Managing hydrogen bonding in the clathrate hydrate of the 1-pentanol guest molecule†
Abstract
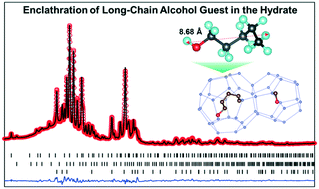
It remains a difficult task to predict the hydrate structure and conformation of potential guest molecules in one of the three canonical hydrate lattices. 1-Pentanol is characteristic of molecules that lie at the margin of suitability as a guest molecule for the structure II hydrate phase, both because of its size and the presence of a hydrophilic OH group that tends to destabilize the clathrate hydrate framework. To manage the destabilizing influence of guest–host hydrogen bonding, some years ago a more robust framework was developed by doping the hydrate with NH4+ and F− ions in order to direct the guest hydrophilic groups away from the cage water molecules. Attempts to enclathrate 1-pentanol in the NH4F doped clathrate were successful, but, surprisingly, the clathrate, as determined by PXRD, proved to be structure II (sII), even though the 1-pentanol appears to be too large to fit the pseudo-spherical 51264 cage. This suggested that the doped clathrate not only managed the guest–host hydrogen bonding network, but also forced the guest into a very compact conformation to fit the pseudospherical sII large cage (51264). These features were investigated further with 13C NMR and molecular dynamics simulations to identify the most likely guest conformation of 1-pentanol in the sII large cage.
- This article is part of the themed collection: Crystal Engineering Techniques


 Please wait while we load your content...
Please wait while we load your content...