visticot, J. Chem. Phys., 1995, 103, 957 CrossRef CAS.
- C. Gée, M. A. Gaveau, J. M. Mestdagh, M. Osborne, O. Sublemontier and J.-P. Visticot, J. Phys. Chem., 1996, 100, 13421–13427 CrossRef.
- C. Gée, M. A. Gaveau, O. Sublemontier, J. M. Mestdagh and J.-P. Visticot, J. Chem. Phys., 1997, 107, 4194 CrossRef.
- M. A. Osborne, M. A. Gaveau, C. Gee, O. Sublemontier, J. M. Mestdagh and J.-P. Visticot, J. Chem. Phys., 1997, 106, 1449 CrossRef CAS.
- W. H. Breckenridge, P. R. Fournier, M. A. Gaveau, J. M. Mestdagh and J.-P. Visticot, Chem. Phys. Lett., 2002, 364, 225–230 CrossRef CAS.
- M. Briant, M. A. Gaveau, J. M. Mestdagh and J.-P. Visticot, J. Chem. Phys., 2000, 112, 1744 CrossRef CAS.
- M. Briant, P. R. Fournier, M. A. Gaveau, J. M. Mestdagh, B. Soep and J. P. Visticot, J. Chem. Phys., 2002, 117, 5036 CrossRef CAS.
- M.-A. Gaveau, E. Glogauen, P.-R. Fournier and J.-M. Mestdagh, J. Phys. Chem. A, 2005, 109, 9494 CrossRef CAS.
- S. Awali, L. Poisson, B. Soep, M.-A. Gaveau, M. Briant, C. Pothier, J.-M. Mestdagh, M. B. E. H. Rhouma, M. Hochlaf, V. Mazet and S. Faisan, Phys. Chem. Chem. Phys., 2014, 16, 516–526 RSC.
- S. Awali, M.-A. Gaveau, M. Briant, J.-M. Mestdagh, B. Soep, O. Gobert, R. Maksimenka and L. Poisson, Phys. Chem. Chem. Phys., 2016, 18, 32378–32386 RSC.
- U. Buck, J. Phys. Chem. A, 2002, 106, 10049 CrossRef CAS.
- M. Fárník, N. H. Nahler, U. Buck, P. Slavíček and P. Jungwirth, Chem. Phys., 2005, 315, 161 CrossRef.
- M. Fárník and U. Buck, Int. Rev. Phys. Chem., 2006, 25, 583 Search PubMed.
- C. Vallance, Astrochemistry, World Scientific, Oxford, 2017 Search PubMed.
- E. Herbst and E. F. van Dishoeck, Annu. Rev. Astron. Astrophys., 2009, 47, 427–480 CrossRef CAS.
- T. Hama and N. Watanabe, Chem. Rev., 2013, 113, 8783–8839 CrossRef CAS.
- S. H. Lee, H. Gordon, H. Yu, K. Lehtipalo, R. Haley, Y. Li and R. Zhang, J. Geophys. Res.: Atmos., 2018, 124, 7098–7146 Search PubMed.
- M. Fárník and J. Lengyel, Mass Spectrom. Rev., 2018, 37, 630–651 CrossRef.
- A. R. Ravishankara, Science, 1997, 276, 1058–1065 CrossRef CAS.
- B. J. Finlayson-Pitts and J. N. Pitts, Chemistry of the upper and lower atmosphere, Academic Press, San Diego, 2000 Search PubMed.
- J. H. Seinfeld and S. N. Pandis, Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, John Wiley & Sons Inc., Hoboken, NJ, 3rd edn, 2016 Search PubMed.
- E. M. Dunne, H. Gordon, A. Kürten, J. Almeida, J. Duplissy, C. Williamson, I. K. Ortega, K. J. Pringle, A. Adamov, U. Baltensperger, P. Barmet, F. Benduhn, F. Bianchi, M. Breitenlechner, A. Clarke, J. Curtius, J. Dommen, N. M. Donahue, S. Ehrhart, R. C. Flagan, A. Franchin, R. Guida, J. Hakala, A. Hansel, M. Heinritzi, T. Jokinen, J. Kangasluoma, J. Kirkby, M. Kulmala, A. Kupc, M. J. Lawler, K. Lehtipalo, V. Makhmutov, G. Mann, S. Mathot, J. Merikanto, P. Miettinen, A. Nenes, A. Onnela, A. Rap, C. L. S. Reddington, F. Riccobono, N. A. D. Richards, M. P. Rissanen, L. Rondo, N. Sarnela, S. Schobesberger, K. Sengupta, M. Simon, M. Sipilä, J. N. Smith, Y. Stozkhov, A. Tomé, J. Tröstl, P. E. Wagner, D. Wimmer, P. M. Winkler, D. R. Worsnop and K. S. Carslaw, Science, 2016, 354, 1119–1124 CrossRef CAS.
- P. Nissenson, C. J. H. Knox, B. J. Finlayson-Pitts, L. F. Phillips and D. Dabdub, Phys. Chem. Chem. Phys., 2006, 8, 4700–4710 RSC.
- B. J. Finlayson-Pitts, Phys. Chem. Chem. Phys., 2009, 11, 7760–7779 RSC.
- V. Vaida, J. Chem. Phys., 2011, 135, 020901 CrossRef.
- B. D. Kay, V. Hermann and A. W. Castleman, Chem. Phys. Lett., 1981, 80, 469–474 CrossRef CAS.
- X. Zhang, E. L. Mereand and A. W. Castleman, J. Phys. Chem., 1994, 98, 3554–3557 CrossRef CAS.
- R. S. MacTaylor and A. W. Castleman, J. Atmos. Chem., 2000, 36, 23–63 CrossRef CAS.
- J. J. Gilligan and A. W. Castleman, J. Phys. Chem. A, 2001, 105, 5601–5605 CrossRef CAS.
- G. Niedner-Schatteburg and V. E. Bondybey, Chem. Rev., 2001, 100, 4059–4086 CrossRef.
- O. P. Balaj, C.-K. Siu, I. Balteanu, M. K. Beyer and V. E. Bondybey, Int, J. Mass Spectrom., 2004, 238, 65–74 CAS.
