Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unravelling the nature of citric acid:L-arginine:water mixtures: the bifunctional role of water†
Ana
Roda
 a,
Filipa
Santos
a,
Filipa
Santos
 a,
Yeong Zen
Chua
a,
Yeong Zen
Chua
 b,
Aarti
Kumar
b,
Aarti
Kumar
 c,
Hoang Tam
Do
c,
Hoang Tam
Do
 c,
Alexandre
Paiva
c,
Alexandre
Paiva
 a,
Ana Rita C.
Duarte
a,
Ana Rita C.
Duarte
 a and
Christoph
Held
a and
Christoph
Held
 *c
*c
aLAQV, REQUIMTE, Departamento de Química da Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal. E-mail: a.roda@fct.unl.pt; mfca.santos@campus.fct.unl.pt; alexandre.paiva@fct.unl.pt; aduarte@fct.unl.pt
bInstitute of Physics, University of Rostock, Albert-Einstein-Str. 23-24, 18051 Rostock, Germany
cLaboratory of Thermodynamics, Department of Biochemical and Chemical Engineering, TU Dortmund, 44227 Dortmund, Germany. E-mail: christoph.held@tu-dortmund.de
First published on 4th January 2021
Abstract
The use of water as a component of deep eutectic systems (DES) has raised some questions regarding its influence on the nature of the mixture. Does it form a DES or an aqueous solution and what is the role of water? In this work, the nature of citric acid:L-arginine:water mixtures was explored through phase equilibria studies and spectroscopic analysis. In a first step, PC-SAFT was validated as a predictive tool to model the water influence on the solid liquid equilibria (SLE) of the DES reline using the individual-component approach. Hence, activity coefficients in the ternary systems citric acid:L-arginine:water and respective binary combinations were studied and compared using ePC-SAFT. It was observed that the water-free mixtures citric acid:L-arginine showed positive deviation from Raoult's law, while upon addition of water strong negative deviation from Raoult's law was found, yielding melting depressions around 100 K. Besides these strong interactions, pH was found to become acidic (pH = 3.5) upon water addition, which yields the formation of charged species ([H2Cit]− and [L-arg]+). Thus, the increased interactions between the molecules upon water addition might be caused by several mechanisms such as hydrogen bonding or ionic forces, both being induced by water. For further investigation, the liquid mixtures citric acid:L-arginine:water were studied by FTIR and NMR spectroscopy. FTIR spectra disproved a possible solubility enhancement caused by salt formation between citric acid and L-arginine, while NMR spectra supported the formation of a hydrogen bonding network different from the binary systems citric acid:water and L-arginine:water. Either being a DES or other type of non-ideal solution, the liquefaction of the studied systems is certainly caused by a water-mediator effect based on the formation of charged species and cross interactions between the mixture constituents.
Introduction
First defined by Abbott,1 deep eutectic solvents/systems (DES) are commonly described as mixtures of two or more components. The hydrogen-bonding interactions lead to the formation of a liquid with a lower freezing/melting temperature than the pure constituents.2–5 This concept was also expanded for mixtures with glass transition temperatures instead of melting points, being called Low-Transition-Temperature-Mixtures (LTTMs).6 They have emerged as highly promising systems to be used in several applications due to their versatility and potentially green and sustainable character.2–6Water is recognized as a universal solvent due to its ability in establishing hydrogen-bonding interactions with the solute. In fact “no other molecule has the hydrogen-bonding potential of water”.7 Due to this strong capacity in forming hydrogen bonds, either as hydrogen-bond acceptor or donor, the influence of water on the development of new solvent classes, as the DES, has been explored. Ackhar and co-workers presented a review summarizing some of the studies involving the influence of water in DES, up to 2018. Most of the works reported similar conclusions. Overall, the addition of water to DES up to 50 wt% maintained their original intermolecular network, even after incorporation of water hydrogen-bonds.8 However, above certain amounts, water can solvate the isolated compounds, disrupting the DES supramolecular complex and forming an aqueous solution.8–12 More recent studies also support these findings and further suggest the formation of water-based DES, accompanied by a much deeper melting depression upon water addition compared to the water-free systems.13,14 While these studies are all based on choline chloride-based DES, they highlight the importance of characterizing the water influence on other DES, opening the possibility to include water in the DES preparation as a starting material rather than a solvent.
Recently, mixtures of citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine
L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water of mole ratios 1
water of mole ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 2
7, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 2
7, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 2
8, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 were reported as new DES,15,16 given their low water amounts, between 15 to 25 weight percent. Although the authors named these mixtures as DES, given the complexity of these systems and the presence of water, other possibilities may be considered. Is the water acting as a solvent, forming a regular aqueous solution? Are the citric acid and L-arginine forming a salt? The nature of these mixtures was explored by state-of-the-art parameters commonly used to define thermodynamic properties and the intermolecular interactions of the DESs.17
9 were reported as new DES,15,16 given their low water amounts, between 15 to 25 weight percent. Although the authors named these mixtures as DES, given the complexity of these systems and the presence of water, other possibilities may be considered. Is the water acting as a solvent, forming a regular aqueous solution? Are the citric acid and L-arginine forming a salt? The nature of these mixtures was explored by state-of-the-art parameters commonly used to define thermodynamic properties and the intermolecular interactions of the DESs.17
In this regard, solid–liquid equilibria (SLE) studies are an important tool as they can provide information about the composition, temperature range and intermolecular interactions responsible for the solid–liquid phase transitions. Namely, the melting temperatures and the activity coefficients of a certain mixture allow to evaluate if they present a negative deviation from ideality (Raout's law).18–21 From this, the activity coefficients translate the cross-interactions affinity between the constituents of the mixture, evaluating their ‘predisposition’ to cross-interact. Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) is an equation-of-state-based model that can be applied in modelling the SLE of mixtures considering hard-chain fluids as a reference.18–22 In addition to the short-range interactions considered in PC-SAFT, electrolyte PC-SAFT (ePC-SAFT) is a variant that also accounts for long-range interactions promoted by charged species.23 Further, PC-SAFT was considered to be highly accurate in a recent review comparing several thermodynamic modelling techniques.22
Beyond the thermodynamic properties, the establishment and type of intermolecular interactions responsible for the liquefaction of these mixtures must be addressed. Fourier transform infrared (FTIR) and nuclear magnetic resonance (NMR) spectroscopy are commonly used for this purpose. FTIR spectroscopy identifies the molecular functional groups of the involved species by specific vibrational frequencies defined by their dipole moment. Further, FTIR can be used to detect intermolecular interactions because they change the dipole moments of the involved functional groups, shifting the respective signals in the FTIR spectra.17,24,25 NMR spectroscopy can be used to identify hydrogen bonding interactions or modifications through shifts in proton NMR spectra.26–28 Additionally, information regarding intermolecular and spatial correlations can be obtained through two-dimensional nuclear Overhauser effect spectroscopy (2D NOESY) analysis.26
In the present work we characterized the SLE and other physicochemical properties of the ternary system citric acid:L-arginine:water through the introduced techniques while presenting some limitations of these tools towards distinction between a DES from a regular solution.
