Symmetrical and unsymmetrical thiazole-based ESIPT derivatives: the highly selective fluorescence sensing of Cu2+ and structure-controlled reversible mechanofluorochromism†
Abstract
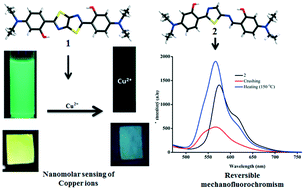
Excited state intramolecular proton transfer (ESIPT) process-based organic fluorophores provide an opportunity to develop large Stokes-shifted multifunctional fluorescence systems for light emitting, chemosensing and bioimaging applications. In this manuscript, ESIPT molecules with two thiazole ring fused symmetrical (1) structures and one thiazole and a Schiff base functional unsymmetrical (2) structure were synthesized, and the impact of their extended π-conjugation was investigated on the fluorescence properties in the solid and solution state as well as metal ions sensing. The strong intramolecular H-bonding and multiple intermolecular H-bonds in the crystal lattice of 1 and 2 lead to ESIPT-induced solid state fluorescence (λmax = 549 (1) and 580 nm (2); ΦF = 7.6 (1) and 6.1% (2)). Interestingly, the molecular assembly of 2 in the solid state facilitated mechanical stimuli-induced reversible fluorescence switching, whereas the flat molecular arrangement in 1 did not show recovery of fluorescence. In contrast, 1 showed strong fluorescence in solution (ΦF = 0.59 in DMF compared to quinine sulphate) due to its symmetrical structure, with strong intramolecular H-bonding, but the PET effect contributed to the very weak fluorescence of 2. The strong solution and solid state fluorescence coupled with the metal chelating functionality of 1 and 2 showed highly selective fluorescence sensing of Cu2+ ions in aqueous solution. 1 and 2 revealed detection limits of 130 and 10 nM, respectively, for Cu2+ ions, which are significantly lower compared to the WHO-recommended level (1.3 mg L−1). The strong solid state fluorescence of 1 and 2 was further used for fabricating filter paper-based fluorescence sensors for the selective sensing of Cu2+ ions.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...