- J. Lengyel, A. Pysanenko and M. Fárník, Atmos. Chem. Phys., 2017, 17, 14171–14180 CrossRef CAS.
- T. I. Yacovitch, T. Wende, L. Jiang, N. Heine, G. Meijer, D. M. Neumark and K. R. Asmis, J. Phys. Chem. Lett., 2011, 2, 2135–2140 CrossRef CAS.
- T. I. Yacovitch, N. Heine, C. Brieger, T. Wende, C. Hock, D. M. Neumark and K. R. Asmis, J. Chem. Phys., 2012, 136, 241102 CrossRef.
- T. I. Yacovitch, N. Heine, C. Brieger, T. Wende, C. Hock, D. M. Neumark and K. R. Asmis, J. Phys. Chem. A, 2013, 117, 7081–7090 CrossRef CAS.
- N. Heine and K. S. Asmis, Int. Rev. Phys. Chem., 2015, 34, 1–34 Search PubMed.
- V. Poterya, J. Lengyel, A. Pysanenko, P. Svrčková and M. Fárník, J. Chem. Phys., 2014, 141, 074309 CrossRef CAS.
- V. Poterya, J. Kočišek, J. Lengyel, P. Svrčková, A. Pysanenko, D. Hollas, P. Slavíček and M. Fárník, J. Phys. Chem. A, 2014, 118, 4740–4749 CrossRef CAS.
- M. Fárník and V. Poterya, Front. Chem., 2014, 2, 4 Search PubMed.
- J. Kočišek, A. Pysanenko, M. Fárník and J. Fedor, J. Phys. Chem. Lett., 2016, 7, 3401–3405 CrossRef.
- A. Pysanenko, E. Pluhařová, I. S. Vinklárek, J. Rakovský, V. Poterya, J. Kočišek and M. Fárník, Phys. Chem. Chem. Phys., 2020, 22, 15312–15320 RSC.
- J. Fedor, V. Poterya, A. Pysanenko and M. Fárník, J. Chem. Phys., 2011, 135, 104305 CrossRef CAS.
- J. Lengyel, J. Kočišek, V. Poterya, A. Pysanenko, P. Svrčková, M. Fárník, D. Zaouris and J. Fedor, J. Chem. Phys., 2012, 137, 034304 CrossRef CAS.
- J. Lengyel, A. Pysanenko, V. Poterya, P. Slavíček, M. Fárník, J. Kočišek and J. Fedor, Phys. Rev. Lett., 2014, 112, 113401 CrossRef CAS.
- J. Fedor, J. Kočišek, V. Poterya, O. Votava, A. Pysanenko, L. Lipciuc, T. N. Kitsopoulos and M. Fárník, J. Chem. Phys., 2011, 134, 154303 CrossRef CAS.
- K. Grygoryeva, J. Rakovský, O. Votava and M. Fárník, J. Chem. Phys., 2018, 149, 094303 CrossRef CAS.
- J. Lengyel, A. Pysanenko, J. Kočišek, V. Poterya, C. Pradzynski, T. Zeuch, P. Slavíček and M. Fárník, J. Phys. Chem. Lett., 2012, 3, 3096–3109 CrossRef CAS.
- J. Kočišek, J. Lengyel and M. Fárník, J. Chem. Phys., 2013, 138, 124306 CrossRef.
- P. Rubovič, A. Pysanenko, J. Lengyel, D. Nachtigallová and M. Fárník, J. Phys. Chem. A, 2016, 120, 4720–4730 CrossRef.
- A. Pysanenko, F. Gámez, M. Fárník, J. Chalabala and P. Slavíček, J. Phys. Chem. A, 2020, 124, 3038–3047 CrossRef CAS.
- J. Lengyel, A. Pysanenko, V. Poterya, J. Kočišek and M. Fárník, Chem. Phys. Lett., 2014, 612, 256–261 CrossRef CAS.
- D. Šmídová, J. Lengyel, A. Pysanenko, J. Med, P. Slavíček and M. Fárník, J. Phys. Chem. Lett., 2015, 6, 2865–2869 CrossRef.
- J. Lengyel, J. Kočišek, M. Fárník and J. Fedor, J. Phys. Chem. C, 2016, 120, 7397–7402 CrossRef CAS.
- D. Šmídová, J. Lengyel, J. Kočišek, A. Pysanenko and M. Fárník, Int. J. Mass Spectrom., 2017, 421, 144–149 CrossRef.
- P. Svrčková, A. Pysanenko, J. Lengyel, P. Rubovič, J. Kočišek, V. Poterya, P. Slavíček and M. Fárník, Phys. Chem. Chem. Phys., 2015, 17, 25734–25741 RSC.
- K. Grygoryeva, J. Kubečka, A. Pysanenko, J. Lengyel, P. Slavíček and M. Fárník, J. Phys. Chem. A, 2016, 120, 4139–4146 CrossRef CAS.
- T. E. Gough, M. Mengel, P. A. Rowntree and G. Scoles, J. Chem. Phys., 1985, 83, 4958 CrossRef CAS.
- S. Grebenev, J. P. Toennies and A. F. Vilesov, Science, 1998, 279, 2083 CrossRef CAS.
- G. Scoles and K. K. Lehmann, Science, 2000, 287, 2429 CrossRef CAS.
- E. Lugovoj, J. P. Toennies and A. F. Vilesov, J. Chem. Phys., 2000, 112, 8217 CrossRef CAS.
- M. Fárník and J. P. Toennies, J. Chem. Phys., 2005, 122, 014307 CrossRef.
- J. M. Merritt, S. Rudić and R. E. Miller, J. Chem. Phys., 2006, 124, 084301 CrossRef CAS.
- S. Denifl, F. Zappa, I. Mähr, F. Ferreira da Silva, A. Aleem, A. Mauracher, M. Probst, J. Urban, P. Mach, A. Bacher, O. Echt, T. D. Märk and P. Scheier, Angew. Chem., Int. Ed., 2009, 121, 9102–9105 CrossRef.