Experimental
Chemicals
Citric acid monohydrate [5949-29-1] (≥99.5% purity) was purchased from PanReac AppliChem (Barcelona, Spain). L-Arginine [74-79-3] (≥98% purity) was acquired from Sigma Aldrich (St. Louis, MO, USA). The ternary mixtures of citric acid:L-arginine:water were prepared by heating and stirring, as described by Santos et al.15 Deionized water was used for the preparation of these mixtures. Anhydrous high purity nitrogen was used for DSC measurements. Sodium hydroxide pellets (NaOH, >99% purity) [1310-73-2] and hydrochloric acid 37% (HCl) [7647-01-0] were bought from NormaxChem (Marinha Grande, Portugal) and dimethyl sulfoxide-d6 (DMSO-d6, 99,96% D) [2206-27-1] from Eurisotop (St. Aubin, France).Methods
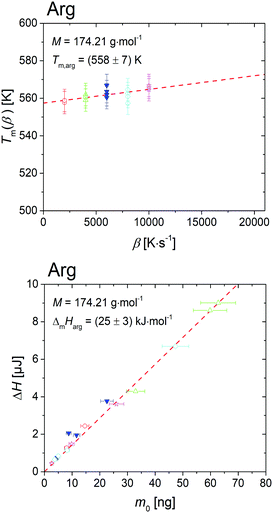
The crystallized sample L-arginine was heated with high scanning rates, β varying from 2000 K s−1 to 10000 K s−1 without sample degradation. The onset temperature of melting peak for scanning rates, Tm(β), is shown in Fig. 1 (top), where the melting temperature of L-arginine was defined at zero heating rate, i.e. Tm,arg = Tm(β → 0), taking into consideration the thermal lag and possible super-heating.31,32Fig. 1 (bottom) shows the enthalpy ΔH of the melting peak of L-arginine with respect to sample mass, m0. The melting enthalpy of L-arginine is denoted as the slope of the linear fit through zero, regardless of the scanning rates.29,30,33,34 The experimentally determined melting properties of L-arginine in this work are summarized in Table 1.
DSC Q200 from TA Instruments Inc. was used to determine the melting temperature of citric acid. The sample was sealed in an aluminium hermetic pan/lid, and it was submitted to two cooling/heating cycles between 183 and 473 K, at a rate of 10 K min−1, at a nitrogen at flow rate of 50 mL min−1. The thermal analysis of the ternary mixture citric acid:L-arginine:water was made using the same method. The thermic events were acquired through the TA Universal Analysis 2000 (4.7.0.2) software.
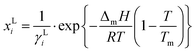 | (1) |
For the SLE modelling of the ternary mixtures reline:water and citric acid:L-arginine:water, eqn (1) was resolved to T, to predict the influence of water addition in the melting temperature of the ternary mixtures at fixed ratios of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (reline) or citric acid:L-arginine. The melting properties used in this work are summarized in Table 1.
urea (reline) or citric acid:L-arginine. The melting properties used in this work are summarized in Table 1.
PC-SAFT or ePC-SAFT was applied for the determination of the activity coefficients (γi), using the following equations:
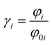 | (2) |
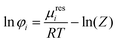 | (3) |
| ares = ahc + adisp + aassoc + aion | (4) |
| kij = kaij + kTij (T – 298.15 K) | (5) |
Results and discussion
Solid–liquid equilibria
Regarding the ternary mixtures of citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine
L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, thermal events were acquired for ratios between 1
water, thermal events were acquired for ratios between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 1
7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (molar). The mixtures have glass transition temperatures (Tg) ranging from 217 to 238 K, respectively.
4 (molar). The mixtures have glass transition temperatures (Tg) ranging from 217 to 238 K, respectively.
Moreover, the mixtures with higher water content (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 1
7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) were found to have melting peaks of around 260 K. This phenomenon did not occur for the mixtures with lower water content (1
6) were found to have melting peaks of around 260 K. This phenomenon did not occur for the mixtures with lower water content (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4).
4).
Within the studied range of temperature and molar ratios, no other thermal events were detected. Since no experimental Tm values for the ternary mixtures could be obtained, it is not possible to apply a thermodynamic correlation model (e.g. common activity coefficient models) for SLE modelling. Thus, eqn (1) requires a predictive model for the determination of activity coefficients. PC-SAFT was used for this purpose. As quantitative validation is impossible for citric acid:L-arginine:water, in a first step the SLE for reline:water was predicted, for which experimental data is available in literature.45
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio), was experimentally studied by Meng et al.45 In the present work, PC-SAFT was used to model the SLE using the individual-component approach; that is, the three components ChCl:urea:water were explicitly considered for modelling. The pure-component parameters are available in the literature, see Table 3. One binary parameter between ChCl and urea was fitted in this work to match the composition of reline at the eutectic temperature, listed in Table 4. As observed in Fig. 2, PC-SAFT allows quantitatively predicting the decrease in the melting temperature (ΔT) of reline upon addition of water; the result is called ‘prediction’ as no parameter was adjusted to the experimental data of the ternary mixture ChCl:urea:water. This validates the use of PC-SAFT to predict the influence of water on melting temperatures. Thus, the individual-component approach within PC-SAFT is a powerful approach to predict SLE for systems that lack experimental data, such as for citric acid:L-arginine:water.
2 molar ratio), was experimentally studied by Meng et al.45 In the present work, PC-SAFT was used to model the SLE using the individual-component approach; that is, the three components ChCl:urea:water were explicitly considered for modelling. The pure-component parameters are available in the literature, see Table 3. One binary parameter between ChCl and urea was fitted in this work to match the composition of reline at the eutectic temperature, listed in Table 4. As observed in Fig. 2, PC-SAFT allows quantitatively predicting the decrease in the melting temperature (ΔT) of reline upon addition of water; the result is called ‘prediction’ as no parameter was adjusted to the experimental data of the ternary mixture ChCl:urea:water. This validates the use of PC-SAFT to predict the influence of water on melting temperatures. Thus, the individual-component approach within PC-SAFT is a powerful approach to predict SLE for systems that lack experimental data, such as for citric acid:L-arginine:water.
| Mixture | Molar ratio | Physical state | pH |
|---|---|---|---|
| a Diluted with H2O until a translucid liquid was obtained. b Diluted with water and HCl until a translucid liquid at pH around 3.5 was obtained. | |||
Citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
White solid paste | — |
Citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
White solid paste | — |
Citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
Translucid liquid | 4.76 |
Citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
Translucid liquid | 3.58 ± 0.15 |
Citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
Translucid liquid | 0.46 |
Citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O pH 3.5 H2O pH 3.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
White solid paste | — |
Citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O pH 3.5 H2O pH 3.5 |
—a | Translucid liquid | 3.62 |
L-Arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
White solid paste | — |
L-Arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O pH 3.5 H2O pH 3.5 |
—b | Translucid liquid | 3.43 |
| Compound | σ i (Å) | u i /kB | m seg i | ε AiBi/kB | k AiBi | N HBD/NHBA | Ref. |
|---|---|---|---|---|---|---|---|
| a Temperature-dependent segment diameter used according to ref. 67. b Pure-component parameters of their charged species set equal to the neutral molecules. | |||||||
| Urea | 2.4456 | 368.2302 | 4.244115 | 3068.31 | 0.0010 | 1/1 | 35 |
| ChCl | 2.3678 | 228.0702 | 13.01519 | 8000.00 | 0.2000 | 1/1 | 66 |
| Water | 2.7927a | 353.9449 | 1.204659 | 2425.67 | 0.04509 | 1/1 | 67 |
| L-Arginineb | 2.6572 | 349.7065 | 9.90818 | 2555.45 | 0.03926 | 3/1 | 38 |
| Citric acidb | 2.7230 | 227.1800 | 8.54600 | 2488.00 | 0.04400 | 4/4 | 20 |
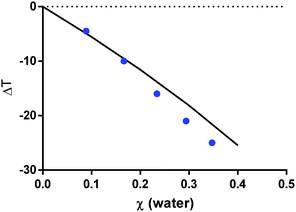 | ||
| Fig. 2 Decrease of the melting temperature at the eutectic composition of reline upon water addition. Symbols correspond to experimental data45 while the line corresponds to the PC-SAFT prediction results using the melting properties from Table 1 and the PC-SAFT parameters from Tables 2 and 3. ΔT = 0 correspond to the binary mixture without water. | ||
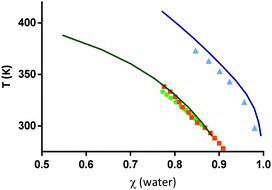 | ||
| Fig. 3 Solubility data of L-arginine in water (triangles46) and of citric acid in water (squares;47 circles48). Symbols correspond to experimental data while the lines correspond to PC-SAFT modelling results using the melting properties from Table 1 and the PC-SAFT parameters from Tables 2 and 3. χ represents the mole fraction. | ||
The SLE of the binary mixture citric acid:L-arginine was predicted with PC-SAFT using the parameters from Table 3 and the results are shown in Fig. 4. A qualitatively similar phase behaviour to the mixture L-arginine:water can be observed for citric acid:L-arginine, translating the low solubility of L-arginine in both, citric acid and water. From these, the predicted eutectic point of the mixture citric acid:L-arginine (χ(citric acid) ∼ 0.96 with a Tm ∼ 425.4 K) is almost equivalent to Tm of pure citric acid (426.9 K). Moreover, the values of the activity coefficients of citric acid and L-arginine in this mixture are greater than one (Fig. 6, χ(water) = 0), indicating rather weak attractive cross-interactions between citric acid and L-arginine. Besides, it presents a positive deviation from the ideal SLE calculations (blue dashed line), strengthening the lack of interactions between citric acid:L-arginine in a binary system.
