The influence of Fe3+ doping on thermally induced crystallization and phase evolution of amorphous calcium phosphate†
Abstract
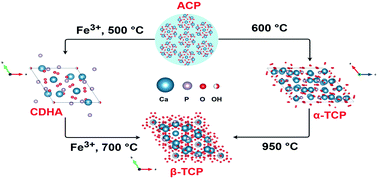
The present study investigates thermally induced crystallization and phase evolution of amorphous calcium phosphate (ACP) partially substituted with Fe3+ ions (M/P = 1.5 : 1). It was demonstrated that the presence of Fe3+ ions radically changes the crystallization behavior of ACP and completely prevents the formation of α-tricalcium phosphate (α-TCP, Ca3(PO4)2), which is the first crystalline phase obtained from non-substituted ACP upon thermal treatment. Surprisingly, calcium deficient hydroxyapatite (CDHA) was obtained instead of α-TCP. Such unusual crystallization behavior was observed with a doping level as low as 0.1 mol% with respect to Ca ions. Moreover, it was shown that the presence of Fe3+ ions significantly reduces the crystallization temperature of ACP, which started already at around 400 °C, whereas pristine ACP crystallizes to α-TCP only at around 600 °C. In the presence of Fe3+ ions β-TCP was also obtained at reduced temperature, compared to the formation from non-substituted ACP. The temperature of the complete conversion of CDHA to β-TCP was found to be dependent on the concentration of Fe3+ ions. The crystallization process and phase evolution were monitored employing thermal analysis, XRD, FTIR, Raman, EPR and Mössbauer spectroscopies.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...