- M. Gatchell, M. Goulart, L. Kranabetter, M. Kuhn, P. Martini, B. Rasul and P. Scheier, Phys. Chem. Chem. Phys., 2018, 20, 7739–7745 RSC.
- A. Gutiérrez-Quintanilla, M. Briant, E. Mengesha, M.-A. Gaveau, J.-M. Mestdagh, B. Soep, C. Crépin and L. Poisson, Phys. Chem. Chem. Phys., 2018, 20, 28658–28666 RSC.
- L. Tiefenthaler, J. Ameixa, P. Martini, S. Albertini, L. Ballauf, M. Zankl, M. Goulart, F. Laimer, K. von Haeften, F. Zappa and P. Scheier, Rev. Sci. Instrum., 2020, 91, 033315 CrossRef CAS.
- L. F. Gomez, K. R. Ferguson, J. P. Cryan, C. Bacellar, R. M. P. Tanyag, C. Jones, S. Schorb, D. Anielski, A. Belkacem, C. Bernando, R. Boll, J. Bozek, S. Carron, G. Chen, T. Delmas, L. Englert, S. W. Epp, B. Erk, L. Foucar, R. Hartmann, A. Hexemer, M. Huth, J. Kwok, S. R. Leone, J. H. S. Ma, F. R. N. C. Maia, E. Malmerberg, S. Marchesini, D. M. Neumark, B. Poon, J. Prell, D. Rolles, B. Rudek, A. Rudenko, M. Seifrid, K. R. Siefermann, F. P. Sturm, M. Swiggers, J. Ullrich, F. Weise, P. Zwart, C. Bostedt, O. Gessner and A. F. Vilesov, Science, 2014, 345, 906–909 CrossRef CAS.
- O. Gessner and A. F. Vilesov, Annu. Rev. Phys. Chem., 2019, 70, 173–198 CrossRef CAS.
- F. Huisken and M. Stemmler, J. Chem. Phys., 1993, 98, 7680 CrossRef CAS.
- M. Ahmed, C. J. Apps, C. Hughes, N. E. Watt and J. C. Whitehead, J. Phys. Chem. A, 1997, 101, 1250 CrossRef CAS.
- M. Ahmed, C. J. Apps, R. Buesnel, C. Hughes, H. Hillier, N. E. Watt and J. C. Whitehead, J. Phys. Chem. A, 1997, 101, 1250 CrossRef CAS.
- R. Moro, R. Rabinovitch and V. V. Kresin, Rev. Sci. Instrum., 2005, 76, 056104 CrossRef.
- R. Moro, R. Rabinovitch and V. V. Kresin, J. Chem. Phys., 2005, 123, 074301 CrossRef.
- C. P. Schulz, R. Haugstätter, H. U. Tittes and I. V. Hertel, Phys. Rev. Lett., 1986, 57, 1703–1706 CrossRef CAS.
- C. Nitsch, C. P. Schulz, A. Gerber, W. Zimmermann-Edling and I. V. Hertel, Z. Phys. D, 1992, 22, 651 CrossRef CAS.
- C. Bobbert, S. Schütte, C. Steinbach and U. Buck, Eur. Phys. J. D, 2002, 19, 183–192 CAS.
- U. Buck, C. C. Pradzinski, T. Zeuch, J. M. Dieterich and B. Hartke, Phys. Chem. Chem. Phys., 2014, 16, 6859–6871 RSC.
- B. L. Yoder, J. H. Litman, P. W. Forysinski, J. L. Corbett and R. Signorell, J. Phys. Chem. Lett., 2011, 2, 2623–2628 CrossRef CAS.
- P. Forysinski, P. Zielke, D. Luckhaus, J. Corbett and R. Signorell, J. Chem. Phys., 2011, 134, 094314 CrossRef.
- M. Huttula, M.-H. Mikkelä, M. Tchaplyguine and O. Björneholm, J. Electron Spectrosc. Relat. Phenom., 2010, 181, 145–149 CrossRef CAS.
- C. Zhang, T. Andersson, S. Svensson, O. Björneholm, M. Huttula, M.-H. Mikkelä, M. Tchaplyguine and G. Öhrwall, J. Chem. Phys., 2011, 134, 124507 CrossRef.
- C. Zhang, T. Andersson, S. Svensson, O. Björneholm, M. Huttula, M.-H. Mikkelä, D. Anin, M. Tchaplyguine and G. Öhrwall, J. Phys. Chem. A, 2012, 116, 12104–12111 CrossRef CAS.
- L. Hautala, K. Jänkälä, M.-H. Mikkelä, M. Tchaplyguine and M. Huttula, Phys. Chem. Chem. Phys., 2015, 17, 7012–7022 RSC.
- L. Hautala, K. Jänkälä, T. Löytynoja, M.-H. Mikkelä, N. L. Prisle, M. Tchaplyguine and M. Huttula, Phys. Rev. B, 2017, 95, 045402 CrossRef.
- L. Partanen, M.-H. Mikkelä, M. Huttula, M. Tchaplyguine, C. Zhang, T. Andersson and O. Björneholm, J. Chem. Phys., 2013, 138, 044301 CrossRef.
- L. Hautala, K. Jänkälä, M.-H. Mikkelä, P. Turunen, N. L. Prisle, M. Patanen, M. Tchaplyguine and M. Huttula, Phys. Chem. Chem. Phys., 2017, 19, 25158–25167 RSC.
- J. Cuvellier, P. Meynadier, P. de Pujo, O. Sublemontier, J.-P. Visticot, J. Berlande, A. Lallement and J.-M. Mestdagh, Z. Phys. D, 1991, 21, 265 CrossRef CAS.
- J. H. Litman, B. L. Yoder, B. Schläppi and R. Signorell, Phys. Chem. Chem. Phys., 2013, 15, 940–949 RSC.
- M. Ahmed and O. Kostko, Phys. Chem. Chem. Phys., 2020, 22, 2713–2737 RSC.
- J. Farges, M. F. de Feraudy, B. Raoult and G. Torchet, Surf. Sci., 1981, 106, 95 CrossRef CAS.