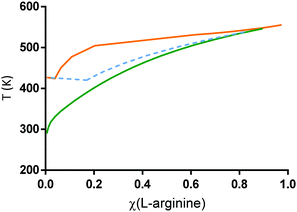 | ||
| Fig. 4 PC-SAFT modelling of the SLE for the binary mixtures L-arginine:water (green line) and L-arginine:citric acid (orange line) using the melting properties from Table 1 and the PC-SAFT parameters from Tables 2 and 3. The dashed blue line represents the ideal SLE of L-arginine:citric acid based on eqn (1). | ||
The PC-SAFT prediction shown in Fig. 4 supports the fact that a DES is not formed between citric acid and L-arginine. Although experimental validation by DSC was not possible for this binary system, it can be reported that this mixture was prepared in the lab and it was found to be solid at room temperature. This scenario changes upon water addition. As reported by Santos et al. and Roda et al., ternary mixtures of citric acid:L-arginine:water at certain compositions are liquid at room temperature.15,16 In the present work, the SLE of these mixtures was studied (Fig. 5).
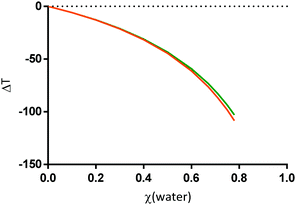 | ||
Fig. 5 PC-SAFT prediction of the influence of water on Tm (expressed as ΔT) upon water addition to the mixture citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine 1 L-arginine 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar), using the melting properties from Table 1 and the PC-SAFT parameters from Tables 2 and 3. Orange line: ΔT for the solubility of citric acid in the mixture; green line: ΔT for the solubility of L-arginine in the mixture. ΔT = 0 corresponds to the binary mixture without water. Data from Table S1 (ESI†). 1 (molar), using the melting properties from Table 1 and the PC-SAFT parameters from Tables 2 and 3. Orange line: ΔT for the solubility of citric acid in the mixture; green line: ΔT for the solubility of L-arginine in the mixture. ΔT = 0 corresponds to the binary mixture without water. Data from Table S1 (ESI†). | ||
The PC-SAFT predictions from Fig. 5 might not be quantitatively correct, since it was not possible to experimentally determine the Tm. Nevertheless, as the model showed to qualitatively predict the ΔT for reline upon water addition (Fig. 2), a qualitatively correct prediction of the SLE using PC-SAFT is expected also for modelling the water influence on the mixture of citric acid:L-arginine:water. Hence, PC-SAFT was used to model the ΔT of the system citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine (at 1
L-arginine (at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar composition) upon water addition. As observed in Fig. 5, there is a pronounced decrease of ΔT with water addition to the system citric acid
1 molar composition) upon water addition. As observed in Fig. 5, there is a pronounced decrease of ΔT with water addition to the system citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine. In comparison to the binary citric acid:L-arginine, there is a Tm reduction of about 100 K for the ternary mixture of citric acid
L-arginine. In comparison to the binary citric acid:L-arginine, there is a Tm reduction of about 100 K for the ternary mixture of citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine
L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (molar, χ(water) = 0.78). This deep reduction in Tm could be compliable with the formation of a DES, but it might also be just the formation of a regular solution, which will be discussed in the following paragraphs.
7 (molar, χ(water) = 0.78). This deep reduction in Tm could be compliable with the formation of a DES, but it might also be just the formation of a regular solution, which will be discussed in the following paragraphs.
The PC-SAFT predictions shown in Fig. 3 and 5 (binary and ternary mixtures containing water, L-arginine, citric acid) assumed presence of only neutral species; charges were neglected, as application of eqn (1) requires that the same species are present in the solid or liquid state. However, the pH of the mixture and the pKa of the molecules define their charge, which can deeply influence interactions and solubility behaviour. When both, L-arginine and citric acid are mixed with water, different pH values and thus, different species will occur. Fig. S1 (ESI†) shows the molecular species of citric acid or L-arginine expected to be present according to pH. Considering the case of the mixture citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine
L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 molar ratio, the measured pH was about 3.6 (Table 2). As observed in Fig. S1 (ESI†),49 at this pH, the majority of L-arginine molecules will be positively charged, [L-arginine]+; whereas for citric acid a mixture of the dehydrogenated form, [citrate]− and its neutral specie is expected. It is known that the solubility of citric acid is only slightly affected by pH changes (calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (©1994–2020 ACD/Labs)). However, the solubility changes of L-arginine species from the isoelectric region (neutral species) to pH values between 2 and 9 ([L-arginine]+) are more significant and must be considered to describe more realistically the interactions between [L-arginine]+, water and citric acid at these pH values. This was accounted for by predicting activity coefficients by ePC-SAFT. The pure-component parameters of the charged species were inherited from the neutral molecules, and a kij for the binary [L-arginine]+:water (Table 4) was adjusted according to previous works by solving dissociation equilibria and solubility.39–41 For [citrate]:water the kij was considered to be the same as the neutral citric acid:water, given their similar behaviour in terms of solubility. The results are shown in Fig. 6–8. Activity coefficients equal to one correspond to an ideal behaviour while negative (or positive) deviations translate into a higher (or lower) interaction affinity between the constituents of the mixture, respectively.
7 molar ratio, the measured pH was about 3.6 (Table 2). As observed in Fig. S1 (ESI†),49 at this pH, the majority of L-arginine molecules will be positively charged, [L-arginine]+; whereas for citric acid a mixture of the dehydrogenated form, [citrate]− and its neutral specie is expected. It is known that the solubility of citric acid is only slightly affected by pH changes (calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (©1994–2020 ACD/Labs)). However, the solubility changes of L-arginine species from the isoelectric region (neutral species) to pH values between 2 and 9 ([L-arginine]+) are more significant and must be considered to describe more realistically the interactions between [L-arginine]+, water and citric acid at these pH values. This was accounted for by predicting activity coefficients by ePC-SAFT. The pure-component parameters of the charged species were inherited from the neutral molecules, and a kij for the binary [L-arginine]+:water (Table 4) was adjusted according to previous works by solving dissociation equilibria and solubility.39–41 For [citrate]:water the kij was considered to be the same as the neutral citric acid:water, given their similar behaviour in terms of solubility. The results are shown in Fig. 6–8. Activity coefficients equal to one correspond to an ideal behaviour while negative (or positive) deviations translate into a higher (or lower) interaction affinity between the constituents of the mixture, respectively.
