Open Access Article
Open Access ArticlePd-catalyzed allylative dearomatisation using Grignard reagents†
Cosimo
Boldrini
 and
Syuzanna R.
Harutyunyan
and
Syuzanna R.
Harutyunyan

Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, Groningen, 9747 AG, The Netherlands. E-mail: s.harutyunyan@rug.nl
First published on 25th October 2021
Abstract
Pd-catalyzed allylative dearomatisation of naphthyl halides is shown to be feasible by employing Grignard reagents. The high reactivity of the nucleophile allows for fast reactions and low catalyst loading, while a plethora of successfully substituted compounds illustrate the broad scope. Five membered heteroaromatic compounds are also demonstrated to be reactive under similar conditions.
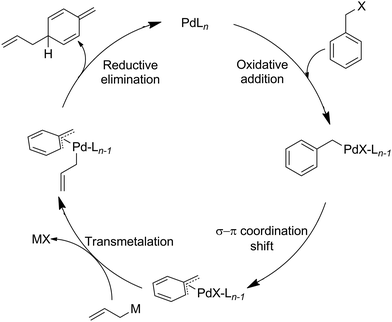
Dearomatisation reactions of arenes are an effective mechanism to convert simple planar aromatic compounds into chiral alicyclic systems.1 These complex, three-dimensional molecular scaffolds are important synthetic building blocks for both natural product synthesis and drug discovery.1,2 Despite the long history (dating back to 1885) and wide utilization of dearomatisation reactions in organic synthesis, the development of general and efficient methodologies lags far behind when compared to typical aromatic substitution reactions. Dearomatisation strategies3 include reductive,4 oxidative,5 enzymatic,6 transition-metal-mediated,7 as well as cycloaddition-based8 methodologies. Often these processes involve electron-biased molecules such as phenols9 and azines10 to achieve dearomatisation of the corresponding aromatic molecules. Methodologies that involve electronically unbiased arenes, especially in intermolecular processes, are limited.3,11 An interesting example of such a transformation is the palladium-catalyzed allylative dearomatisation of benzylic systems.12 In 2001, Yamamoto et al. elegantly demonstrated dearomatisation of benzylic halides employing stannanes as allylating reagents.13,14 This transformation relies on the formation of a π-benzyl intermediate (Scheme 1) after the oxidative addition of the palladium catalyst to the benzylic halides, which then evolves – through transmetalation and reductive elimination – to the dearomatised species.12 Previously a mechanism was proposed that allows for a close interaction between the allyl and the p-carbon through a rearrangement in the palladium coordination.13 However, a recent computational study indicated that an alternative pathway might be more feasible.15 Following the original report by Yamamoto et al. more reports were published on allylative dearomatisation of benzylic systems.
Bao et al. demonstrated that both allyl silanes and allyl boronic esters can be used as nucleophiles to achieve similar transformations.16 Then Yamaguchi et al. reported the dearomative allylation of benzyl phosphates with allyl borates,17 as well as a series of beautiful dearomative three component reactions of haloarenes, in which the π-benzyl complex is generated by the Pd-catalyzed insertion of diazo-compounds.18 Interestingly, nitrogen based as well as malonates and arylacetonitriles based nucleophiles were also reported to support addition to the π-benzyl intermediate,19,20 although most protocols require subsequent in situ rearomatisation of the formed semibenzene to achieve good conversions. All of these methodologies result in good yields; however, the low reactivity of the reported nucleophiles give rise to longer reaction times (12–24 h) and typically necessitate a relatively high catalyst loading, specific phosphine-ligands and high temperatures.12,16,17
We envisioned that all these issues could be addressed by using an allylic Grignard reagent as a highly reactive and readily available allylating agent. While there is a clear advantage of using Grignard reagents in dearomatisation of benzylic systems, a plethora of new issues could arise from the use of such a reactive organometallic reagent, among which direct uncatalyzed benzyl coupling reactions and Pd-catalyzed homo-coupling of the benzyl halide, triggered by the reductive potential of the Grignard reagent.21 Furthermore, it is known that the presence of strong bases can lead to rearomatisation of the initial product.19 In fact, bases are often used to trigger rearomatisation in order to achieve higher conversions.19
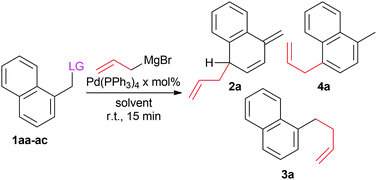
Keeping all of this in mind, we started our investigation by studying the addition of allyl magnesium bromide to various naphthyl halides (1aa–1ac) as model compounds (Table 1).