- J. Farges, M. F. de Feraudy, B. Raoult and G. Torchet, J. Chem. Phys., 1983, 78, 5067 CrossRef CAS.
- G. Torchet, P. Schwartz, J. Farges, M. F. de Feraudy and B. Raoult, J. Chem. Phys., 1983, 79, 6196 CrossRef CAS.
- J. Farges, M. F. de Feraudy, B. Raoult and G. Torchet, J. Chem. Phys., 1986, 84, 3491 CrossRef CAS.
- G. Torchet, M. F. de Feraudy and B. Raoult, J. Chem. Phys., 1995, 103, 3074–3083 CrossRef CAS.
- U. Buck and R. Krohne, J. Chem. Phys., 1996, 105, 5408–5415 CrossRef CAS.
- A. D. Martino, M. Benslimane, M. Châtelet, C. Crozes, F. Padère and H. Vach, Z. Phys. D, 1993, 27, 185 CrossRef.
- U. Buck and H. Meyer, Phys. Rev. Lett., 1984, 52, 109 CrossRef CAS.
- U. Buck and H. Meyer, J. Chem. Phys., 1986, 84, 4854 CrossRef CAS.
- U. Buck, J. Phys. Chem., 1988, 92, 1023 CrossRef CAS.
- R. Moro, R. Rabinovitch, C. Xia and V. V. Kresin, Phys. Rev. Lett., 2006, 97, 123401 CrossRef.
- H. Bieker, J. Onvlee, M. Johny, L. He, T. Kierspel, S. Trippel, D. A. Horke and J. Küpper, J. Phys. Chem. A, 2019, 123, 7486–7490 CrossRef CAS.
- B. S. Kamerin, J. W. Niman and V. V. Kresin, J. Chem. Phys., 2020, 153, 081101 CrossRef.
- J. Lengyel, A. Pysanenko, P. Rubovič and M. Fárník, Eur. Phys. J. D, 2015, 69, 269 CrossRef.
- M. Macler and Y. K. Bae, J. Phys. Chem. A, 1997, 101, 145 CrossRef CAS.
- J. Vigué, P. Labastie and F. Calvo, Eur. Phys. J. D, 2000, 8, 265 CrossRef.
- O. F. Hagena, Surf. Sci., 1981, 106, 101–116 CrossRef CAS.
- O. F. Hagena, Z. Phys. D, 1987, 4, 291–299 CrossRef CAS.
- O. F. Hagena, Rev. Sci. Instrum., 1992, 63, 2374–2379 CrossRef CAS.
- C. Huang, V. V. Kresin, A. Pysanenko and M. Fárník, J. Chem. Phys., 2016, 145, 104304 CrossRef.
- V. Buch, S. Bauerecker, J. P. Devlin, U. Buck and J. K. Kazimirski, Int. Rev. Phys. Chem., 2004, 23, 375 Search PubMed.
- C. Li, M. Lippe, J. Krohn and R. Signorell, J. Chem. Phys., 2019, 151, 094305 CrossRef.
- S. Zamith, P. Feiden, P. Labastie and J.-M. L'Hermite, Phys. Rev. Lett., 2010, 104, 103401 CrossRef.
- S. Zamith, P. Feiden, P. Labastie and J.-M. L'Hermite, J. Chem. Phys., 2010, 133, 154305 CrossRef.
- S. Zamith, G. de Tournadre, P. Labastie and J.-M. L'Hermite, J. Chem. Phys., 2013, 138, 034301 CrossRef.
- S. Zamith, P. Feiden, P. Labastie and J.-M. L'Hermite, J. Chem. Phys., 2014, 141, 139901 CrossRef.
- H. Vehkamäki, Classical Nucleation Theory in Multicomponent Systems, Springer-Verlag, Berlin, 2006 Search PubMed.
- R. Zhang, A. Khalizov, L. Wang, M. Hu and W. Xu, Chem. Rev., 2012, 112, 1957–2011 CrossRef CAS.
- B. E. Wyslouzil and J. Wölk, J. Chem. Phys., 2016, 145, 211702 CrossRef.
- J. Wölk, R. Strey, C. H. Heath and B. E. Wyslouzil, J. Chem. Phys., 2002, 117, 4954 CrossRef.
- A. Manka, D. Brus, A.-P. Hyvärinen, H. Lihavainen, J. Wölk and R. Strey, J. Chem. Phys., 2010, 132, 244505 CrossRef.
- A. Manka, H. Pathak, S. Tanimura, J. Wölk, R. Strey and B. E. Wyslouzil, Phys. Chem. Chem. Phys., 2012, 14, 4505 RSC.
- J. Wölk and R. Strey, J. Phys. Chem. B, 2001, 105, 11683–11701 CrossRef.
- V. I. Kalikmanov, J. Chem. Phys., 2006, 124, 124505 CrossRef CAS.
- M. Kulmala, Atmos. Res., 2010, 98, 201 CrossRef CAS.
- H. Vehkamäki, M. J. McGrath, T. Kurtén, J. Julin, K. E. J. Lehtinen and M. Kulmala, J. Chem. Phys., 2012, 136, 094107 CrossRef.
- R. J. Weber, J. J. Marti, P. H. McMurry, F. L. Eisele, D. J. Tanner and A. Jefferson, J. Geophys. Res., 1997, 102, 4375–4385 CrossRef CAS.
- R. J. Weber, P. H. McMurry, R. L. Mauldin, D. J. Tanner, F. L. Eisele, A. D. Clarke and V. N. Kapustin, Geophys. Res. Lett., 1999, 26, 307–310 CrossRef CAS.
- S.-L. Sihto, M. Kulmala, V.-M. K. M. D. Maso, T. Petäjä, I. Riipinen, H. Korhonen, F. A. R. Janson, M. Boy, A. Laaksonen and K. E. J. Lehtinen, Atmos. Chem. Phys., 2006, 6, 4079–4091 CrossRef CAS.
- M. Kulmala, I. Riipinen, M. Sipila, H. E. Manninen, T. Petaja, H. Junninen, M. D. Maso, G. Mordas, A. Mirme, M. Vana, A. Hirsikko, L. Laakso, R. M. Harrison, I. Hanson, C. Leung, K. E. J. Lehtinen and V.-M. Kerminen, Science, 2007, 318, 89–92 CrossRef CAS.