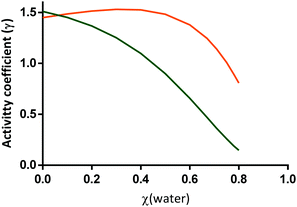 | ||
Fig. 6 Activity coefficients of citric acid (green line) and [L-arginine]+ (orange line) when water is added to the mixture citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine 1 L-arginine 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar), predicted by ePC-SAFT using the parameters from Tables 2 and 3. Data from Table S2 (ESI†). 1 (molar), predicted by ePC-SAFT using the parameters from Tables 2 and 3. Data from Table S2 (ESI†). | ||
As observed in Fig. 6 the activity coefficients of citric acid (γcit) in the ternary mixture gradually decrease upon water addition. The decrease from γcit = 1.51 to values lower than 1, namely for χ(water) = 0.78 with a γcit = 0.19, highly emphasizes the negative deviation of the mixture from Raoult's law. For [L-arginine]+, a positive and almost constant deviation from ideality is observed for water molar ratios up to 0.71. Above this water content, the activity coefficient of [L-arginine]+ (γarg+) decreases pronouncedly, reaching negative deviation from Raoult's law when χ(water) ≥ 0.78. The lower γcit in comparison to γarg+ indicates a higher contribution of citric acid to the melting depression of the mixture50 and a higher affinity to establish cross-interactions in the mixture.
Until now, the results were discussed using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of citric acid
1 molar ratio of citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine. The influence of water on the Tm of the mixture might depend also on the ratio of citric acid:L-arginine. Thus, the activity coefficient of citric acid in the ternary mixture was also predicted for different L-arginine:citric acid ratios at constant water contents (Fig. 7).
L-arginine. The influence of water on the Tm of the mixture might depend also on the ratio of citric acid:L-arginine. Thus, the activity coefficient of citric acid in the ternary mixture was also predicted for different L-arginine:citric acid ratios at constant water contents (Fig. 7).
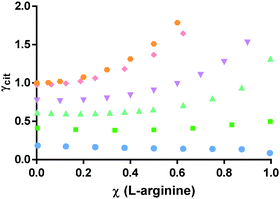 | ||
| Fig. 7 Activity coefficients of citric acid in the mixture citric acid:L-arginine:water, at different relative molar fraction of L-arginine with respect to citric acid and constant water molar ratios. Circles: χ(water) = 0.8; squares: χ(water) = 0.7; triangles: χ(water) = 0.6; inverted triangles: χ(water) = 0.5; rhombi: χ(water) = 0.2; pentagons: χ(water) = 0.01. PC-SAFT results listed in Tables S3–S8 (ESI†). | ||
Analysing the scenario where water is added to pure citric acid (χ(L-arginine) = 0), there is a decrease of γcit for increasing water amounts, with negative deviations from ideality (γcit < 1) when χ(water) > 0.2. This situation is regularly explained by water solvation effect. It is also observed that, at low water content, the addition of L-arginine increases the activity coefficient of citric acid in the mixture, probably by inducing competition between species. This behavior is less noticeable as the χ(water) increases up to 0.6 and changes significantly for χ(water) ≥ 0.7. At this water amount or higher, the γcit seems to stabilize into an almost constant value for all the L-arginine ratios in the mixture. Further, an even more distinct behavior was observed for χ(water) = 0.8, where the addition of L-arginine seems to slightly favour the interactions of citric acid in the mixture. For that water content, the γarg+ (Fig. 8) shows a positive deviation from Raoult's law for χ(L-arginine)≤ 0.05, that shifts to a negative deviation from ideality, when χ(L-arginine) ≥ 0.1. This emphasizes that the addition of L-arginine to the mixture citric acid:L-arginine:water above a certain amount causes increased cross interactions, but only at high water content. This is a non-regular solution effect.
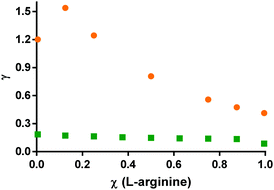 | ||
| Fig. 8 Activity coefficients of [L-arginine]+ (circles) and citric acid (squares) in the mixture citric acid:L-arginine:water, at a χ(water) = 0.8 and different relative molar ratios of L-arginine with respect to citric acid. PC-SAFT results listed in Tables S3 and S9 (ESI†). | ||
Interestingly, the mixtures of citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine
L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water previously reported to be liquid (1
water previously reported to be liquid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 2
7, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 2
7, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 2
8, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 mol ratios15,16) correspond to water molar contents between 0.7 and 0.8, which coincide within the range where this ‘non-regular’ behavior was detected. Moreover, the lowest γcit values predicted by the ePC-SAFT model and respective ratios, are in accordance with the formation of liquid phase mixtures at the ratios previously reported and herein explored.
9 mol ratios15,16) correspond to water molar contents between 0.7 and 0.8, which coincide within the range where this ‘non-regular’ behavior was detected. Moreover, the lowest γcit values predicted by the ePC-SAFT model and respective ratios, are in accordance with the formation of liquid phase mixtures at the ratios previously reported and herein explored.
Despite the possibility that the mixture citric acid:L-arginine:water might be a solution rather than a DES, the ‘non-regular’ behaviour observed for the activity coefficients within the only ratios reported to form a homogenous liquid phase has not yet been explained. It is certain that the liquid state is stabilized by water mediation, but also preponderantly influenced by the citric acid and L-arginine contents. Still, the type of network interactions responsible for this phenomenon are not known. Thus, from these data and the ePC-SAFT prediction alone it is not possible to postulate the type of mixture formed (DES, solution, salt), as different scenarios might fit the results presented.
Given the complexity of these systems and the impossibility to measure the SLE, spectroscopy analysis was additionally performed to further characterize the mixtures and to better understand their nature.
Physicochemical analysis
Aiming to complement the investigation on the nature of the mixtures citric acid:L-arginine:water, several binary and ternary mixtures involving these components were prepared for comparison and analysed through spectroscopic techniques. The mixture citric acid:L-arginine:water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (mol) was chosen as a model to compare with aqueous solutions of citric acid or L-arginine, in the same ratio or at the same pH (around 3.5) of the ternary mixture.
7 (mol) was chosen as a model to compare with aqueous solutions of citric acid or L-arginine, in the same ratio or at the same pH (around 3.5) of the ternary mixture.
Furthermore, ternary mixtures with increasing citric acid molar ratio from 0.1 to 1 were prepared and analysed by FTIR; those that were liquid were also analysed by NMR in order to evaluate the role of citric acid in the mixture. The mixtures prepared, their ratios, physical state and pH are reported in Table 2.
From the ternary mixtures tested, it was observed that only the ones with citric acid molar ratios ≥0.5 formed translucid liquids, with pH < 7. Since the pH alone might be the reason for the solubility increase of L-arginine in water, a mixture of the same ratio of L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) was prepared and acidified with HCl rather than citric acid, to identify the role of pH. It was observed that this mixture did not lead to the formation of a liquid at room temperature, neither before nor after acidification. The other way around was also evaluated: changing the pH of the binary citric acid
7) was prepared and acidified with HCl rather than citric acid, to identify the role of pH. It was observed that this mixture did not lead to the formation of a liquid at room temperature, neither before nor after acidification. The other way around was also evaluated: changing the pH of the binary citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (translucid liquid at pH 0.5) to pH = 3.5 using NaOH instead of L-arginine caused the formation of a white solid paste at room temperature. These observations support that the liquid formation is not only a function of pH, but it is additionally influenced by the involving species. In this case, the mixture of L-arginine and citric acid in the presence of water seems to have unique properties in addition to pH to promote their liquefaction. One possibility could be complexation of the oppositely charged species [H2Cit]− and [Arg]+ (Fig. S1, ESI†) in water yielding a salt that might have higher water solubility than the non-charged precursor molecules. Hence, this hypothesis was addressed in the following FTIR section.
