Adjacent N→O and C–NH2 groups — a highly efficient amphoteric structure for energetic materials resulting from tautomerization proved by crystal engineering†
Abstract
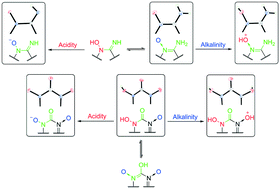
Existing amphoteric energetic materials (EMs) are very scarce, and their amphoteric structures are difficult to reproduce and protonate. Herein, adjacent N→O and C–NH2 groups (O←N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2) were found to be a highly efficient and fairly balanced amphoteric energetic structure for EMs by crystal engineering and are the first discovered amphoteric structure caused by tautomerization to the best of our knowledge. As the O←N
C–NH2) were found to be a highly efficient and fairly balanced amphoteric energetic structure for EMs by crystal engineering and are the first discovered amphoteric structure caused by tautomerization to the best of our knowledge. As the O←N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2 structure has existed in many synthesized nitrogen-rich EMs, our work will greatly enrich amphoteric EMs.
C–NH2 structure has existed in many synthesized nitrogen-rich EMs, our work will greatly enrich amphoteric EMs.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...