| Entry | LG | x mol% | Solvent | 2a/3a/4a | Yieldb (%) |
|---|---|---|---|---|---|
| a General reaction conditions, 1aa–ac (0.3 mmol), allylMgBr (1.25 equiv., 1 M in Et2O), Pd(PPh3)4 (x mol%), in the specified solvent (1.0 mL, 0.3 M), reaction time 15 minutes at r.t. b Isolated yield. c 2 h reaction time. d Reaction performed on 1 mmol scale. e Reaction performed on 10 mmol scale. | |||||
| 1 | Cl (1aa) | 5 | CH2Cl2 | >99/<1/<1 | 94 |
| 2 | Br (1ab) | 5 | CH2Cl2 | >99/<1/<1 | 93 |
| 3 | F (1ac) | 5 | CH2Cl2 | 60/40/<1 | 54 |
| 4c | Cl | 0 | CH2Cl2 | 0/100/0 | 92 |
| 5 | Cl | 1 | CH2Cl2 | >99/<1/<1 | 94 |
| 6d | Cl | 0.5 | CH2Cl2 | >99/<1/<1 | 93 |
| 7d | Cl | 0.1 | CH2Cl2 | 85/15/<1 | 81 |
| 8d | Cl | 0.1 | 2-Me-THF | >99/<1/<1 | 95 |
| 9e | Cl | 0.1 | 2-Me-THF | >99/<1/<1 | 97 |
As stated before, the use of a Grignard reagent as nucleophile could lead, aside from the desired dearomatised product 2, to products 3 and 4, derived from an uncatalysed SN2 type reaction and the rearomatisation of the initial product, respectively. We expected that the ratio of compound 2 to 3 would be affected by the nature of the leaving group (LG) and the amount of the Pd-catalyst, whereas the formation of the rearomatised product 4 will depend on the reaction time and the excess of Grignard reagent. Several combinations of palladium sources (such as Pd(OAc)2 and Pd2(dba)3) and electron rich phosphine ligands (such as PCy3 and 4-(dimethylamino)phenyldiphenylphosphine) were tested, but the best results were obtained with Pd(PPh3)4 without any additional ligand. Because of this reason, as well as due to its stability and availability, this Pd source was chosen as our catalyst of choice. Chloride or bromide based benzylic substrate (entries 1 and 2) yielded the desired product 2a with full selectivity and high yield, in just 15 minutes at room temperature. Even fluoride proved to act as LG (entry 3), albeit with lower selectivity, most probably due to a slower oxidative addition of the palladium in this case. As expected, the reaction without catalyst (entry 4) yielded only the side product 3a.
Encouraged by these results, we were interested in the possibility of lowering the high catalyst loading usually employed in the reported protocols. Using CH2Cl2 as a solvent (entries 5–7) the catalyst loading could be reduced by a factor 10 (0.5 mol%) before the chemoselectivity of the reaction was negatively affected. However, switching from CH2Cl2 to 2-Me-THF (entry 8) allowed full chemoselectivity to be reached with only 0.1 mol% of catalyst (∼1000 TON), with complete conversion of the substrate in under 15 minutes. This is attributed to the peculiar properties of 2-Me-THF, which is on the one hand a good solvating agent so it will form strong interactions with the Grignard, but is at the same time also bulky so that the reactivity of the Grignard will be somewhat reduced.22 As expected, the short reaction time allowed us to avoid the formation of rearomatised products.