7 (translucid liquid at pH 0.5) to pH = 3.5 using NaOH instead of L-arginine caused the formation of a white solid paste at room temperature. These observations support that the liquid formation is not only a function of pH, but it is additionally influenced by the involving species. In this case, the mixture of L-arginine and citric acid in the presence of water seems to have unique properties in addition to pH to promote their liquefaction. One possibility could be complexation of the oppositely charged species [H2Cit]− and [Arg]+ (Fig. S1, ESI†) in water yielding a salt that might have higher water solubility than the non-charged precursor molecules. Hence, this hypothesis was addressed in the following FTIR section.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, Fig. 9b), a broader peak is observed for the guanidine amines due to their transition to the delocalized charge state. At pH 3.5 (Fig. 9c), the primary amine is also charged causing the amine peaks to overlap around 3323 cm−1. Additionally, the O–H stretching of L-arginine in its pure form (3057 cm−1, Fig. 9a) shifts to higher frequencies due to the establishment of hydrogen bonding interactions with the surrounding media (Fig. 9b–d). Regarding citric acid, the most important vibrations are from the O–H and C
7, Fig. 9b), a broader peak is observed for the guanidine amines due to their transition to the delocalized charge state. At pH 3.5 (Fig. 9c), the primary amine is also charged causing the amine peaks to overlap around 3323 cm−1. Additionally, the O–H stretching of L-arginine in its pure form (3057 cm−1, Fig. 9a) shifts to higher frequencies due to the establishment of hydrogen bonding interactions with the surrounding media (Fig. 9b–d). Regarding citric acid, the most important vibrations are from the O–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the carboxylic groups, centred at 3297 and 1719 cm−1, respectively. When mixed with water at pH 3.5, the O–H vibrations are broadened and shifted, and the maximum is at 3340 cm−1.
O stretching of the carboxylic groups, centred at 3297 and 1719 cm−1, respectively. When mixed with water at pH 3.5, the O–H vibrations are broadened and shifted, and the maximum is at 3340 cm−1.
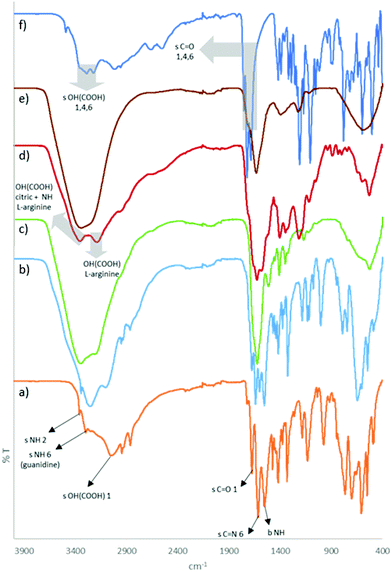 | ||
Fig. 9 FTIR spectra of (a) pure L-arginine (orange line); (b) L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mol (light blue line); (c) L-arginine 7 mol (light blue line); (c) L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O pH = 3.5 (green line); (d) citric acid H2O pH = 3.5 (green line); (d) citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mol (red line); (e) citric acid 7 mol (red line); (e) citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mol (brown line); (f) pure citric acid (dark blue line). “s” and “b” refer to stretching and bending vibrations, respectively. Numbers attributed according to the chemical structures from Fig. S2 (ESI†). 7 mol (brown line); (f) pure citric acid (dark blue line). “s” and “b” refer to stretching and bending vibrations, respectively. Numbers attributed according to the chemical structures from Fig. S2 (ESI†). | ||
Comparing the citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water and L-arginine
water and L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures at a pH = 3.5 with the ternary system of citric acid
water mixtures at a pH = 3.5 with the ternary system of citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine
L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, it is possible to observe a combined contribution of the same O–H and N–H stretching peaks (Fig. 9c–e, dashed lines). This was also observed for the other analysed ratios of citric acid:L-arginine:water (Fig. S3, ESI†). If the liquids formed by the combination of citric acid, L-arginine and water were promoted by the ionic complexation of [H2Cit]− and [Arg]+, the dipole moments of those functional groups would be changed. However, different vibrations in the FTIR spectra were not observed, refuting the salt formation hypothesis.
7, it is possible to observe a combined contribution of the same O–H and N–H stretching peaks (Fig. 9c–e, dashed lines). This was also observed for the other analysed ratios of citric acid:L-arginine:water (Fig. S3, ESI†). If the liquids formed by the combination of citric acid, L-arginine and water were promoted by the ionic complexation of [H2Cit]− and [Arg]+, the dipole moments of those functional groups would be changed. However, different vibrations in the FTIR spectra were not observed, refuting the salt formation hypothesis.
To sum up, the liquid is neither a case of salt formation nor exclusively induced by the acidic pH value. Thus, other L-arginine:citric acid interactions mediated by water must be the reason for the deep melting point depression of the ternary system, probably H-bonding interactions. This was further explored by NMR analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mixture were compared with the ternary systems of citric acid
7 mixture were compared with the ternary systems of citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine
L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 0.5
7 and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (Fig. 10).
7 (Fig. 10).
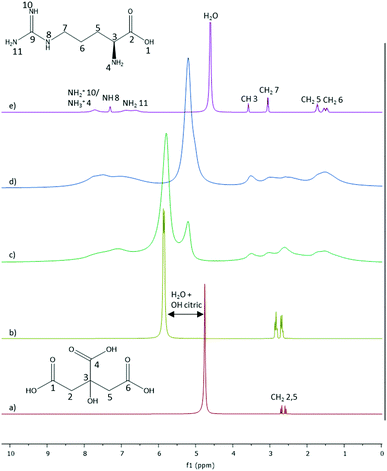 | ||
Fig. 10
1H NMR spectra of (a) citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O pH = 3.5; (b) citric acid H2O pH = 3.5; (b) citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7; (c) citric acid 7; (c) citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7; (d) citric acid 7; (d) citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 0.5 H2O 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7; (e) L-arginine 7; (e) L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O pH = 3.5. The functional groups of the protons detected were identified and numbers attributed according to the respective chemical structures (see species distribution in Fig. S2, ESI†). H2O pH = 3.5. The functional groups of the protons detected were identified and numbers attributed according to the respective chemical structures (see species distribution in Fig. S2, ESI†). | ||
For the system L-arginine:water at pH = 3.5, it was possible to identify the protons from water and most of the protons of the L-arginine's amine and alkyl groups (Fig. 10e); whereas for the citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water at pH = 3.5 or at the molar ratio 1
water at pH = 3.5 or at the molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 it was only possible to detect the alkyl groups and a contribution of the OH-group protons overlapped with the protons from water. This influence of the OH group was attributed due to the downfield shift of the concentrated solution of citric acid (1
7 it was only possible to detect the alkyl groups and a contribution of the OH-group protons overlapped with the protons from water. This influence of the OH group was attributed due to the downfield shift of the concentrated solution of citric acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 molar) (5.79 ppm, Fig. 10b) in comparison to its diluted solution at pH 3.5 (4.79 ppm, Fig. 10a). Interestingly, the same peak (5.79 ppm) appears in the case of the ternary mixture 1
7 molar) (5.79 ppm, Fig. 10b) in comparison to its diluted solution at pH 3.5 (4.79 ppm, Fig. 10a). Interestingly, the same peak (5.79 ppm) appears in the case of the ternary mixture 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (Fig. 10c), but it is broader. Further, it has a second peak at 5.20 ppm, also appearing in the ternary system 0.5
7 (Fig. 10c), but it is broader. Further, it has a second peak at 5.20 ppm, also appearing in the ternary system 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. Given that the only difference from the samples of Fig. 10b to c was the addition of L-arginine, the appearance of a new peak is certainly caused by hydrogen-bonding interactions with the L-arginine molecules, in addition to the ones between citric acid and water. Regarding the broadening-effect observed for the ternary mixtures in comparison to the binary solutions, it might be related to the samples’ high viscosity. According to Stokes–Einstein–Debye law, high viscosity mixtures lead to slower rotational molecular diffusion59–62 and longer T2 relaxation times.63,64 These translate into broader NMR peaks, as previously reported for viscous DES mixtures.65
7. Given that the only difference from the samples of Fig. 10b to c was the addition of L-arginine, the appearance of a new peak is certainly caused by hydrogen-bonding interactions with the L-arginine molecules, in addition to the ones between citric acid and water. Regarding the broadening-effect observed for the ternary mixtures in comparison to the binary solutions, it might be related to the samples’ high viscosity. According to Stokes–Einstein–Debye law, high viscosity mixtures lead to slower rotational molecular diffusion59–62 and longer T2 relaxation times.63,64 These translate into broader NMR peaks, as previously reported for viscous DES mixtures.65
Moreover, in the NOESY spectra of the system citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine
L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 there are cross peaks between the protons from the citric acid alkyl groups and the protons of L-arginine chemical groups (Fig. 11) that may indicate spatial correlation. Similar correlations were observed for the mixture 0.5
7 there are cross peaks between the protons from the citric acid alkyl groups and the protons of L-arginine chemical groups (Fig. 11) that may indicate spatial correlation. Similar correlations were observed for the mixture 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (Fig. S4, ESI†). This may support that in addition to the expected interactions between H2O–H2O, citric acid–H2O, citric acid–citric acid, L-arginine–H2O and L-arginine–L-arginine, further citric acid–L-arginine and/or mutual interactions between the three components might occur. However, given that the cross peaks are in the same phase as the diagonal peaks (positive, red peaks), this might also be a case of spin diffusion due to the high viscosity. Still, since viscosity is a direct cause of mixing the three components, it is highly probable that they are spatially close and interacting. Combined with the evidence from 1H NMR analysis that there is a change in the hydrogen bonding network when citric acid and L-arginine are mixed with water in comparison to their independent water solutions, these findings might be compliable with the formation of a supramolecular network, which is characteristic from DES. To properly address the interactions-network between the components that allowed to form the liquid ternary mixtures, dynamic molecular studies would have to be performed. Additionally, magic-angle spinning (MAS) NMR has recently been described as a good technique to overcome some of the conventional NMR limitations in the characterization of viscous mixtures, like DES.65 This MAS NMR technique also allows to analyse and compare both liquid and solid mixtures, which, in the field of DES, might be highly useful in understanding those interactions between the involved molecules that are responsible for the mixture liquefaction.