- T. Peter, Annu. Rev. Phys. Chem., 1997, 48, 785–822 CrossRef CAS.
- J. Lengyel, M. Ončák, J. Fedor, J. Kočišek, A. Pysanenko, M. K. Beyer and M. Fárník, Phys. Chem. Chem. Phys., 2017, 19, 11753–11758 RSC.
- J. Lengyel, J. Fedor and M. Fárník, Phys. Chem. Chem. Phys., 2019, 21, 8691–8697 RSC.
- A. Pysanenko, J. Lengyel and M. Fárník, J. Chem. Phys., 2017, 148, 154301 CrossRef.
- J. Lengyel, A. Pysanenko, K. Fárníková, E. Pluhařová and M. Fárník, J. Phys. Chem. Lett., 2020, 11, 2101–2105 CrossRef CAS.
- B. J. Finlayson-Pitts and J. N. Pitts, Chemistry of the upper and lower atmosphere, Academic Press, San Diego, 2000, p. 969 Search PubMed.
- T. Huthwelker, M. Ammann and T. Peter, Chem. Rev., 2006, 106, 1375–1444 CrossRef CAS.
- P. Davidovits, C. E. Kolb, L. R. Williams, J. T. Jayne and D. R. Worsnop, Chem. Rev., 2006, 106, 1323–1354 CrossRef CAS.
- C. E. Kolb, R. A. Cox, J. P. D. Abbatt, M. Ammann, E. J. Davis, D. J. Donaldson, B. C. Garrett, C. George, P. T. Griffiths, D. R. Hanson, M. Kulmala, G. McFiggans, U. Poschl, I. Riipinen, M. J. Rossi, Y. Rudich, P. E. Wagner, P. M. Winkler, D. R. Worsnop and C. D. O'Dowd, Atmos. Chem. Phys., 2010, 10, 10561–10605 CrossRef CAS.
- R. Y. Zhang, I. Suh, J. Zhao, D. Zhang, E. C. Fortner, X. X. Tie, L. T. Molina and M. J. Molina, Science, 2004, 304, 1487–1490 CrossRef CAS.
- A. Laaksonen, M. Kulmala, C. D. O'Dowd, J. Joutsensaari, P. Vaattovaara, S. Mikkonen, K. E. J. Lehtinen, L. Sogacheva, M. Dal Maso, P. Aalto, T. Petäjä, A. Sogachev, Y. J. Yoon, H. Lihavainen, D. Nilsson, M. C. Facchini, F. Cavalli, S. Fuzzi, T. Hoffmann, F. Arnold, M. Hanke, K. Sellegri, B. Umann, W. Junkermann, H. Coe, J. D. Allan, M. R. Alfarra, D. R. Worsnop, M. L. Riekkola, T. Hyötyläinen and Y. Viisanen, Atmos. Chem. Phys., 2008, 8, 2657–2665 CrossRef CAS.
- J. N. Smith, M. J. Dunn, T. M. VanReken, K. Iida, M. R. Stolzenburg, P. H. McMurry and L. G. Huey, Geophys. Res. Lett., 2008, 35, L04808 Search PubMed.
- I. Riipinen, T. Yli-Juuti, J. R. Pierce, T. Petäjä, D. R. Worsnop, M. Kulmala and N. M. Donahue, Nat. Geosci., 2012, 5, 453–458 CrossRef CAS.
- N. M. Donahue, I. K. Ortega, W. Chuang, I. Riipinen, F. Riccobono, S. Schobesberger, J. Dommen, U. Baltensperger, M. Kulmala, D. R. Worsnop and H. Vehkamäki, Faraday Discuss., 2013, 165, 91–104 RSC.
- J. Kirkby, J. Curtius, J. Almeida, E. Dunne, J. Duplissy, S. Ehrhart, A. Franchin, S. Gagne, L. Ickes, A. Kürten, A. Kupc, A. Metzger, F. Riccobono, L. Rondo, S. Schobesberger, G. Tsagkogeorgas, D. Wimmer, A. Amorim, F. Bianchi, M. Breitenlechner, A. David, J. Dommen, A. Downard, M. Ehn, R. C. Flagan, S. Haider, A. Hansel, D. Hauser, W. Jud, H. Junninen, F. Kreissl, A. Kvashin, A. Laaksonen, K. Lehtipalo, J. Lima, E. R. Lovejoy, V. Makhmutov, S. Mathot, J. Mikkilä, P. Minginette, S. Mogo, T. Nieminen, A. Onnela, P. Pereira, T. Petäajä, R. Schnitzhofer, J. H. Seinfeld, M. Sipilä, Y. Stozhkov, F. Stratmann, A. Tomé, J. Vanhanen, Y. Viisanen, A. Vrtala, P. E. Wagner, H. Walther, E. Weingartner, H. Wex, P. M. Winkler, K. S. Carslaw, D. R. Worsnop, U. Baltensperger and M. Kulmala, Nature, 2011, 476, 429–433 CrossRef CAS.
- S. Chee, N. Myllys, K. C. Barsanti, B. M. Wong and J. N. Smith, J. Phys. Chem. A, 2019, 123, 5640–5648 CrossRef CAS.
- S. Schobesberger, A. Franchin, F. Bianchi, L. Rondo, J. Duplissy, A. Kürten, I. K. Ortega, A. Metzger, R. Schnitzhofer, J. Almeida, A. Amorim, J. Dommen, E. M. Dunne, M. Ehn, S. Gagné, L. Ickes, H. Junninen, A. Hansel, V.-M. Kerminen, J. Kirkby, A. Kupc, A. Laaksonen, K. Lehtipalo, S. Mathot, A. Onnela, T. Petäjä, F. Riccobono, F. D. Santos, M. Sipilä, A. Tomé, G. Tsagkogeorgas, Y. Viisanen, P. E. Wagner, D. Wimmer, J. Curtius, N. M. Donahue, U. Baltensperger, M. Kulmala and D. R. Worsnop, Atmos. Chem. Phys., 2015, 15, 55–78 CrossRef.
