Substituent effect on the thermophysical properties and thermal dissociation behaviour of 9-substituted anthracene derivatives†
Abstract
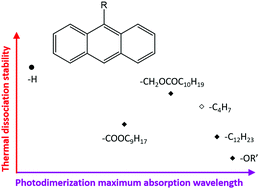
The chemical structure and location of substituents on anthracene derivatives influence the electron balance of the aromatic system, thus determining the wavelengths at which light is absorbed, which results in the photochemically induced dimerization or monomerization. Here, the thermal dissociation kinetics of 7 photodimers of 9-substituted anthracene derivatives are studied using a combination of spectroscopic and calorimetric techniques in the condensed state and compared to scarce literature data on thermal dissociation of other anthracene derivatives. The length and chemical structure of the substituent chains have a clear impact on the melting temperatures of the anthracene derivatives and corresponding photodimers. The crystallinity of the photodimers and monomers in turn influences the thermal dissociation kinetics. The thermal dissociation behaviour and previously published photochemistry data are related to the electronic effects of the substituents by means of the Hammett parameters. Stronger electron-withdrawing effects result in larger red shifts of the maximum wavelength λmax for the photodimerization of the anthracene derivatives. It is also shown that for the studied substitutions on the 9-position of anthracene, the higher the magnitude of the electronic effect – both electron-donating and electron-withdrawing – the faster the thermal dissociation kinetics and thus the lower the thermal stability. The strong electronic effects of the substituents on the thermal and photochemical reactivity of the anthracene derivatives and their photodimers allow tuning of the thermal or photochemical responsiveness, e.g. for polymer networks.


 Please wait while we load your content...
Please wait while we load your content...