7 (Fig. S4, ESI†). This may support that in addition to the expected interactions between H2O–H2O, citric acid–H2O, citric acid–citric acid, L-arginine–H2O and L-arginine–L-arginine, further citric acid–L-arginine and/or mutual interactions between the three components might occur. However, given that the cross peaks are in the same phase as the diagonal peaks (positive, red peaks), this might also be a case of spin diffusion due to the high viscosity. Still, since viscosity is a direct cause of mixing the three components, it is highly probable that they are spatially close and interacting. Combined with the evidence from 1H NMR analysis that there is a change in the hydrogen bonding network when citric acid and L-arginine are mixed with water in comparison to their independent water solutions, these findings might be compliable with the formation of a supramolecular network, which is characteristic from DES. To properly address the interactions-network between the components that allowed to form the liquid ternary mixtures, dynamic molecular studies would have to be performed. Additionally, magic-angle spinning (MAS) NMR has recently been described as a good technique to overcome some of the conventional NMR limitations in the characterization of viscous mixtures, like DES.65 This MAS NMR technique also allows to analyse and compare both liquid and solid mixtures, which, in the field of DES, might be highly useful in understanding those interactions between the involved molecules that are responsible for the mixture liquefaction.
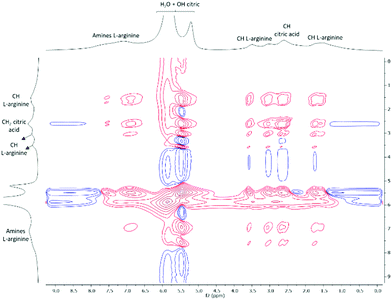 | ||
Fig. 11 2D NOESY spectra of the mixture citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-arginine L-arginine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mol. The functional groups of the protons identified are aligned with the respective peaks. 7 mol. The functional groups of the protons identified are aligned with the respective peaks. | ||
Still, from our findings, it is noticeable that the interactions between the water molecules and the functional groups of citric acid and L-arginine, namely, amine, carboxyl and hydroxyl groups may play a major role in establishing those interactions.
Conclusions and final remarks
The ternary mixtures of citric acid:L-arginine:water previously reported in the literature as DES, have been questioned as whether or not they are regular solutions due to the water present in their composition. This work shows that these mixtures are prone to strong non-ideal behaviour induced by water addition. Water has a bifunctional role as it causes acidic environment (formation of charged species) and it acts as hydrogen-bonding mediator for the L-arginine and citric acid. As the phase behaviour depends on the ratio of the mixture's constituents, the thermodynamic model ePC-SAFT was used as a qualitative predictive model to predict the influence of water on the SLE. This prediction tool is particularly useful as it was not possible to obtain experimental SLE data for the system, due to decomposition, slow melting or to ultra-low melting points. Within this context, the influence of water on citric acid:L-arginine mixtures was studied in terms of SLE and activity coefficients. Novel melting properties of L-arginine were first determined through FSC, as this was required for SLE modelling. ePC-SAFT predicted a transition from positive to negative deviation from Raoult's law upon addition of water to citric acid:L-arginine at certain ratios. This rendered an estimated deep decrease in the Tm of these mixtures upon water addition as well as increased intermolecular interactions. Furthermore, the hypothesis of salt formation and pH influence to explain the deep decrease of Tm were explored. However, both could be contradicted (by pH control measurements without either citric acid or L-arginine and by the FTIR analysis). Finally, the formation of an altered hydrogen-bonding network was confirmed by NMR spectroscopy. The observed non-ideal behaviour of the mixture and the hydrogen-bonding formation in the mixture are consistent with (i) the hypothesis of DES formation and (ii) the occurrence of strong non-ideal solvation of the citric acid and L-arginine species in water. In this scenario (considering the high potential of water to establish hydrogen-bonding interactions) it is challenging to distinguish between non-ideal solvation and DES formation. Both phenomena are mainly promoted by hydrogen-bonding interactions between the constituents, causing a depression on the melting temperatures. To the best of our knowledge, the only difference reported in the literature that differentiates a DES from a regular solution is the structural network organization. Whereas DES are recognized by the supramolecular complexes formed between its constituents, aqueous solutions are considered when solvation of the isolated components occurs.8–14 Thus, if distinguishable within the available state-of-the-art tools, this would only be possible by molecular dynamic simulations as activity-coefficient analyses rely on non-local spatially-averaged properties. Still, from the present work, important features of the ternary systems were characterized, and the crucial bifunctional role of water was postulated (formation of charged species and mediated hydrogen-bonded interactions between citric acid and L-arginine). Therefore, either a DES or a non-ideal aqueous solution, these ternary mixtures have new properties and might be considered as independent systems from conventional solvents.In sum, it was shown that despite meeting the definition for a DES, still such definition is not sharp enough for complex aqueous mixtures and other liquid-based mixtures, as the current concepts of solvation and DES formation overlap. For this reason, this work aims to instigate the scientific community in investing in the study of this type of mixtures while seeking for additional tools that may contribute to a comprehensive characterization and understanding of their nature.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union's Horizon 2020 (European Research Council) under grant agreement no. ERC-2016-CoG 725034. This work was supported by the Associate Laboratory for Green Chemistry-LAQV which is financed by national funds from FCT/MCTES (UID/QUI/50006/2019). Further, C. H. and Y. Z. C. thank German Science Foundation (DFG) for funding (HE 7165/6-1 and CH 1922/1-1).References
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Novel solvent properties of choline chloride/urea mixtures, Chem. Commun., 2003, 70–71 RSC.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jérôme, Deep eutectic solvents: Syntheses, properties and applications, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, Natural deep eutectic solvents - Solvents for the 21st century, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS.