- J. Almeida, S. Schobesberger, A. Kürten, I. K. Ortega, O. Kupiainen-Määttä, A. P. Praplan, A. Adamov, A. Amorim, F. Bianchi, M. Breitenlechner, A. David, J. Dommen, N. M. Donahue, A. Downard, E. Dunne, J. Duplissy, S. Ehrhart, R. C. Flagan, A. Franchin, R. Guida, J. Hakala, A. Hansel, M. Heinritzi, H. Henschel, T. Jokinen, H. Junninen, M. Kajos, J. Kangasluoma, H. Keskinen, A. Kupc, T. Kurtén, A. N. Kvashin, A. Laaksonen, K. Lehtipalo, M. Leiminger, J. Leppä, V. Loukonen, V. Makhmutov, S. Mathot, M. J. McGrath, T. Nieminen, T. Olenius, A. Onnela, T. Petäjä, F. Riccobono, I. Riipinen, M. Rissanen, L. Rondo, T. Ruuskanen, F. D. Santos, N. Sarnela, S. Schallhart, R. Schnitzhofer, J. H. Seinfeld, M. Simon, M. Sipilä, Y. Stozhkov, F. Stratmann, A. Tomé, J. Tröstl, G. Tsagkogeorgas, P. Vaattovaara, Y. Viisanen, A. Virtanen, A. Vrtala, P. E. Wagner, E. Weingartner, H. Wex, C. Williamson, D. Wimmer, P. Ye, T. Yli-Juuti, K. S. Carslaw, M. Kulmala, J. Curtius, U. Baltensperger, D. R. Worsnop, H. Vehkamäki and J. Kirkby, Nature, 2013, 502, 359–363 CrossRef CAS.
- B. A. McGuire, Astrophys. J., Suppl. Ser., 2018, 239(17), 1–48 Search PubMed.
- P. D. Holtom, C. E. Bennett, Y. Osamura, N. J. Mason and R. I. Kaiser, Astrophys. J., 2005, 626, 940–952 CrossRef CAS.
- E. F. van Dishoeck, E. Herbst and D. A. Neufeld, Chem. Rev., 2013, 113, 9043–9085 CrossRef CAS.
- S. Pilling, E. Seperuelo Duarte, E. F. da Silveira, E. Balanzat, H. Rothard, A. Domaracka and P. Boduch, Astron. Astrophys., 2010, 509, A87 CrossRef.
- F. Borget, F. Duvernay, G. Danger, P. Theulé, J.-B. Bossa, V. Vinogradoff, F. Mispelaer, S. Müller, D. Grote and T. Chiavassa, J. Phys. Org. Chem., 2015, 28, 163–169 CrossRef CAS.
- M. J. Abplanalp and R. I. Kaiser, Phys. Chem. Chem. Phys., 2019, 21, 16949–16980 RSC.
- M. P. Bernstein, S. A. Sandford, L. J. Allamandola, J. S. Gillette, S. J. Clemett and R. N. Zare, Science, 1999, 283, 1135–1138 CrossRef CAS.
- E. Rauls and L. Hornekær, Astrophys. J., 2008, 679, 531–536 CrossRef CAS.
- A. Candian, J. Zhen and A. G. G. M. Tielens, Phys. Today, 2018, 71, 38–43 CrossRef CAS.
- A. K. Lemmens, S. Gruet, A. L. Steber, J. Antony, S. Grimme, M. Schnell and A. Rijs, Phys. Chem. Chem. Phys., 2019, 21, 3414–3422 RSC.
- C. Joblin, L. Dontot, G. A. Garcia, F. Spiegelman, M. Rapacioli, L. Nahon, P. Parneix, T. Pino and P. Bréchignac, J. Phys. Chem. Lett., 2017, 8, 3697–3702 CrossRef CAS.
- S. Zamith, M.-C. Ji, J.-M. L'Hermite, C. Joblin, L. Dontot, M. Rapacioli and F. Spiegelman, J. Chem. Phys., 2019, 151, 194303 CrossRef.
- H. A. B. Johansson, H. Zettergren, A. I. S. Holm, F. Seitz, H. T. Schmidt, P. Rousseau, A. aawicki, M. Capron, A. Domaracka, E. Lattouf, S. Maclot, R. Maisonny, B. Manil, J.-Y. Chesnel, L. Adoui, B. A. Huber and H. Cederquist, Phys. Rev. A: At., Mol., Opt. Phys., 2011, 84, 043201 CrossRef.
- R. Delaunay, M. Gatchell, P. Rousseau, A. Domaracka, S. Maclot, Y. Wang, M. H. Stockett, T. Chen, L. Adoui, M. Alcamí, F. Martín, H. Zettergren, H. Cederquist and B. A. Huber, J. Phys. Chem. Lett., 2015, 6, 1536–1542 CrossRef CAS.
- M. Gatchell, M. H. Stockett, N. de Ruette, L. G. T. Chen, R. F. Nascimento, M. Wolf, E. K. Anderson, R. Delaunay, V. Vizcaino, P. Rousseau, L. Adoui, B. A. Huber, H. T. Schmidt, H. Zettergren and H. Cederquist, Phys. Rev. A: At., Mol., Opt. Phys., 2015, 92, 050702 CrossRef.
- M. Mitsui, N. Ando and A. Nakajima, J. Phys. Chem. A, 2007, 111, 9644–9648 CrossRef CAS.
- N. Ando, M. Mitsui and A. Nakajima, J. Chem. Phys., 2008, 128, 154318 CrossRef.
- M. Goulart, M. Kuhn, B. Rasul, J. Postler, M. Gatchell, H. Zettergren, P. Scheier and O. Echt, Phys. Chem. Chem. Phys., 2017, 19, 27968–27973 RSC.
- M. Miyazaki, A. Fujii, T. Ebata and N. Mikami, J. Phys. Chem. A, 2004, 108, 10656–10660 CrossRef CAS.
- Y. M. Ibrahim, M. Meot-Ner, E. H. Alshraeh, M. S. El-Shall and S. Scheiner, J. Am. Chem. Soc., 2005, 127, 7053–7064 CrossRef CAS.