Lastly, we performed the reaction on 10 mmol (1.77 g, entry 9), scale and found full conversion towards the dearomatised product 2a in 15 minutes with excellent chemoselectivity and high isolated yield (97%). Interestingly, under similar catalytic condition, reactions with non-allylic Grignard (e.g. EtMgBr, PhMgBr, vinylMgBr) yielded only mixture of SN2 product (3) and homo-coupling of the halide.
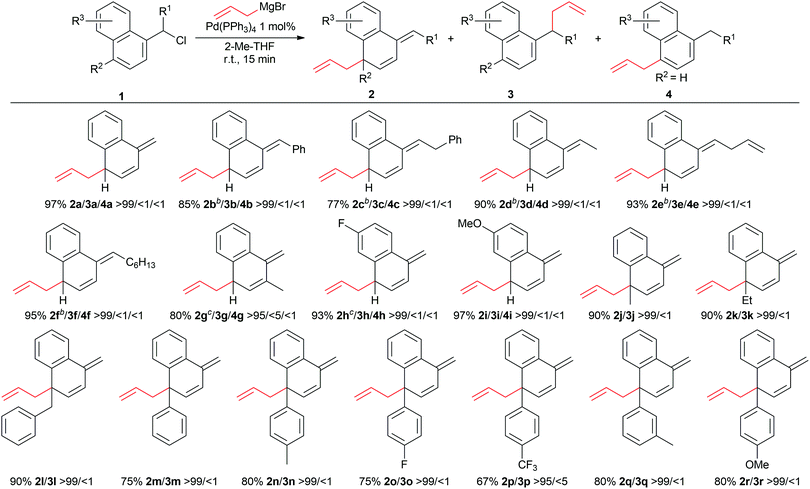
With the optimized reaction conditions in hand, we studied how the outcome of the reaction is influenced by different substitution patterns of aromatic substrates (Table 2).
| a General reaction conditions 1 (0.3 mmol), allylMgBr (1.25 equiv., 1 M in Et2O), Pd(PPh3)4 (1 mol%), in 2-Me-THF (1.0 mL, 0.3 M) for 15 minutes at r.t.; unless otherwise stated, reported yields are isolated yields of 2. b Only (E)-stereoisomer is formed in the reaction. c Product rearomatises during purification, yield determined by 1H NMR analysis of crude products. |
|---|
|
First, we looked at various substitutions in the benzylic position (2a–2f). Both in the presence of non-bulky alkyl chain and phenyl substituents, the dearomatised products were obtained in high yields and complete selectivity. In all the reactions, the E configuration of the tri-substituted external double bond was found (confirmed by NOESY NMR), in line with the previous report.17 Substitution at the ortho position (2g) slightly decreased the yield, whereas substituents on the other aromatic ring seem to be well tolerated (2h, 2i). The p-unsubstituted substrates (2a, 2i) don’t rearomatise during the reaction, despite the presence of an excess of Grignard reagent. Various p-substituted substrates were tested as well, which could result in decreased yields. To our delight, however, p-Me, p-Et and p-Bn substituted substrates all afforded the corresponding dearomatised products (2j–l) with excellent conversions and yields in under 15 minutes. The presence of an aromatic ring in the para position was also well tolerated (2m–2r). Electron withdrawing and electron donating groups do not have a strong impact on the outcome of the dearomatisation. However, the presence of an ortho substituent on the phenyl ring inhibited the reaction, most likely due to the increased steric bulk around the reactive center. Moreover, it is interesting to note that in aryl substituted substrates (2m–2r) the selectivity drops when performing the reaction in CH2Cl2. Benzyl chloride showed also some reactivity in this dearomatisation protocol, albeit with a much lower selectivity. The reaction in CH2Cl2 led to a mixture of dearomatised and benzyl coupling products (2/3), with the latter always the major product. On the other hand, the reaction in 2-Me-THF always yielded primarily the homo-coupling product.