- A. Paiva, A. A. Matias and A. R. C. Duarte, How do we drive deep eutectic systems towards an industrial reality ?, Curr. Opin. Green Sustain. Chem., 2018, 11, 81–85 CrossRef.
- M. Francisco, A. Van Den Bruinhorst and M. C. Kroon, Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents, Angew. Chem., Int. Ed., 2013, 52, 3074–3085 CrossRef CAS.
- D. L. Nelson and M. M. Cox, Lehninger Principles of Biochemistry, W. H. Freeman, New York, 7th edn, 2017 Search PubMed.
- T. El Achkar, S. Fourmentin and H. Greige-Gerges, Deep eutectic solvents: An overview on their interactions with water and biochemical compounds, J. Mol. Liq., 2019, 288, 111028 CrossRef CAS.
- Y. Dai, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Tailoring properties of natural deep eutectic solvents with water to facilitate their applications, Food Chem., 2015, 187, 14–19 CrossRef CAS.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution, Angew. Chem., Int. Ed., 2017, 56, 9782–9785 CrossRef CAS.
- H. Passos, D. J. P. Tavares, A. M. Ferreira, M. G. Freire and J. A. P. Coutinho, Are Aqueous Biphasic Systems Composed of Deep Eutectic Solvents Ternary or Quaternary Systems ?, ACS Sustainable Chem. Eng., 2016, 4, 2881–2886 CrossRef CAS.
- J. Baz, C. Held, J. Pleiss and N. Hansen, Thermophysical properties of glyceline-water mixtures investigated by molecular modelling, Phys. Chem. Chem. Phys., 2019, 21, 6467–6476 RSC.
- N. López-Salas, J. M. Vicent-Luna, S. Imberti, E. Posada, M. J. Roldán, J. A. Anta, S. R. G. Balestra, R. M. M. Castro, S. Calero, R. J. Jiménez-Riobóo, M. C. Gutiérrez, M. L. Ferrer and F. del Monte, Looking at the ‘Water-in-Deep-Eutectic-Solvent’ System: A Dilution Range for High Performance Eutectics, ACS Sustainable Chem. Eng., 2019, 7, 17565–17573 CrossRef.
- L. Sapir and D. Harries, Restructuring a Deep Eutectic Solvent by Water: The Nanostructure of Hydrated Choline Chloride/Urea, J. Chem. Theory Comput., 2020, 16, 3335–3342 CrossRef CAS.
- F. Santos, M. I. P. S. Leitão and A. R. C. Duarte, Properties of Therapeutic Deep Eutectic Solvents of L-Arginine and Ethambutol for Tuberculosis Treatment, Molecules, 2018, 24, 55 CrossRef.
- A. Roda, F. Santos, A. A. Matias, A. Paiva and A. R. C. Duarte, Design and processing of drug delivery formulations of therapeutic deep eutectic systems for tuberculosis, J. Supercrit. Fluids, 2020, 161, 104826 CrossRef CAS.
- G. Degam, Deep Eutectic Solvents Synthesis, Characterization and Applications in Pretreatment of Lignocellulosic Biomass, Electronic Theses and Dissertations, 2020, p. 1156 Search PubMed.
- P. V. A. Pontes, E. A. Crespo, M. A. R. Martins, L. P. Silva, C. M. S. S. Neves, G. J. Maximo, M. D. Hubinger, E. A. C. Batista, S. P. Pinho, J. A. P. Coutinho, G. Sadowski and C. Held, Measurement and PC-SAFT modeling of solid-liquid equilibrium of deep eutectic solvents of quaternary ammonium chlorides and carboxylic acids, Fluid Phase Equilib., 2017, 448, 69–80 CrossRef CAS.
- E. A. Crespo, L. P. Silva, A. R. Martins, L. Fernandez, J. Ortega, O. Ferreira, G. Sadowski, C. Held, S. P. Pinho and J. A. P. Coutinho, Characterization and Modeling of the Liquid Phase of Deep Eutectic Solvents Based on Fatty Acids/Alcohols and Choline Chloride, Ind. Eng. Chem. Res., 2017, 56, 12192–12202 CrossRef CAS.
- E. A. Crespo, L. P. Silva, M. A. R. Martins, M. Bülow, O. Ferreira, G. Sadowski, C. Held, S. P. Pinho and J. A. P. Coutinho, The Role of Polyfunctionality in the Formation of [Ch]Cl-Carboxylic Acid-Based Deep Eutectic Solvents, Ind. Eng. Chem. Res., 2018, 57, 11195–11209 CrossRef CAS.
- M. A. R. Martins, E. A. Crespo, P. V. A. Pontes, L. P. Silva, M. Bülow, G. J. Maximo, E. A. C. Batista, C. Held, S. P. Pinho and J. A. P. Coutinho, Tunable Hydrophobic Eutectic Solvents Based on Terpenes and Monocarboxylic Acids, ACS Sustainable Chem. Eng., 2018, 6, 8836–8846 CrossRef CAS.
- I. I. I. Alkhatib, D. Bahamon, F. Llovell, M. R. M. Abu-Zahra and L. F. Vega, Perspectives and guidelines on thermodynamic modelling of deep eutectic solvents, J. Mol. Liq., 2020, 298, 112183 CrossRef CAS.
- C. Held, T. Reschke, S. Mohammad, A. Luza and G. Sadowski, ePC-SAFT revised, Chem. Eng. Res. Des., 2014, 92, 2884–2897 CrossRef CAS.
- T. Aissaoui and I. M. AlNashef, Neoteric FT-IR Investigation on the Functional Groups of Phosphonium-Based Deep Eutectic Solvents, Pharm. Anal. Acta, 2015, 6, 1–3 Search PubMed.
- H. Ghaedi, M. Ayoub, S. Sufian, B. Lal and Y. Uemura, Thermal stability and FT-IR analysis of Phosphonium-based deep eutectic solvents with different hydrogen bond donors, J. Mol. Liq., 2017, 242, 395–403 CrossRef CAS.
- L. Meneses, F. Santos, A. R. Gameiro, A. Paiva and A. R. C. Duarte, Preparation of binary and ternary deep eutectic systems, J. Visualized Exp., 2019, 2019, 1–5 Search PubMed.
- P. A. Frey and W. W. Cleland, Are there strong hydrogen bonds in aqueous solutions?, Bioorg. Chem., 1998, 26, 175–192 CrossRef CAS.
- M. J. Roldán-Ruiz, R. J. Jiménez-Riobóo, M. C. Gutiérrez, M. L. Ferrer and F. del Monte, Brillouin and NMR spectroscopic studies of aqueous dilutions of malicine: Determining the dilution range for transition from a “water-in-DES” system to a “DES-in-water” one, J. Mol. Liq., 2019, 284, 175–181 CrossRef.
- H. T. Do, Y. Z. Chua, J. Habicht, M. Klinksiek, M. Hallermann, D. Zaitsau, C. Schick and C. Held, Melting properties of peptides and their solubility in water. Part 1: Dipeptides based on glycine or alanine, RSC Adv., 2019, 9, 32722–32734 RSC.
- Y. Z. Chua, H. T. Do, C. Schick, D. Zaitsau and C. Held, New experimental melting properties as access for predicting amino-acid solubility, RSC Adv., 2018, 8, 6365–6372 RSC.
- G. W. H. Höhne, H. K. Cammenga, W. Eysel, E. Gmelin and W. Hemminger, The temperature calibration of scanning calorimeters, Thermochim. Acta, 1990, 160, 1–12 CrossRef.
- A. Toda, M. Hikosaka and K. Yamada, Superheating of the melting kinetics in polymer crystals: A possible nucleation mechanism, Polymer, 2002, 43, 1667–1679 CrossRef CAS.
