Molecular engineering of carbazole–acrylonitrile fluorophores: substituent-dependent optical properties and mechanochromism†
Abstract
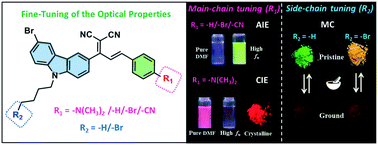
Controlling the fluorescence properties of organic molecules in the aggregation state and understanding the structure–activity relationship are important for developing excellent luminophors with tunable solid emission. Herein, a series of carbazole based donor/acceptor (D/A) type fluorophores functionalized with different substituents (–N(CH3)2, –H, –Br, –CN) were designed and synthesized. The optical measurements show that the compounds exhibit unique solid luminescence because the substituents influence the aggregated packing mode. Interestingly, the compounds CZ–H, CZ–Br, and CZ–CN with –H, –Br, and –CN substituents display aggregation-induced emission (AIE) originating from the restriction of the molecular vibration and rotation. The compound CZ–N with the –N(CH3)2 substituent exhibits crystallization-induced emission (CIE) derived from the packing differences and an increase in crystallinity from amorphous to crystalline states. Furthermore, the N-butylcarbazole unit of CZ–N, CZ–H, CZ–Br and CZ–CN was replaced by the N-bromidebutylcarbazole unit to obtain the corresponding polybromide compounds Br–CZ–N, Br–CZ–H, Br–CZ–Br, and Br–CZ–CN. Notably, the solid emission of these four compounds is largely red-shifted to longer wavelength regions (λFL = 558–618 nm) compared to those without a bromide substituent in the N-butyl chain of carbazole, owing to the bromine induced unique molecular conformation and packing arrangements in the aggregation state. Moreover, because of the diverse fluorescence properties of the eight compounds in various aggregated forms, they exhibit reversible fluorescence response to the transition of solid phases between crystalline and amorphous states under the mechanical force stimuli through breaking the multi-intermolecular interactions.
- This article is part of the themed collection: Database Analysis


 Please wait while we load your content...
Please wait while we load your content...