- I. K. Attaha, S. P. Platt, M. Meot-Ner, M. S. El-Shall, S. G. Aziz and A. O. Alyoubi, Chem. Phys. Lett., 2014, 613, 45–53 CrossRef.
- K. Chatterjee and O. Dopfer, Phys. Chem. Chem. Phys., 2017, 19, 32262–32271 RSC.
- B. Xu, T. Stein, U. Ablikim, L. Jiang, J. Hendrix, M. Head-Gordon and M. Ahmed, Faraday Discuss., 2019, 217, 414–433 RSC.
- J. Bouwman, S. Horst and J. Oomens, ChemPhysChem, 2018, 19, 3211–3218 CrossRef CAS.
- M. A. R. George, M. Förstel and O. Dopfer, Angew. Chem., Int. Ed., 2020, 59, 12098–12104 CrossRef CAS.
- X. Biquard, O. Sublemontier, J. P. Visticot, J. M. Mestdagh, P. Meynadier, M. A. Gaveau and J. Berlande, Z. Phys. D, 1994, 30, 45–52 CrossRef CAS.
- M. Lewerenz, B. Schilling and J. P. Toennies, J. Chem. Phys., 1995, 102, 8191 CrossRef CAS.
- A. Pysanenko, A. Habartová, P. Svrčková, J. Lengyel, V. Poterya, M. Roeselová, J. Fedor and M. Fárník, J. Phys. Chem. A, 2015, 119, 8991–8999 CrossRef CAS.
- M. P. Bernstein, J. P. Dworkin, S. A. Sandford, G. W. Cooper and L. J. Allamandola, Nature, 2002, 416, 401–403 CrossRef CAS.
- G. M. M. Caro, U. J. Meierhenrich, W. A. Schutte, B. Barbier, A. A. Segovia, H. Rosenbauer, W. H.-P. Thiemann, A. Brack and J. M. Greenberg, Nature, 2002, 416, 403–406 CrossRef.
- N. Goldman and I. Tamblyn, J. Phys. Chem. A, 2013, 117, 5124–5131 CrossRef CAS.
- A. Pysanenko, J. Kočišek, V. Poterya and M. Fárník, J. Phys. Chem. A, 2017, 121, 1069–1077 CrossRef CAS.
- K. Grygoryeva, M. Ončák, A. Pysanenko and M. Fárník, Phys. Chem. Chem. Phys., 2019, 21, 8221–8227 RSC.
- F. Gámez, A. Pysanenko, M. Fárník and M. Ončák, Phys. Chem. Chem. Phys., 2019, 21, 19201–19208 RSC.
- V. Poterya, M. Fárník, P. Slavíček, U. Buck and V. V. Kresin, J. Chem. Phys., 2007, 126, 071101 CrossRef.
- M. Fárník, A. Pysanenko, K. Moriová, L. Ballauf, P. Scheier, J. Chalabala and P. Slavíček, J. Phys. Chem. A, 2018, 122, 8458–8468 CrossRef.
- A. Pysanenko, F. Gámez, K. Fárníková, E. Pluhařová and M. Fárník, J. Phys. Chem. A, 2019, 123, 7201–7209 CrossRef CAS.
- R. Schinke, Photodissociation dynamics, Cambridge University Press, Cambridge, 1993, pp. 8–10 Search PubMed.
- H. Sato, Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 2004, 100, 73–98 RSC.
- A. L. Harris, J. K. Brown and C. B. Harris, Ann. Rev. Phys. Chem., 1988, 39, 341 CrossRef CAS.
- R. B. Gerber, A. B. McCoy and A. García-Vela, Ann. Rev. Phys. Chem., 1994, 45, 275–314 CrossRef CAS.
- R. Alimi and R. B. Gerber, Phys. Rev. Lett., 1990, 64, 1453 CrossRef CAS.
- S. Solomon, R. R. Garcia, F. S. Rowland and D. J. Wuebbles, Nature, 1986, 321, 755 CrossRef CAS.
- S. Solomon, Rev. Geophys., 1999, 37, 275–316 CrossRef CAS.
- K. Kawamura, Y. Imai and L. A. Barrie, Atmos. Environ., 2005, 39, 599–614 CrossRef CAS.
- R. Bianco and J. Hynes, Acc. Chem. Res., 2006, 39, 159 CrossRef CAS.
- A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2002, 4, 4 RSC.
- A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2003, 107, 1557 CrossRef CAS.
- A. L. Sobolewski and W. Domcke, J. Chem. Phys., 2005, 122, 184320 CrossRef.
- A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2007, 9, 3818 RSC.
- F. Uhlig, O. Marsalek and P. Jungwirth, Phys. Chem. Chem. Phys., 2011, 13, 14003–14009 RSC.
- M. Ončák, P. Slavíček, V. Poterya, M. Fárník and U. Buck, J. Phys. Chem. A, 2008, 112, 5344–5353 CrossRef.
- V. Poterya, J. Fedor, A. Pysanenko, O. Tkáč, J. Lengyel, M. Ončák, P. Slavíček and M. Fárník, Phys. Chem. Chem. Phys., 2011, 13, 2250–2258 RSC.
- M. Ončák, P. Slavíček, M. Fárník and U. Buck, J. Phys. Chem. A, 2011, 115, 6155–6168 CrossRef.
- V. Poterya, J. Kočišek, A. Pysanenko and M. Fárník, Phys. Chem. Chem. Phys., 2014, 16, 421–429 RSC.
- B. Brutschy, J. Phys. Chem., 1990, 94, 8637–8647 CrossRef CAS.
- C. G. Nolte, P. A. Solomon, T. Fall, L. G. Salmon and G. R. Cass, Environ. Sci. Technol., 1997, 31, 2547–2553 CrossRef CAS.
- A. J. Eugene and M. I. Guzmán, J. Phys. Chem. A, 2017, 121, 2924–2935 CrossRef CAS.
