Co-crystal of Ti4Ni2 and Ti8Ni4 clusters with enhanced photochemical properties†
Abstract
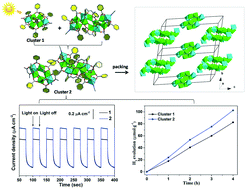
Many titanium-oxo clusters (TOCs) with various structure types have been reported, but research on co-crystallized TOCs still remains very rare. Herein, we successfully isolated heterometallic {Ti8Ni4+ Ti4Ni2} with co-crystal arrangement. Because of the Ni-to-TiO and Ti4Ni2-to-Ti8Ni4 charge transfer, cluster {Ti8Ni4+ Ti4Ni2} shows wide light absorption and narrow band gap based on the ultraviolet-visible spectra and density functional theory calculations. Moreover, the cluster co-crystallization and more metal centers in cluster {Ti8Ni4+ Ti4Ni2} endow it with better photocurrent response and photocatalytic hydrogen evolution activity than {Ti4Ni2} alone. This work not only provides a new model for co-crystallized TOCs but also stimulates research interest in their photochemical performances.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...