Sandwich-like low-sensitive nitroamine explosives stabilized by hydrogen bonds and π–π stacking interactions†
Abstract
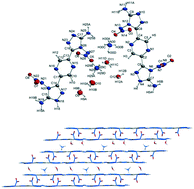
Nitroamine-containing compounds are a class of high energy materials usually exhibiting high impact sensitivity. Exploring low-sensitive nitroamine explosives is meaningful since explosives have helped to reduce labor costs and enhanced production efficiency greatly. In this study, two new nitroamine explosives, 2,6-bis(3-amino-4-nitramino 1,2,4-triazol-5-yl)-pyridine (2a) and 2,5-bis(3-amino-4-nitramino-1,2,4-triazol-5-yl)-pyrazine (2b), and their salts were designed and synthesized. The molecular structures of compound 2a and ammonium salt of 2b (4b) were confirmed using single-crystal X-ray diffraction. It is found that their molecular backbones stack into a layered structure while the nitroamine groups locate between layers leading to a sandwich-like structure. Low impact sensitivity of all the materials is verified with standard BAM measurements. Salts 4a, 4b, and 5b exhibit good heat resistance. Theoretical calculations show that all the compounds have high positive enthalpies of formation (from 647.9 to 1052.7 kJ mol−1) which offer them good detonation performances. In addition, Hirshfeld and IGM (independent gradient model) analyses were carried out to discover the intermolecular interaction of 2a and 4b and have clarified the relationship between the structures and molecular stabilities.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...