Bulk supercooled water versus adsorbed films on silica surfaces: specific heat by Monte Carlo simulation
Abstract
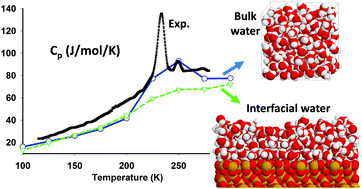
Between 150 and 230.6 K, bulk supercooled water freezes upon cooling, and amorphous ice crystallizes upon heating: bulk water thus exists only in its stable ice form. To circumvent this problem, experiments are generally performed on water adsorbed in SiO2 based porous systems. In this work, we take advantage of Monte Carlo simulations to explore this metastable supercooled region inaccessible to experiments. Using three rigid, non-polarizable water models, namely SPC, TIP4P and TIP4P/2005, we investigate the isobaric specific heat capacity (Cp), between 100 and 300 K, of bulk water and water films of few monolayers adsorbed on different SiO2 surfaces: a smooth surface, a non-hydroxylated (0001) surface of quartz, and a fully hydroxylated (001) surface of cristobalite. As Cp is directly related to the entropy fluctuations and we focus on low temperatures, the convergence of the Monte Carlo simulations is a critical point of this work. Also, due to the small mass of the hydrogen atoms, quantum corrections are taken into account, and lead to an excellent agreement of the simulated and experimental Cp values at low temperature (100 K region). Altogether, we conclude that, in bulk, Cp is shown to exhibit a broad peak around 225 K for the SPC and TIP4P models, and around 250 K for the TIP4P/2005 model, in qualitative agreement with the experimentally observed features in Cp measurements. For interfacial water, in all cases, the broad Cp peak disappears. This result, at odds with experimental observations, suggests that disorder and hydrogen bonding at the interface (not yet taken into account) have a fundamental role in confined water transitions.


 Please wait while we load your content...
Please wait while we load your content...