- P. Cebe, D. Thomas, J. Merfeld, B. P. Partlow, D. L. Kaplan, R. G. Alamo, A. Wurm, E. Zhuravlev and C. Schick, Heat of fusion of polymer crystals by fast scanning calorimetry, Polymer, 2017, 126, 240–247 CrossRef CAS.
- X. Hu, W. K. Raja, B. An, O. Tokareva, P. Cebe and D. L. Kaplan, Stability of silk and collagen protein materials in space, Sci. Rep., 2013, 3, 3428 CrossRef.
- C. Held, T. Neuhaus and G. Sadowski, Compatible solutes: Thermodynamic properties and biological impact of ectoines and prolines, Biophys. Chem., 2010, 152, 28–39 CrossRef CAS.
- L. Fernandez, L. P. Silva, M. A. R. Martins, O. Ferreira, J. Ortega, S. P. Pinho and J. A. P. Coutinho, Indirect assessment of the fusion properties of choline chloride from solid-liquid equilibria data, Fluid Phase Equilib., 2017, 448, 9–14 CrossRef CAS.
- V. Meltzer and E. Pincu, Thermodynamic study of binary mixture of citric acid and tartaric acid, Cent. Eur. J. Chem., 2012, 10, 1584–1589 CAS.
- C. Held, L. F. Cameretti and G. Sadowski, Measuring and Modeling Activity Coefficients in Aqueous Amino-Acid Solutions, Ind. Eng. Chem. Res., 2011, 50, 131–141 CrossRef CAS.
- L. Lange, K. Lehmkemper and G. Sadowski, Predicting the aqueous solubility of pharmaceutical cocrystals as a function of pH and temperature, Cryst. Growth Des., 2016, 16, 2726–2740 CrossRef CAS.
- L. Lange, M. Schleinitz and G. Sadowski, Predicting the Effect of pH on Stability and Solubility of Polymorphs, Hydrates, and Cocrystals, Cryst. Growth Des., 2016, 16, 4136–4147 CrossRef CAS.
- K. Wysoczanska, E. A. MacEdo, G. Sadowski and C. Held, Solubility Enhancement of Vitamins in Water in the Presence of Covitamins: Measurements and ePC-SAFT Predictions, Ind. Eng. Chem. Res., 2019, 58, 21761–21771 CrossRef CAS.
- CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press LLC, Bosa Roca, United States, 82nd edn, 2001, p. 1 Search PubMed.
- I. M. Weiss, C. Muth, R. Drumm and H. O. K. Kirchner, Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine, BMC Biophys., 2018, 11, 1–15 CrossRef.
- R. C. Wilhoit and D. Shiao, Thermochemistry of Biologically Important Compounds. Heats of Combustion of Solid Organic Acids, J. Chem. Eng. Data, 1964, 9, 595–599 CrossRef CAS.
- X. Meng, K. Ballerat-Busserolles, P. Husson and J.-M. Andanson, Impact of water on the melting temperature of urea + choline chloride deep eutectic solvent, New J. Chem., 2016, 40, 4492–4499 RSC.
- J. P. Amend and H. C. Helgeson, Solubilities of the common L-α-amino acids as a function of temperature and solution pH, Pure Appl. Chem., 1997, 69, 935–942 CAS.
- A. Apelblat and E. Manzurola, Solubility of oxalic, malonic, succinic, adipic, maleic, malic, citric, and tartaric acids in water from 278.15 to 338.15 K, J. Chem. Thermodyn., 1987, 19, 317–320 CrossRef CAS.
- H. Yang and J. H. Wang, Solubilities of 3-Carboxy-3-hydroxypentanedioic acid in Ethanol, Butan-1-ol, Water, Acetone, and Methylbenzene, J. Chem. Eng. Data, 2011, 56, 1449–1451 CrossRef CAS.
- I. G. R. Gutz, CurTiPot – pH and Acid–Base Titration Curves: Analysis and Simulation freeware, version 4.2, http://www.iq.usp.br/gutz/Curtipot_.html Search PubMed.
- A. Alhadid, L. Mokrushina and M. Minceva, Modeling of Solid–Liquid Equilibria in Deep Eutectic Solvents: A Parameter Study, Molecules, 2019, 24, 1–19 CrossRef.
- Merck, IR Spectrum Table & Chart, https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html, (accessed 23 July 2020).
- T. Mallik and T. Kar, Growth and characterization of nonlinear optical L-arginine dihydrate single crystals, J. Cryst. Growth, 2005, 285, 178–182 CrossRef CAS.
- S. Gowri, J. Sathiyabama and S. Rajendran, Corrosion inhibition effect of carbon steel in sea water by L-Arginine-Zn2+ system, Int. J. Chem. Eng., 2014, 11, 1–9 Search PubMed.
- A. M. Petrosyan and R. P. Sukiasyan, Vibrational spectra of L-arginine nitrates, J. Mol. Struct., 2008, 874, 51–56 CrossRef CAS.
- P. Mikes, A. Brož, A. Sinica, N. Asatiani and L. Bacakova, In vitro and in vivo testing of nanofibrous membranes doped with alaptide and L-arginine for wound treatment, Biomed. Mater., 2020, 1–26 Search PubMed.
- P. Pimpang, R. Sumang and S. Choopun, Effect of concentration of citric acid on size and optical properties of fluorescence graphene quantum dots prepared by tuning carbonization degree, Chiang Mai J. Sci., 2018, 45, 2005–2014 CAS.
- L. C. Bichara, H. E. Lans, E. G. Ferrer, M. B. Gramajo and S. A. Brandán, Vibrational study and force field of the citric acid dimer based on the SQM methodology, Adv. Phys. Chem., 2011, 2011, 1–10 CrossRef.
- N. T. Thuy and D. Le Minh, Size effect on the structural and magnetic properties of nanosized perovskite LaFeO3 prepared by different methods, Adv. Mater. Sci. Eng., 2012, 2012, 1–6 CrossRef.
- G. G. Stokes, Volume the Ninth, Trans. Cambridge Philos. Soc., 1856, 9, 5 Search PubMed.
- A. Einstein, Zur Theorie der Brownschen Bewegung, Ann. Phys., 1906, 324, 371–381 CrossRef.
- P. Debye, Polar Molecules, Dover Publications, New York, USA, 1929 Search PubMed.
- G. H. Koenderink, H. Zhang, D. G. A. L. Aarts, M. P. Lettinga, A. P. Philipse and G. Nägele, On the validity of Stokes–Einstein–Debye relations for rotational diffusion in colloidal suspensions, Faraday Discuss., 2003, 123, 335–354 RSC.
- J. L. Dote, D. Kivelson and R. N. Schwartz, A molecular quasi-hydrodynamic free-space model for molecular rotational relaxation in liquids, J. Phys. Chem., 1981, 85, 2169–2180 CrossRef CAS.
- L. Zhang and M. L. Greenfield, Rotational relaxation times of individual compounds within simulations of molecular asphalt models, J. Chem. Phys., 2010, 132, 184502 CrossRef.
- S. K. Mann, T. N. Pham, L. L. McQueen, J. R. Lewandowski and S. P. Brown, Revealing Intermolecular Hydrogen Bonding Structure and Dynamics in a Deep Eutectic Pharmaceutical by Magic-Angle Spinning NMR Spectroscopy, Mol. Pharmaceutics, 2020, 17, 622–631 CrossRef CAS.
- L. F. Zubeir, C. Held, G. Sadowski and M. C. Kroon, PC-SAFT modeling of CO2 solubilities in deep eutectic solvents, J. Phys. Chem. B, 2016, 120, 2300–2310 CrossRef CAS.
- L. F. Cameretti and G. Sadowski, Modeling of aqueous amino acid and polypeptide solutions with PC-SAFT, Chem. Eng. Process., 2008, 47, 1018–1025 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cp04992a |
| This journal is © the Owner Societies 2021 |