When using branched allyl Grignard (Scheme 2), their ambident nucleophilicity can result in different products. In this context, the reaction of 1aa with 2-butenyl magnesium chloride, which has two different reactive sites, resulted in two products (2ab and 2ac) with an 8 to 2 ratio (the highest selectivity reported so far for this type of allylation).16,17
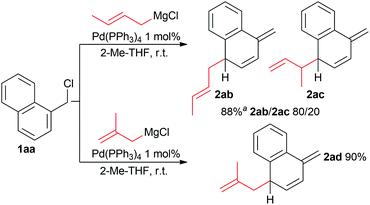 | ||
| Scheme 2 Reaction of branched allyl. aProduct rearomatises during purification, yield determined by 1H NMR analysis of crude products. | ||
On the other hand, the dearomatised product 2ad was obtained with 90% yield when using 2-methyl-2-propenyl magnesium chloride with two equivalent reactive sites, although a slight excess of the Grignard reagent was required to reach full conversion. Interestingly, 1-methyl-2-propenyl magnesium chloride afforded the products 2ab and 2ac in the same ratio as 2-butenyl magnesium chloride. In line with what was previously reported, compounds with substituents at the para position that can act as a leaving group led to double allylation product 2s (Scheme 3).15
As expected, when less than 2 equivalents of the Grignard reagent were added, the reaction yielded a mixture of mono- and bis-allylated products, as well as unreacted starting material. We then focused our attention on expanding this protocol to five membered heteroaromatic compounds (Scheme 4), since such aromatic cores and their alicyclic derivatives find many applications in the synthesis of biologically active molecules.23
 | ||
| Scheme 4 Reaction of heteroaromatic compounds. aProduct rearomatises during purification, yield determined by 1H NMR analysis of crude products. | ||
Using the optimized reaction conditions, the dearomative allylation of both N-tosylated pyrrole (5a) and thiophene (5b) yielded the corresponding products 6a and 6b in high yields, while only side products were obtained with furan derived substrate.
To conclude, the use of Grignard reagents in the Pd-catalyzed allylative dearomatisation of naphthyl halides allows for fast reactions (minutes) with low catalyst loading, which can be easily scaled up. The dearomatised compounds are obtained with good to excellent yield and complete selectivity, even with p-unsubstituted benzylic halides. The protocol can also be applied to five membered heteroaromatic compounds.
Financial support from the European Research Council (S. R. H. Grant No. 773264, LACOPAROM) is acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) C. Zheng and S. You, ACS Cent. Sci., 2021, 7, 432–444 CrossRef CAS PubMed; (b) L. Williams, Y. Bhonoah, L. A. Wilkinson and J. W. Walton, Chem. – Eur. J., 2021, 27, 3650 CrossRef CAS PubMed; (c) W. C. Wertjes, E. H. Southgate and D. Sarlah, Chem. Soc. Rev., 2018, 47, 7996 RSC.
- (a) S. P. Roche and J. A. Porco, Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS PubMed; (b) C. Zheng and S. You, Nat. Prod. Rep., 2019, 36, 1589 RSC; (c) Z. Wang, Org. Biomol. Chem., 2020, 18, 4354 RSC.
- C. F. Pigge, in Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds, ed. Mortier, J., John Wiley & Sons, Inc, 1st edn, 2016, ch. 15 Search PubMed.
- (a) P. W. Rabideau and Z. Marcinow, Org. React., 1992, 42, 1 CAS; (b) P. J. Dyson, Dalton Trans., 2003, 2964 RSC; (c) A. Gualandi and D. Savoia, RSC Adv., 2016, 6, 18419 RSC; (d) J. A. Leitch, T. Rogova, F. Duarte and D. J. Dixon, Angew. Chem., Int. Ed., 2020, 59, 4121 CrossRef CAS PubMed.
