Hydrogen bonding from crystalline water mediates the hydration/dehydration of mequitazine glycolate†
Abstract
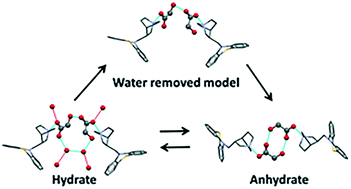
The structural transition behaviors of the hydration and dehydration of mequitazine glycolate (MQZ–GLC) after exposure to heat and relative humidity have yet not been clarified, although our previous study demonstrated that mequitazine can be hydrated with glycolic acid. In this study, the hydration and dehydration behavior of MQZ–GLC were investigated using crystal structure analysis and water adsorption/desorption measurements. Dynamic vapor sorption measurements revealed that the MQZ–GLC anhydrate undergoes irreversible hydration above a certain humidity level. The crystal structure of the anhydrate was identified via structural analysis using in situ powder diffraction (PXRD) measurements and dispersion-corrected density functional theory (DFT-D) calculations. The structural characterization of the hydrate/anhydrate of MQZ–GLC showed that the formation of a hydrogen-bonding network by bridging crystalline water stabilized the hydrophilic layer of the hydrate more than that of the anhydrate. Furthermore, the geometry optimization of the dehydration model and the lattice energy calculations revealed that the conformational change during the dehydration process and the transition to the hydrate form in the solid phase are energetically induced.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...