Collision-induced dissociation of xylose and its applications in linkage and anomericity identification†
Abstract
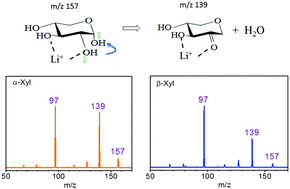
Collision-induced dissociation (CID) of α-xylose and β-xylose were studied using mass spectrometry and quantum chemistry calculations. Three dissociation channels, namely loss of metal ions, dehydration, and cross-ring dissociation were found. The major dissociation channel of sodium adducts is the loss of sodium ions, and the minor dissociation channels are dehydration and cross-ring dissociation. By contrast, dehydration and cross-ring dissociation are the major dissociation channels of lithium adducts, and the corresponding dissociation mechanisms can be used to determine the anomericity and linkages of xylose in oligosaccharides. These mechanisms include (1) the dehydration branching ratio can be used to differentiate the anomericity of xylose and xylose in oligosaccharides because α-xylose has a larger branching ratio of dehydration than β-xylose, (2) various cross-ring dissociation reactions can be used to identify linkage positions. The oligosaccharide with xylose at the reducing end is predicted to undergo 0,2X, 0,3X, and 0,2A cross-ring dissociation for the 1 → 2, 1 → 3, and 1 → 4 linkages, respectively. Application of these mechanisms to determine the anomericity and linkage positions of xylobiose was demonstrated.


 Please wait while we load your content...
Please wait while we load your content...