- A. E. Reed Harris, A. Pajunoja, M. Cazaunau, A. Gratien, E. Pangui, A. Monod, E. C. Griffith, A. Virtanen, J. F. Doussin and V. Vaida, J. Phys. Chem. A, 2017, 121, 3327–3339 CrossRef CAS.
- W. A. Brown and A. S. Bolina, Mon. Not. R. Astron. Soc., 2007, 374, 1006–1014 CrossRef CAS.
- P. Ehrenfreund, L. d'Hendecourt, E. Dartois, M. Jourdain de Muizon, M. Breitfellner, J. L. Puget and H. J. Habing, Icarus, 1997, 130, 1–15 CrossRef CAS.
- E. L. Gibb, D. C. B. Whittet, A. C. A. Boogert and A. G. G. M. Tielens, Astrophys. J., Suppl. Ser., 2004, 151, 35–73 CrossRef CAS.
- K. M. Pontoppidan, E. F. van Dishoeck and E. Dartois, Astron. Astrophys., 2004, 426, 925–940 CrossRef CAS.
- N. Biver, D. Bockelée-Morvan, P. Colom, J. Crovisier, J. K. Davies, W. R. F. Dent, D. Despois, E. Gérard, E. Lellouch, H. Rauer, R. Moreno and G. Paubert, Science, 1997, 275, 1915–1918 CrossRef CAS.
- K. I. Öberg, R. T. Garrot, E. F. van Dishoeck and H. Linnartz, Astron. Astrophys., 2009, 504, 891–913 CrossRef.
- K.-J. Chuang, G. Fedoseev, S. Ippolo, E. F. van Dishoeck and H. Linnartz, Mon. Not. R. Astron. Soc., 2016, 455, 1702–1712 CrossRef CAS.
- J. Cernicharo, N. Marcelino, E. Roueff, M. Gerin, A. Jiménez-Escobar and G. M. M. Noz Caro, Astrophys. J., 2012, 759, L43 CrossRef.
- C. Bermudez, S. Bailleux and J. Cernicharo, Astron. Astrophys., 2017, 598, A9 CrossRef.
- I. I. Fabrikant, S. Eden, N. J. Mason and J. Fedor, Advances In Atomic, Molecular, and Optical Physics, Elsevier Inc., Academic Press, 2017, vol. 66, pp. 545–657 Search PubMed.
- T. D. Märk, Int. J. Mass Spectrom. Ion Processes, 1991, 107, 143–163 CrossRef.
- O. Ingólfsson, F. Weik and E. Illenberger, Int. J. Mass Spectrom., 1996, 155, 1–68 CrossRef.
- I. I. Fabrikant, Eur. Phys. J. D, 2018, 72, 96 CrossRef.
- A. A. Viggiano and F. Arnold, Planet. Space Sci., 1981, 29, 895–906 CrossRef CAS.
- D. Smith and P. Španěl, Mass Spectrom. Rev., 1995, 14, 255–278 CrossRef CAS.
- F. Arnold, Space Sci. Rev., 2008, 137, 225–239 CrossRef CAS.
- O. P. Balaj, C.-K. Siu, I. Balteanu, M. K. Beyer and V. E. Bondybey, Chem. – Eur. J., 2004, 10, 4822–4830 CrossRef CAS.
- S. J. Retmeier, O. P. Balaj, V. E. Bondybey and M. K. Beyer, Int. J. Mass Spectrom., 2006, 249, 106–111 CrossRef.
- J. Lengyel, C. van der Linde, M. Fárník and M. K. Beyer, Phys. Chem. Chem. Phys., 2016, 18, 23910–23915 RSC.
- C. van der Linde, W. K. Tang, C.-K. Siu and M. K. Beyer, Phys. Chem. Chem. Phys., 2018, 20, 10838–10845 RSC.
- A. Herburger, E. Barwa, M. Ončák, J. Heller, C. van der Linde, D. M. Neumark and M. K. Beyer, J. Am. Chem. Soc., 2019, 141, 18000–18003 CrossRef CAS.
- Q.-B. Lu and T. E. Madey, J. Chem. Phys., 1999, 111, 2861 CrossRef CAS.
- Q.-B. Lu and L. Sanche, Phys. Rev. Lett., 2001, 87, 078501 CrossRef CAS.
- Q.-B. Lu, Phys. Rep., 2010, 487, 141–167 CrossRef CAS.
- R. Müller, Phys. Rev. Lett., 2003, 91, 058502 CrossRef.
- J.-U. Grooß and R. Müller, Atmos. Environ., 2011, 45, 3508–3514 CrossRef.
- J.-U. Grooß and R. Müller, Atmos. Environ., 2013, 68, 350 CrossRef.
- R. Müller and J.-U. Grooß, Int. J. Mod. Phys. B, 2014, 28, 1482001 CrossRef.
- Q.-B. Lu, Int. J. Mod. Phys. B, 2014, 28, 1475003 CrossRef CAS.
- S. Versick, G. P. Stiller, T. von Clarmann, T. Reddmann, N. Glatthor, U. Grabowski, M. Höpfner, S. Kellmann, M. Kiefer, A. Linden, R. Ruhnke and H. Fischer, Atmos. Chem. Phys., 2012, 12, 4923–4933 CrossRef CAS.
- M. J. Loeffler, U. Raut, R. A. Vidal, R. A. Baragiola and R. W. Carlson, Icarus, 2006, 180, 265–273 CrossRef CAS.
- S. Nandi, A. Sanov, N. Delaney, J. Faeder, R. Parson and W. C. Lineberger, J. Phys. Chem., 1998, 102, 8827 CrossRef CAS.
- J. Takagi and K. Ishigure, Nucl. Sci. Eng., 1985, 89, 177–186 CrossRef CAS.
- G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513–886 CrossRef CAS.
- H. Christensen, K. Sehested and T. Løgager, Radiat. Phys. Chem., 1994, 43, 527–531 CrossRef CAS.
- M. Knapp, O. Echt, D. Kreisle and E. Recknagel, J. Phys. Chem., 1987, 91, 2601–2607 CrossRef CAS.
- U. Pöschl, Angew. Chem., Int. Ed., 2005, 44, 7520–7540 CrossRef.
| This journal is © the Owner Societies 2021 |