Influences of nano-effect on the thermodynamic properties of solid–liquid interfaces: theoretical and experimental researches
Abstract
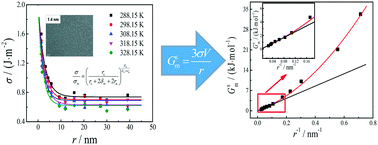
The unique physical and chemical properties of nanoparticles are closely related to the interfacial properties, which depend on the nano-effect. Herein, we theoretically analyzed the relationships of interfacial thermodynamic properties with particle size and discussed the influencing mechanism and regularities of nano-effect on interfacial thermodynamic properties. Experimentally, nano-Ag with different radii (1.4 nm to 39.1 nm) was prepared by the liquid phase reduction method. The interfacial tension, its temperature coefficient, and the interfacial thermodynamic properties of the interface between nano-Ag and its electrolyte solution were determined by an electrochemical method. Both the theoretical analysis and the experimental results show that nano-effect has a great influence on the interfacial properties of nanoparticles. Nano-effect mainly consists of interfacial area effect and interfacial tension effect. With the decreasing particle size, nano-effect increases; interfacial Gibbs energy, interfacial internal energy, interfacial enthalpy, and interfacial entropy increase. When the radius exceeds 10 nm, the interfacial area effect is the crucial influencing factor for interfacial thermodynamic properties, and there exist linear relations of these interfacial thermodynamic properties and the reciprocal of radius. When the radius is less than 10 nm, both interfacial area effect and interfacial tension effect (including the effect of interfacial tension and its temperature coefficient) influence interfacial thermodynamic properties, resulting in deviations from the linear relationships, and the smaller the particle size, the more obvious the deviation. In addition, for nano-Ag, when the radius is reduced to 1.5 nm, the influence of interfacial area effect and interfacial tension effect on interfacial thermodynamic properties are nearly equal, and interfacial tension effect will be crucial with continuing decrease of the particle size. This research can provide significant fundamental insight into size-dependent interfacial properties and explain and predict the regularities of the effect of particle size on thermodynamic properties in various physical and chemical processes.
- This article is part of the themed collection: Nanomaterials

 Please wait while we load your content...
Please wait while we load your content...