- (a) Y. Tamura, T. Yakura, J. Haruta and Y. Kita, J. Org. Chem., 1987, 52, 3927 CrossRef CAS; (b) L. Pouysegu, D. Deffieux and S. Quideau, Tetrahedron, 2010, 66, 2235 CrossRef CAS; (c) W.-T. Wu, L. Zhang and S.-L. You, Chem. Soc. Rev., 2016, 45, 1570 RSC.
- (a) P. A. Peixoto, M. El Assal, I. Chataigner, F. Castet, A. Cornu, R. Coffinier, C. Bosset, D. Deffieux, I. Pouysegu and S. Quideau, Angew. Chem., Int. Ed., 2021, 60, 14967 CrossRef CAS PubMed; (b) Z. Lin, Y. Xue, X. W. Liang, J. Wang, S. Lin, J. Tao, S. L. You and W. Liu, Angew. Chem., Int. Ed., 2021, 60, 8401 CrossRef CAS PubMed.
- (a) A. R. Pape, K. P. Kaliappan and E. P. Kendig, Chem. Rev., 2000, 100, 2917 CrossRef CAS PubMed; (b) M. Rosillo, G. Dominguez and J. Perez-Castells, Chem. Soc. Rev., 2007, 36, 1589 RSC; (c) B. K. Liebov and W. D. Harman, Chem. Rev., 2017, 117, 13721 CrossRef CAS PubMed.
- (a) M. Ohashi, Y. Tanaka and S. Yamada, Tetrahedron Lett., 1977, 18, 3629 CrossRef; (b) S. J. Hamrock and R. S. Sheridan, J. Am. Chem. Soc., 1989, 111, 9247 CrossRef CAS; (c) C. Tang, M. Okumura, H. Deng and D. Sarlah, Angew. Chem., Int. Ed., 2019, 58, 15762 CrossRef CAS PubMed; (d) W. C. Wertjes, M. Okumura and D. Sarlah, J. Am. Chem. Soc., 2019, 141, 163 CrossRef CAS PubMed.
- (a) W. Sun, G. Li, L. Hong and R. Wang, Org. Biomol. Chem., 2016, 14, 2164 RSC; (b) H. Nakayama, S. Harada, M. Kono and T. Nemoto, J. Am. Chem. Soc., 2017, 139, 10188 CrossRef CAS PubMed.
- (a) G. Bertuzzi, L. Bernardi and M. Fochi, Catalysts, 2018, 8, 632 CrossRef; (b) M. W. Gribble, R. Y. Liu and S. L. Buchwald, J. Am. Chem. Soc., 2020, 142, 11252 CrossRef CAS PubMed.
- (a) Y. Guo, T. V. Nguyen and R. M. Koenigs, Org. Lett., 2019, 21, 8814 CrossRef CAS PubMed; (b) K. L. Smith, C. L. Padgett, W. D. Mackay and J. S. Johnson, J. Am. Chem. Soc., 2020, 142, 6449 CrossRef CAS PubMed; (c) B. Zhou, H. Wang, Z. Cao, J. Zhu, R. Liang, X. Hong and T. Jia, Nat. Commun., 2020, 11, 4380 CrossRef CAS PubMed; (d) Y. Cheng, X. Huang, W. Zhuang, Q. Zhao, X. Zhang, T. Mei and S. You, Angew. Chem., Int. Ed., 2020, 59, 18062 CrossRef CAS PubMed.
- (a) S. Zhang, Y. Yamamoto and M. Bao, Adv. Synth. Catal., 2021, 363, 587 CrossRef CAS; (b) B. M. Trost and L. C. Czabaniuk, Angew. Chem., Int. Ed., 2014, 53, 2826 CrossRef CAS PubMed.
