Single metal atom anchored on a CN monolayer as an excellent electrocatalyst for the nitrogen reduction reaction
Abstract
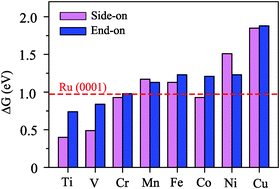
Based on first-principles calculations, we have studied the behavior of single-atom catalysts formed by a series of single metal atoms (from Ti to Cu) and a CN monolayer in nitrogen reduction reactions (NRRs). It was demonstrated that TM atoms could be anchored on CN and Ti@CN has good electrical conductivity, high stability and good catalytic performance. The onset potential of Ti@CN is as low as −0.38 V through the enzymatic mechanism, which well suppresses the competitive hydrogen evolution reaction. In addition, the determinate step of Ti@CN for the N2 reduction reaction is lower than that of the Ru(0001) stepped surface (−0.98 V). We further examine the effect of coordination on activity and propose a single Ti atom anchored on CN as a promising catalyst with high catalytic capability for N2 reduction to NH3. Our work offers a new opportunity and useful guidance for the NRR in an ambient environment.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...