Theoretical insights on the encapsulated hydronium ion mediated supramolecular assembly of nickel(ii) Schiff base complexes: strong hydrogen bonding interaction due to charge transfer from the lone pair of oxygen to the antibonding orbital of the O–H bond†
Abstract
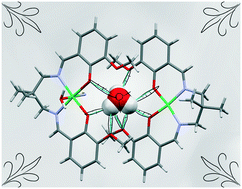
To understand the packing in a particular crystal, we need to investigate supramolecular interactions. Here, we report a hydronium ion encapsulated within a dimeric assembly of two different nickel(II) Schiff base complexes (NiL). The structure has been confirmed by single crystal X-ray diffraction analysis. In the structure, the two NiL complexes are placed in such a way that the outer 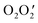 cores form a cavity in which the hydronium ion resides. The crystal packing is mainly directed by strong hydrogen bonding interactions observed between the hydronium ion and NiL complexes but, in addition, weak interactions such as π⋯π and C–H⋯π are also observed. The overall crystal packing and the strong hydrogen bonds due to the encapsulated H3O+ ion are evaluated using DFT calculations. The reason for the strength of these hydrogen bond interactions can be accounted for by charge transfer from the lone pair of oxygen atoms in the two nickel(II) Schiff base complexes to the antibonding orbital of the O–H (of H3O+) bond.
cores form a cavity in which the hydronium ion resides. The crystal packing is mainly directed by strong hydrogen bonding interactions observed between the hydronium ion and NiL complexes but, in addition, weak interactions such as π⋯π and C–H⋯π are also observed. The overall crystal packing and the strong hydrogen bonds due to the encapsulated H3O+ ion are evaluated using DFT calculations. The reason for the strength of these hydrogen bond interactions can be accounted for by charge transfer from the lone pair of oxygen atoms in the two nickel(II) Schiff base complexes to the antibonding orbital of the O–H (of H3O+) bond.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...