- M. Bao, H. Nakamura and Y. Yamamoto, J. Am. Chem. Soc., 2001, 123, 759 CrossRef CAS PubMed.
- S. Zhang, X. Yu, X. Feng, Y. Yamamoto and M. Bao, Chem. Commun., 2015, 51, 3842 RSC.
- (a) F. Azambuja, M. Yang, T. Feoktistova, M. Selvaraju, A. C. Brueckner, M. A. Grove, S. Koley, P. H. Cheong and R. A. Altman, Nat. Chem., 2020, 12, 489 CrossRef PubMed; (b) M. Ling, J. Yuan, Z. Song, J. Gao, M. Cao and H. Xie, Comput. Theor. Chem., 2020, 1191, 113030 CrossRef CAS.
- (a) B. Peng, X. Feng, X. Zhang, S. Zhang and M. Bao, J. Org. Chem., 2010, 75, 2619 CrossRef CAS PubMed; (b) S. Zhang, A. Ullah, Y. Yamamoto and M. Bao, Adv. Synth. Catal., 2017, 359, 2723 CrossRef CAS; (c) S. Zhang, J. Cai, Y. Yamamoto and M. Bao, J. Org. Chem., 2017, 82, 5974 CrossRef CAS PubMed.
- (a) M. Komatsuda, K. Muto and J. Yamaguchi, Org. Lett., 2018, 20, 4354 CrossRef CAS PubMed; (b) A. Yanagimoto, M. Komatsuda, K. Muto and J. Yamaguchi, Org. Lett., 2020, 22, 3423 CrossRef CAS PubMed.
- (a) M. Komatsuda, H. Kato, K. Muto and J. Yamaguchi, ACS Catal., 2019, 9, 8991 CrossRef CAS; (b) H. Kato, I. Musha, M. Komatsuda, K. Muto and J. Yamaguchi, Chem. Sci., 2020, 11, 8779 RSC.
- (a) S. Zhang, Y. Wang, X. Feng and M. Bao, J. Am. Chem. Soc., 2012, 134, 5492 CrossRef CAS PubMed; (b) Y. Xu, M. Zhu, Y. Lin and S. Tian, Org. Lett., 2019, 21, 7169 CrossRef CAS PubMed.
- (a) B. Peng, S. Zhang, X. Yu, X. Feng and M. Bao, Org. Lett., 2011, 13, 5402 CrossRef CAS PubMed; (b) S. Zhang, Y. Yamamoto and M. Bao, J. Org. Chem., 2018, 83, 13981 CrossRef CAS PubMed.
- (a) S. Khan, A. Ghatak and S. Bhar, Tetrahedron Lett., 2015, 56, 2480 CrossRef CAS; (b) Y. Cai, X. Qian and C. Gosmini, Adv. Synth. Catal., 2016, 358, 2427–2430 CrossRef CAS; (c) M.-Z. Zhu, D. Xie and S.-K. Tian, Org. Lett., 2021, 23(17), 6877–6888 CrossRef CAS PubMed.
- (a) M. Sassian and A. Tuulmets, Helv. Chim. Acta, 2003, 86, 82 CrossRef CAS; (b) D. F. Aycock, Org. Process Res. Dev., 2007, 11, 156 CrossRef CAS; (c) S. Monticelli, L. Castoldi, I. Murgia, R. Senatore, E. Mazzeo, J. Wackerlig, E. Urban, T. Langer and V. Pace, Monatsh. Chem., 2017, 148, 37 CrossRef CAS PubMed; (d) W. Zhong, Y. Wu and X. Zhang, J. Chem. Res., 2009, 370 CrossRef CAS.
- (a) I. S. Young, P. D. Thornton and A. Thompson, Nat. Prod. Rep., 2010, 27, 1801 RSC; (b) J. B. Sperry and D. L. Wright, Curr. Opin. Drug Discovery Dev., 2005, 8, 723 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc05609c |
| This journal is © The Royal Society of Chemistry 2021 |